Introduction
Pyrimidine nucleoside phosphorylase is the
collective name for enzymes involved in the metabolism of
pyrimidine nucleosides, which convert thymidine to thymine and
participate in the biosynthesis of pyrimidine nucleosides,
angiogenesis and activation of anti-neoplastic drugs (1). There are two thymidine phosphorylases:
Uridine phosphorylase (UP), which belongs to the family of
glycosyltransferases, specifically the pentosyltransferases; and
thymidine phosphorylase (TP), which belongs to the family of
glycosyltransferases, specifically the pentosyltransferases
(2). UP has uridine and thymidine as
its substrates, and is abundant in mice and rats (2), while TP only has thymidine as its
substrate, and is abundant in humans (2). TP is involved in the synthesis of
nucleic acids, and its activity is increased in cancer cells due to
the additional nucleic acid synthesis required for active cell
proliferation (2). TP has a similar
structure to that of the angiogenic factor platelet-derived
endothelial cell growth factor (PD-ECGF), and promotes vascular
endothelial migration (3).
Another tumor-associated action of TP that has
attracted attention is the activation of 5-fluorouracil (5-FU)
prodrugs (2). 5-FU was first
synthesized by Dushinsky et al (4) in 1957, and its efficacy as an
anti-cancer agent was subsequently established by the fundamental
and clinical studies of Heidelberger et al (5). Since then, its use has been approved for
a variety of tumors, including breast and gastrointestinal cancers
(6–10). Once incorporated into cells by
nucleotide transporters, 5-FU is largely degraded and inactivated
by dihydropyrimidine dehydrogenase (DPD), prior to be excreted in
the urine as α-fluoro-β-alanine, while unchanged 5-FU is
phosphorylated and activated via the same pyrimidine metabolic
pathway that processes uracil (11).
The anti-neoplastic effect of 5-FU generally depends on the
following mechanism: When 5-FU is metabolized to fluorodeoxyuridine
monophosphate (FdUMP) by TP, it forms a strong ternary complex with
thymidylate synthetase and 5,10-methylenetetrahydrofolate (a
reduced folic acid coenzyme), thus inhibiting the conversion of
dUMP to thymidine 5′-monophosphate and interfering with DNA
synthesis (11). 5-FU also causes RNA
dysfunction when it is incorporated into intracellular RNA by
orotate phosphoribosyltransferase (11). Development of 5-FU prodrugs with
various mechanisms of action has enabled the availability of a
number of drugs, including doxifluridine, capecitabine, uracil plus
tegafur (UFT) and titanium silicate-1 (Fig. 1). These prodrugs are designed to
reduce adverse reactions to 5-FU or to exhibit enhanced activity
against tumors with elevated TP expression, since these agents
display an anti-tumor effect upon being converted to 5-FU by TP in
tumor cells (12).
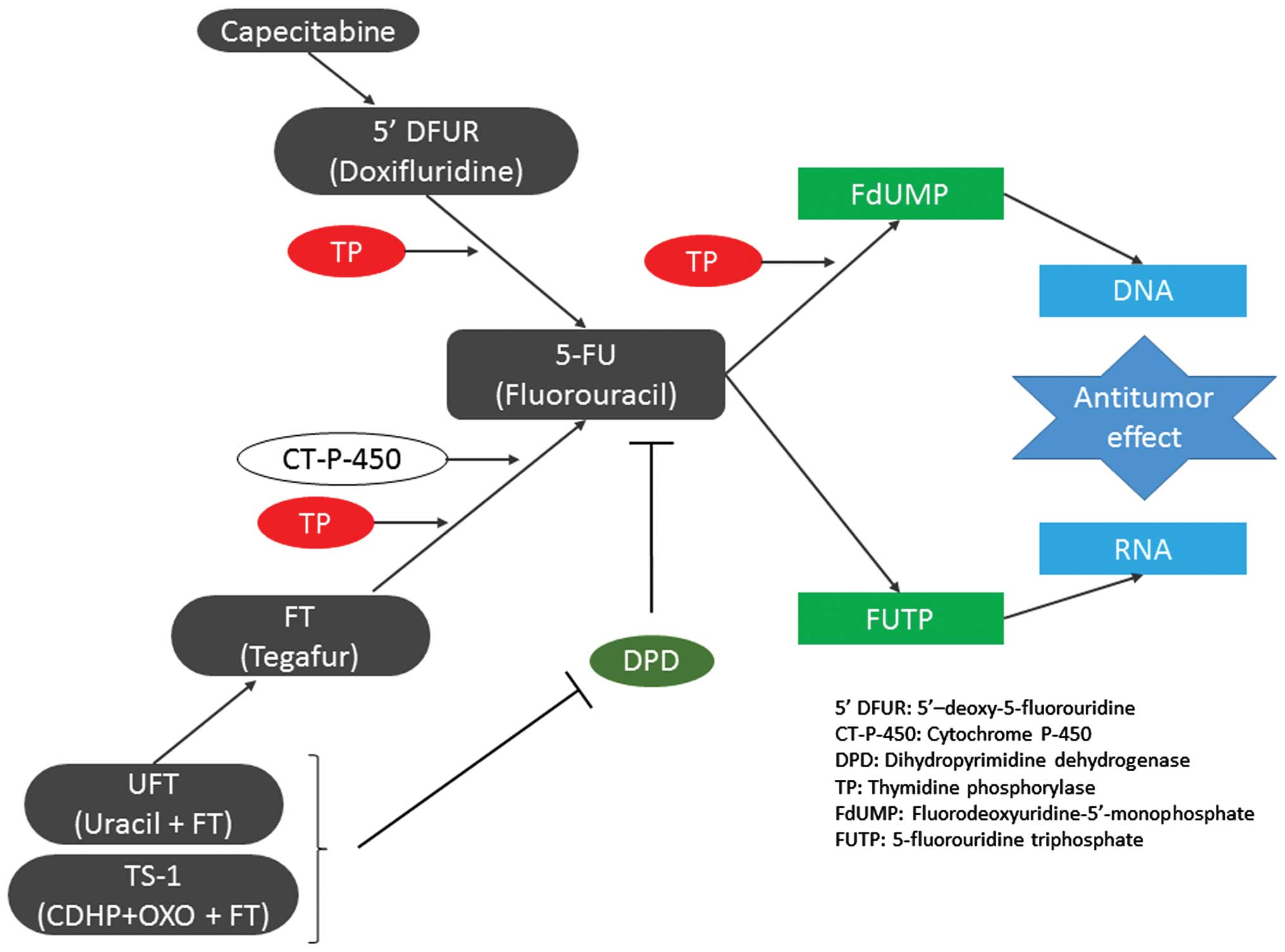 | Figure 1.Effects, summary of metabolic pathways
and antitumor activity of 5-FU and fluorinated pyrimidines.
Capecitabine is absorbed from the gastrointestinal tract,
transformed into doxifluridine in the tumor tissue and subsequently
transformed into 5-FU by TP to exert an antitumor effect. FT was
developed to reduce gastrointestinal toxicity, and is transformed
into 5-FU by cytochrome P-450 mainly in the liver and by TP in the
tumor tissue, where its concentration remains stable for a long
period. Uracil plus FT contains uracil, which has an inhibitory
effect on the degradation of 5-FU. TS-1 contains
5-chloro-2,4-dihydroxypyridine, which has a stronger inhibitory
effect on dihydropyrimidine dehydrogenase than that of uracil.
Oxonate is distributed to the gastrointestinal tract at a high
concentration, and reduces gastrointestinal toxicity by inhibiting
the phosphorylation of 5-FU. 5-FU is transformed into
fluorodeoxyuridine monophosphate in tumor cells, which inactivates
TS-1 and inhibits the synthesis of DNA. It also causes dysfunction
of RNA through 5-fluorouridine 5′-triphosphate. 5′-DFUR,
5′-deoxy-5-fluorouridine; CT-P-450, cytochrome P-450; DPD,
dihydropyrimidine dehydrogenase; TP, thymidine phosphorylase;
FdUMP, fluorodeoxyuridine monophosphate; FUTP, 5-fluorouridine
5′-triphosphate; 5-FU, fluorouracil; OXO, oxonate; CDHP,
5-chloro-2,4-dihydroxypyridine; FT, tegafur; UFT, uracil plus
tegafur; TS-1, titanium silicate. |
TP is an important enzyme that activates 5-FU.
However, there are a limited number of studies on TP expression in
gynecological cancer, with the exception of cervical cancer, for
which 5-FU prodrugs have already been approved (12), and no studies have been performed to
date to compare TP expression among all gynecological tumors. In
the present study, the expression of TP was analyzed in various
types of gynecological cancer, and the expression of TP in these
tumors was compared with the tumor characteristics to explore the
possibility of individualized treatment.
Materials and methods
Patients and tissue specimens
A total of 188 patients who underwent surgery for
gynecological tumors at the Department of Obstetrics and Gynecology
of Tokai University Hospital (Isehara, Kanagawa, Japan) between
February 2002 and January 2010 were enrolled in the present study
(Table I). At the time of surgery for
the benign tumors, samples that were considered normal tissues,
including myometrium, endometrium and ovary in 33 patients, were
resected. The samples were confirmed to be free from gynecological
neoplasm and pathologically diagnosed as normal tissue. The
Institutional Review Board (IRB) for Clinical Research of Tokai
University School of Medicine (Isehara, Kanagawa, Japan) approved
the present study (IRB no. 09R-082). Written informed consent was
obtained from all patients for the use of the resected specimens at
the time of enrollment. The present study was performed in
accordance with the Declaration of Helsinki. Clinicopathological
staging was performed according to the International Federation of
Gynecology and Obstetrics classification (13).
 | Table I.Patient characteristics and TP and DPD
activities. |
Table I.
Patient characteristics and TP and DPD
activities.
| Classification (total
cases, n=188) | Histology | Cases, n | TP average, U/mg
protein (SD) | DPD average, U/mg
protein (SD) | TP/DPD ratio |
|---|
| Uterine cervical
tumors (18) | SCC | 11 | 306.9
(106.9) | 138.2 (66.6) | 2.2 |
|
| AA | 2 | 317.6
(183.7) | 222.4
(205.3) | 1.4 |
|
| MUA | 2 | 52.7 (1.2) | 17.8
(15.0) | 3.0 |
|
| SMCC | 3 | 23.1 (8.0) | 10.4 (2.6) | 2.2 |
| Uterine body tumors
(53) | EMA-G1 | 22 | 68.6
(30.0) | 48.7
(40.0) | 1.4 |
|
| EMA-G2 | 5 | 63.3
(61.6) | 42.1
(33.1) | 1.5 |
|
| EMA-G3 | 6 | 63.0
(79.3) | 34.6
(20.2) | 1.8 |
|
| SEA | 2 | 52.4
(19.2) | 45.9
(21.9) | 1.2 |
|
| Adenomyosis | 2 | 26.2
(15.6) | 33.1
(10.6) | 0.8 |
|
| Leiomyoma | 14 | 12.9 (7.7) | 48.1
(17.5) | 0.3 |
|
| Leiomyosarcoma | 2 | 41.1
(21.4) | 54.9
(26.9) | 0.7 |
| Ovarian tumors
(84) | SEA | 9 | 98.6
(65.8) | 82.7
(70.2) | 1.2 |
|
| CCA | 16 | 115.2 (59.3) | 54.2
(109.6) | 2.1 |
|
| MUA | 16 | 54.4
(44.4) | 63.3
(47.1) | 0.9 |
|
| EMA | 8 | 74.8
(47.9) | 37.2
(12.8) | 2.0 |
|
| Serous BT | 1 | 42.2 | 59.1 | 0.7 |
|
| Mucinous BT | 19 | 17.3
(10.4) | 52.4
(24.2) | 0.3 |
|
| Mucinous
adenoma | 6 | 21.0
(16.9) | 58.7
(19.2) | 0.4 |
|
| Yolk sac tumor | 4 | 24.4
(17.6) | 73.2
(84.5) | 0.3 |
|
| Dysgerminoma | 2 | 109.5 (41.2) | 41.9 (1.2) | 2.6 |
|
| Endometriosis
cyst | 1 | 77.5 | 121.8 | 0.6 |
|
| Mature
teratoma | 1 | 69.5 | 84.7 | 0.8 |
|
| Adenofibroma | 1 | 6.4 | 142.9 | 0.0 |
| Non-neoplastic
lesions (33) | Myometrium | 12 | 32.4
(38.4) | 62.5
(19.5) | 0.5 |
|
| Endometrium | 8 | 27.5
(35.2) | 40.5
(16.3) | 0.7 |
|
| Ovary | 13 | 19.5
(14.7) | 89.3
(55.3) | 0.2 |
Enzyme-linked immunosorbent assay
(ELISA) for TP and DPD activity
TP and DPD activities were measured by a sandwich
ELISA using a Protein Detector ELISA kit (KPL, Inc., Gaithersburg,
MD, USA), according to the manufacturer's protocol. A 96-well plate
was incubated for 1 h at room temperature (RT) with 10 µg/ml
monoclonal mouse anti-TP (catalog no., 1C6-203; Roche Diagnostics
GmbH, Mannheim, Germany) and 10 µg/ml monoclonal mouse anti-DPD
antibodies (Nippon Roche Research Center, Kamakura, Kanagawa,
Japan). Then, the plasmatic compartment was added to each well and
incubated with the antibodies for 1 h. Upon washing the plate with
PBS containing 0.05% Tween-20 (Wako Pure Chemical Industries, Ltd.,
Osaka, Osaka, Japan), incubation was conducted with anti-TP and
anti-DPD antibodies overnight at 4°C. Next, peroxidase-linked
species-specific F(ab')2 fragments of anti-rabbit
immunoglobulin (Ig)G (dilution, 1:10,000; catalog number NA9340; GE
Healthcare Life Sciences, Chalfont, UK) were added to each well for
1 h. Subsequently, a reaction was conducted at RT for 15 min with
substrate solution containing 3,3′,5,5′-tetramethylbenzidine and
H2O2 (TMB Microwell Peroxidase Substrate
system; KPL), and the absorbance at 450 nm was measured using a
microplate reader (3550; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Immunohistochemistry (IHC)
The expression of TP and cluster of differentiation
(CD)34 in gynecological tumors was determined by IHC analysis and
hematoxylin (Merck, Ltd., Tokyo, Japan) and eosin (Muto Pure
Chemicals, Co., Ltd, Tokyo, Japan) staining in all 188 patients.
Tumor sections were deparaffinized in xylene (3 times for 5 min
each) and ethanol (4 times for 5 min each) and heated in 0.01 M
citrate buffer (pH 6.0; Dako, Glostrup, Denmark) for 13 min in a
microwave oven at 99°C. Then, endogenous peroxidase was blocked by
incubation in methanol (Wako Pure Chemical Industries, Ltd.)
containing 0.3% H2O2 (Wako Pure Chemical
Industries, Ltd.) for 30 min at RT. Next, the sections were washed
in 0.01 M phosphate-buffered saline (PBS; Wako Pure Chemical
Industries, Ltd.) for 10 min, prior to be incubated overnight at
4°C with anti-TP (dilution, 1:400) and monoclonal mouse anti-CD34
antibodies (dilution, 1:100; product code, END-L-CE; Novocastra;
Leica Microsystems, Ltd., Milton Keynes, UK). Subsequently, the
sections were washed in 0.01 M PBS and incubated with goat
anti-mouse/rabbit IgG conjugated to a horseradish
peroxidase-labeled dextran polymer (EnVision kit; catalog no.,
K1491; Dako) for 60 min at RT. Upon being washed 20 times in 0.01 M
PBS, the sections were developed in a 3′3-diaminobenzidine solution
(Dojindo Laboratories, Kamimashiki, Kumamoto, Japan) containing
0.006% H2O2 for 3–5 min at RT, and
counterstained with hematoxylin. Protein expression was assessed
semiquantitatively as negative (0% positive, 0), weak (<10%
positive, 1+), intermediate (10–50% positive, 2+) or strong
(>50% positive, 3+).
Analysis of microvessels by monochrome
imaging
The distribution of microvessels was analyzed by
monochrome imaging of sections immunostained with anti-CD34
antibody and mounted on glass slides (Muto Pure Chemicals, Co.,
Ltd). Tumor stromal microvessels with a longer and shorter diameter
of ≤5 mm were counted in five fields (magnification, ×200;
Axiophot; Carl Zeiss, Oberkochen, Germany) for each patient, and
the area occupied by the vessels was measured. Microvessels were
only counted in fields without necrosis that were completely filled
by tissue.
Western blotting
To confirm the results of TP expression detected by
IHC, western blot analysis was performed in four representative
tumors of squamous cell carcinoma (SCC) and clear cell
adenocarcinoma (CCA) of ovary (OV). Total protein from whole tissue
lysates was separated by electrophoresis on NuPAGE Novex 4–12%
Bis-Tris Protein Gels (1.0 mm; 12-well; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and transferred to Immobilon-P
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The running buffer was 20X NuPAGE MES SDS Running Buffer
(Thermo Fisher Scientific, Inc.) and the blotting buffer consisted
of Trizma base (Sigma-Aldrich, St. Louis, MO, USA), glycine
(Nacalai Tesque, Inc., Kyoto, Kyoto, Japan) and methanol. Precision
Plus Protein Standards All Blue (Bio-Rad Laboratories, Inc.) was
used as a marker. Membranes were blocked with 5% skim milk (Wako
Pure Chemical Industries, Ltd.) in PBS containing 0.5% Tween-20
(PBS-T) at RT for 1 h, followed by overnight incubation at 4°C with
anti-TP antibody diluted 1:500 in PBS-T containing 5% skim milk.
Horseradish peroxidase-conjugated anti-mouse/rabbit complexes were
visualized with an ECL Plus kit (GE Healthcare Life Sciences).
Cultured HeLa whole-cell lysates served as positive control. The
HeLa cells were grown and maintained in 75 cm2 tissue
culture flasks in a humidified 5% CO2 atmosphere at
37°C. The cells were cultured in Minimum Essential Media (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-incubated fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 1% penicillin-streptomycin (10,000 U/ml penicillin; 10,000
µg/ml streptomycin; Gibco; Thermo Fisher Scientific, Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
To verify the close association between TP protein
and messenger (m)RNA expression in the limited cases of
gynecological cancer evaluated in the present study, RT-PCR
analysis was performed. TRIzol (500 µl) was used to homogenize the
samples. Subsequently, the samples were centrifuged at 20,379 × g
and 4°C for 15 min, and the aqueous layer was transferred to a
different tube. After performing DNase treatment with DNAse I (RQ1
RNase-Free DNase; Promega Corporation, Madison, WI, USA) overnight
at 37°C, the quantity of total RNA dissolved in DEPC-DW was
measured using an absorbance measuring instrument, and total RNA
was calculated as 5 µg. Complementary DNA was prepared from four
representative tumors using a Ready-To-Go T-primed First-Strand kit
(GE Healthcare Life Sciences). The sequences of the TP primers used
were as follows: 5′-CTGCTGTATCGTGGGTCAGA-3′ and
5′-CAGCGTCTTTGCCAGCTC-3′ (Greiner Bio-One International GmbH,
Tokyo, Japan). In addition, β-actin was amplified with another pair
of primers (5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and
5′-CCTAGAAGCATTTGCGGTGCACGATG-3′; Promega Corporation), and served
as internal standard. PCR was initiated with denaturation at 95°C
for 10 min, followed by 30 cycles of 95°C for 20 sec, 59°C for 30
sec and 72°C for 60 sec, with a final elongation at 72°C for 10
min. PCR products were analyzed by 2% agarose gel electrophoresis
(Wako Pure Chemical Industries, Ltd.), and the gels were stained
with ethidium bromide (Invitrogen; Thermo Fisher Scientific, Inc.).
Tris-acetate-EDTA (50X; Sigma-Aldrich) was used as the running
buffer and 100 bp DNA Ladder (New England Biolabs Japan, Inc.,
Tokyo, Japan) was used as a marker. Mupid (Advance Co., Ltd.,
Tokyo, Japan) was used for electrophoresis. Visualization was
performed using High Performance UV Transilluminator (UVP, Inc.,
Upland, CA, USA). Leiomyoma samples were used as negative controls
and an RT- control (no reverse transcription) was also
performed.
Statistical and prognosis
analysis
The overall survival ratios were calculated by the
Kaplan-Meier method, and the significance of difference in survival
was analyzed by the log-rank test. Quantitative variables are
presented as the mean ± standard deviation. Data were analyzed
using SPSS software version 21.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
TP and DPD activity in various tumors
Cervical tumors
The mean TP activity was elevated in SCC (306.9 U/mg
protein) and adenosquamous carcinoma (AA) (317.6 U/mg protein) of
the cervix, although it was not very high in mucinous
adenocarcinoma (MUA) (52.7 U/mg protein) or small cell carcinoma
(SMCC) (23.1 U/mg protein). The mean DPD activity was also elevated
in SCC (138.2 U/mg protein) and AA (222.4 U/mg protein). However,
the TP/DPD ratios of all cervical tumors did not exhibit major
differences (2.2 for SCC, 1.4 for AA, 3.0 for MUA and 2.2 for
SMCC).
Tumors of the uterine corpus
There were no significant differences in TP activity
among the different grades of endometrioid adenocarcinoma (EMA),
with the mean TP activity being 68.6, 63.3 and 63.0 U/mg protein in
G1, G2 and G3 tumors, respectively. In contrast, DPD activity
decreased as EMA became progressively less differentiated, with the
mean levels being 48.7, 42.1 and 34.6 U/mg protein in G1, G2 and G3
tumors, respectively. DPD activity was significantly higher than TP
activity in leiomyoma (P=0.048), where the mean TP activity was
12.9 U/mg protein, while the mean DPD activity was 48.1 U/mg
protein, with a TP/DPD ratio of 0.3. DPD activity was also higher
than TP activity in leiomyosarcoma, with a TP/DPD ratio of 0.7.
Ovarian tumors
Comparison of the TP/DPD ratios among four
histological types of epithelial ovarian cancer [serous
adenocarcinoma (SEA)-OV, CCA-OV, EMA-OV and MUA-OV] revealed that
the TP/DPD ratio was highest for CCA-OV (2.1), followed by EMA-OV
(2.0) and SEA-OV (1.2). Mean TP activity was the highest in CCA-OV
(115.2 U/mg protein), followed by SEA-OV (98.6 U/mg protein) and
EMA-OV (74.8 U/mg protein) and MUA-OV (54.4 U/mg protein). Among
non-epithelial ovarian tumors, the mean TP activity was 109.5 U/mg
protein in dysgerminoma, where the TP/DPD ratio was also high
(2.6), while the TP/DPD ratios of the other non-epithelial tumors
were ≤1.0.
Non-tumor tissues
In normal tissues, including myometrium, endometrium
and ovary, the mean DPD activity (64.4 U/mg protein) was higher
than the mean TP activity (19.7 U/mg protein), and the TP/DPD ratio
was ≤0.7 (Table I and Fig. 2).
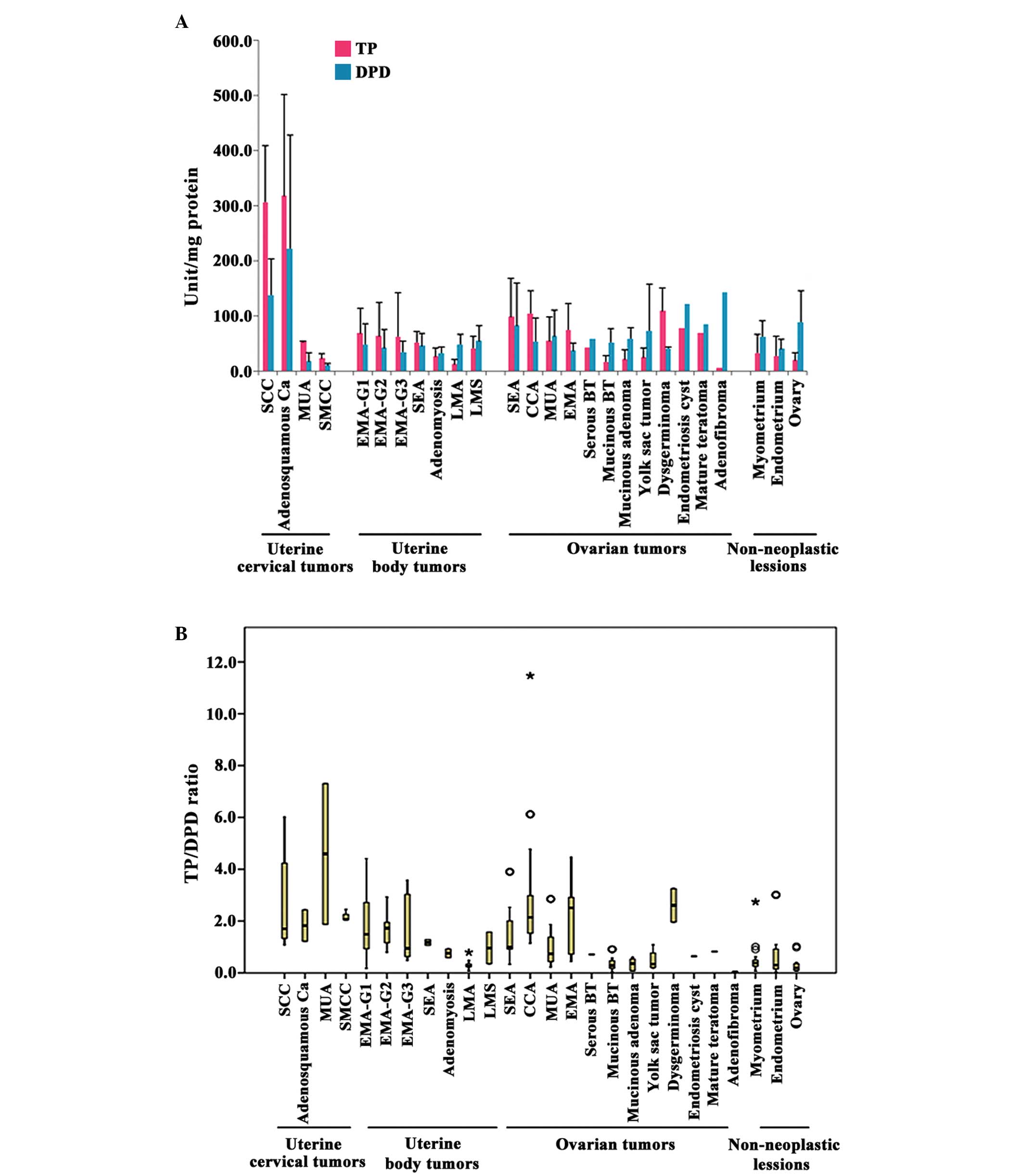 | Figure 2.(A) Enzymatic activity of TP and DPD
(mean levels stratified by histological type) and (B) TP/DPD ratio
in gynecological tumors. The mean enzymatic activity of TP and DPD
was higher in SCC of the cervix and adenosquamous carcinoma
compared with other tumor types. In patients with tumors of other
histological types, the TP levels were often higher than the DPD
levels. As observed in SCC of the cervix, the median TP/DPD ratio
was also high in other malignant tumors, and a number of patients
exhibited a very high TP/DPD ratio. *Outlier point ≥3 × IQR;
ºOutlier point 1.5 × IQR - 3 × IQR. SCC, squamous cell carcinoma;
AA, adenosquamous carcinoma; MUA, mucinous adenocarcinoma; SMCC,
small cell carcinoma; EMA, endometrioid adenocarcinoma; SEA, serous
adenocarcinoma; LMA, leiomyoma; LMS, leiomyosarcoma; CCA, clear
cell adenocarcinoma; BT, borderline tumor; TP, thymidine
phosphorylase; DPD, dihydropyrimidine dehydrogenase; IQR,
interquartile range. |
Prognosis analysis
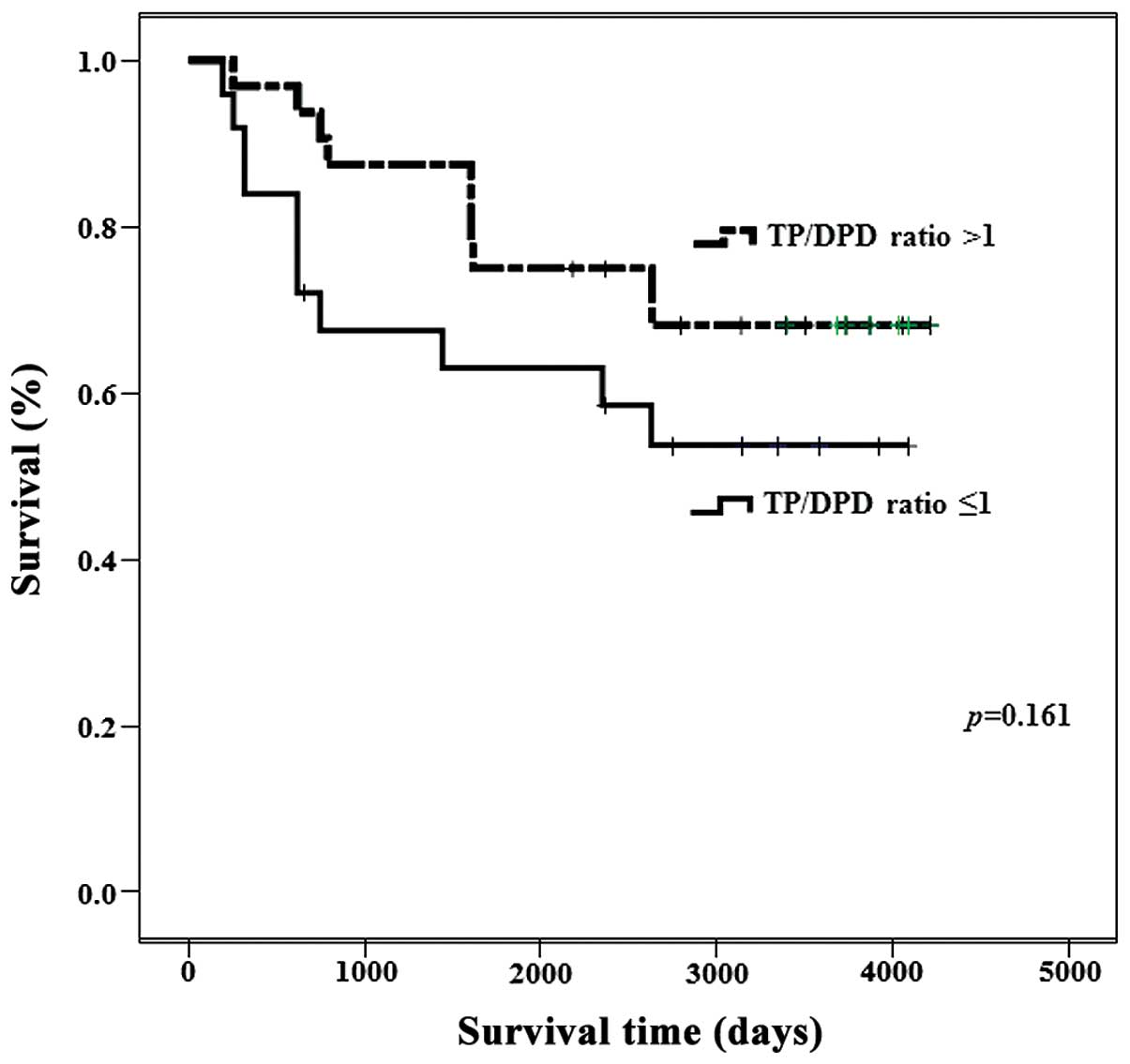
The ovarian tumors with a TP/DPD ratio >1.0
demonstrated poor prognosis (P=0.161; Fig. 3). There were no significant
differences among uterine cervical tumors and uterine body tumors
(data not shown).
IHC analysis of TP expression and
localization
TP expression was observed in a wide range of
uterine tumors, but it was generally strongest in MUA, SCC and
AA.
In tumors of the uterine corpus, TP expression was
strongest in G1 EMA, and TP expression decreased as these tumors
became less differentiated. There were significant differences in
TP expression among G1, G2 and G3 tumors (P<0.01). TP expression
could not be detected by IHC in >50% of leiomyoma cases.
Among ovarian tumors, ELISA also revealed high
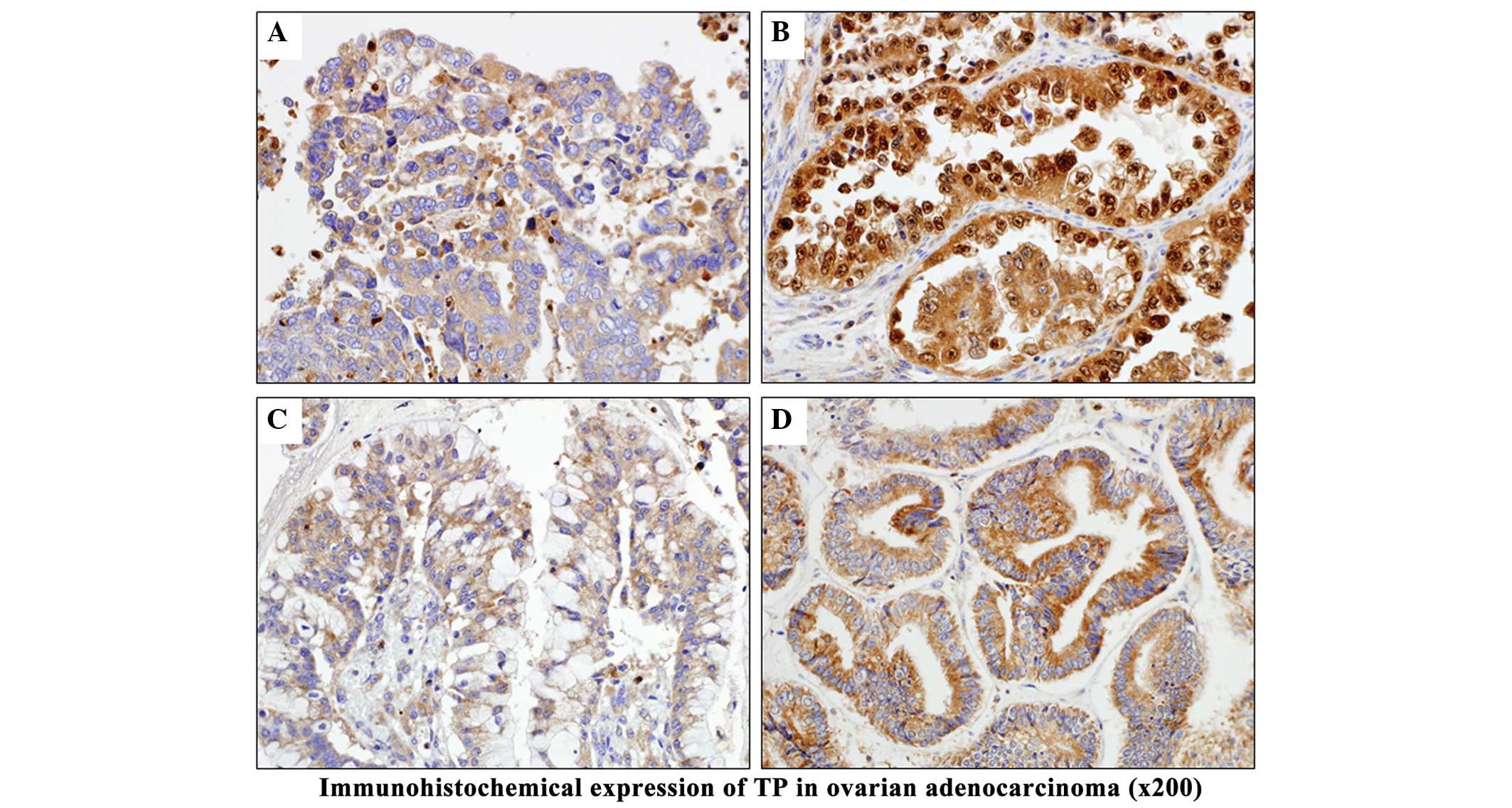
levels of TP expression in EMA-OV, CCA-OV and SEA-OV. In CCA-OV, TP
expression was detected in both the nucleus and cytoplasm of tumor
cells, and was stronger than in the other histological types. TP
expression was not observed in the normal tissues of the majority
of patients. Although TP was predominantly localized to the
cytoplasm, it was often detected in the nucleus when the tumor
exhibited strong expression (Figs. 4
and 5).
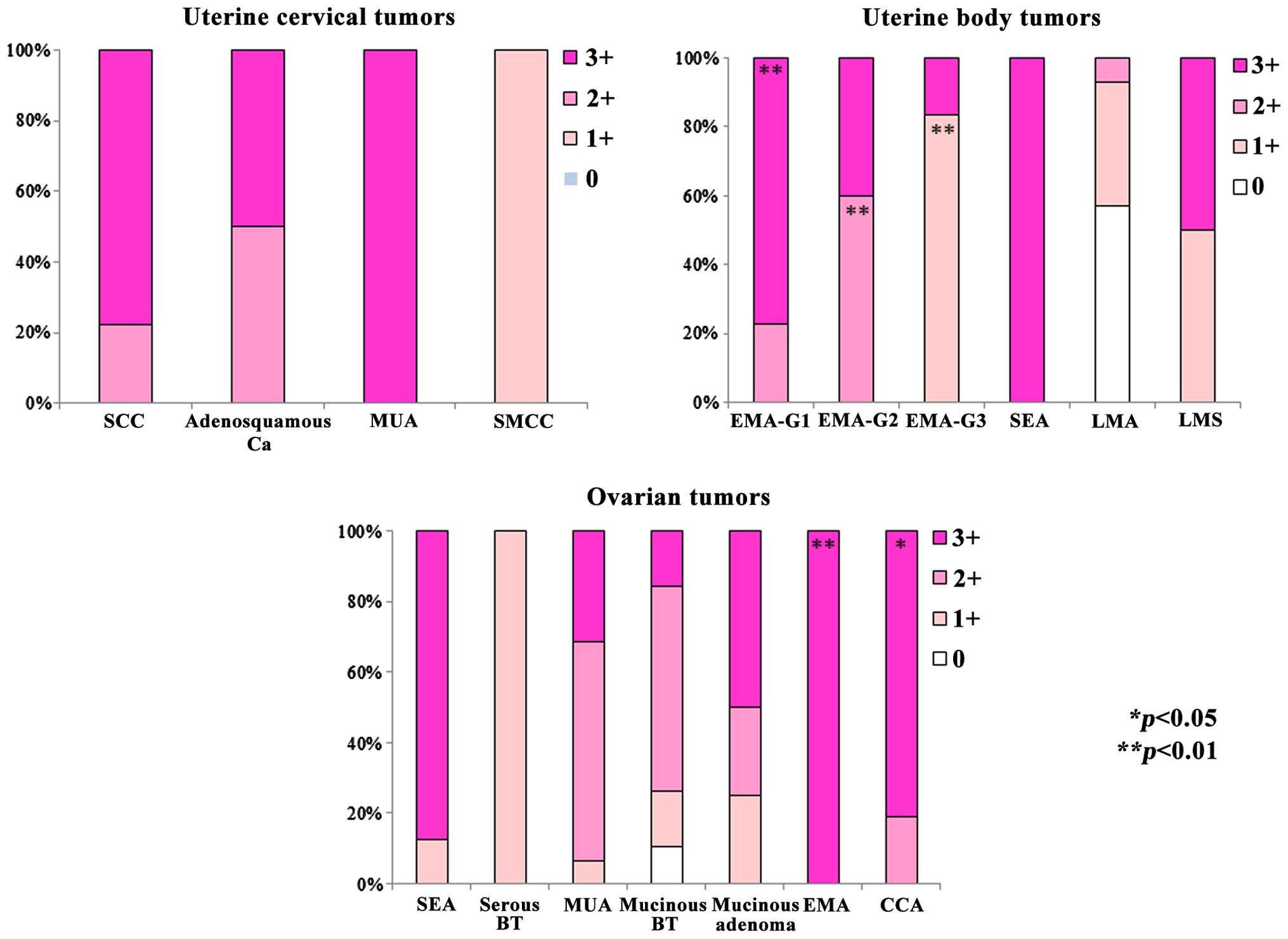 | Figure 5.Comparison of immunohistochemical
findings. All malignant tumors exhibited expression of TP at
different levels, whereas benign tumors were often negative for TP
expression. Significant differences in positive expression rate
were observed among G1, G2 and G3 EMA tumors of the uterus
(**P<0.01). Strong TP expression (3+) was detected in numerous
patients with ovarian tumors such as clear cell adenocarcinoma-OV
and EMA-OV (*P<0.05, **P<0.01). SCC, squamous cell carcinoma;
AA, adenosquamous carcinoma; MUA, mucinous adenocarcinoma; SMCC,
small cell carcinoma; EMA, endometrioid adenocarcinoma; SEA, serous
adenocarcinoma; LMA, leiomyoma; LMS, leiomyosarcoma; BT, borderline
tumor; CCA, clear cell adenocarcinoma; TP, thymidine phosphorylase;
OV, of ovary. |
Monochrome imaging of microvessel
distribution
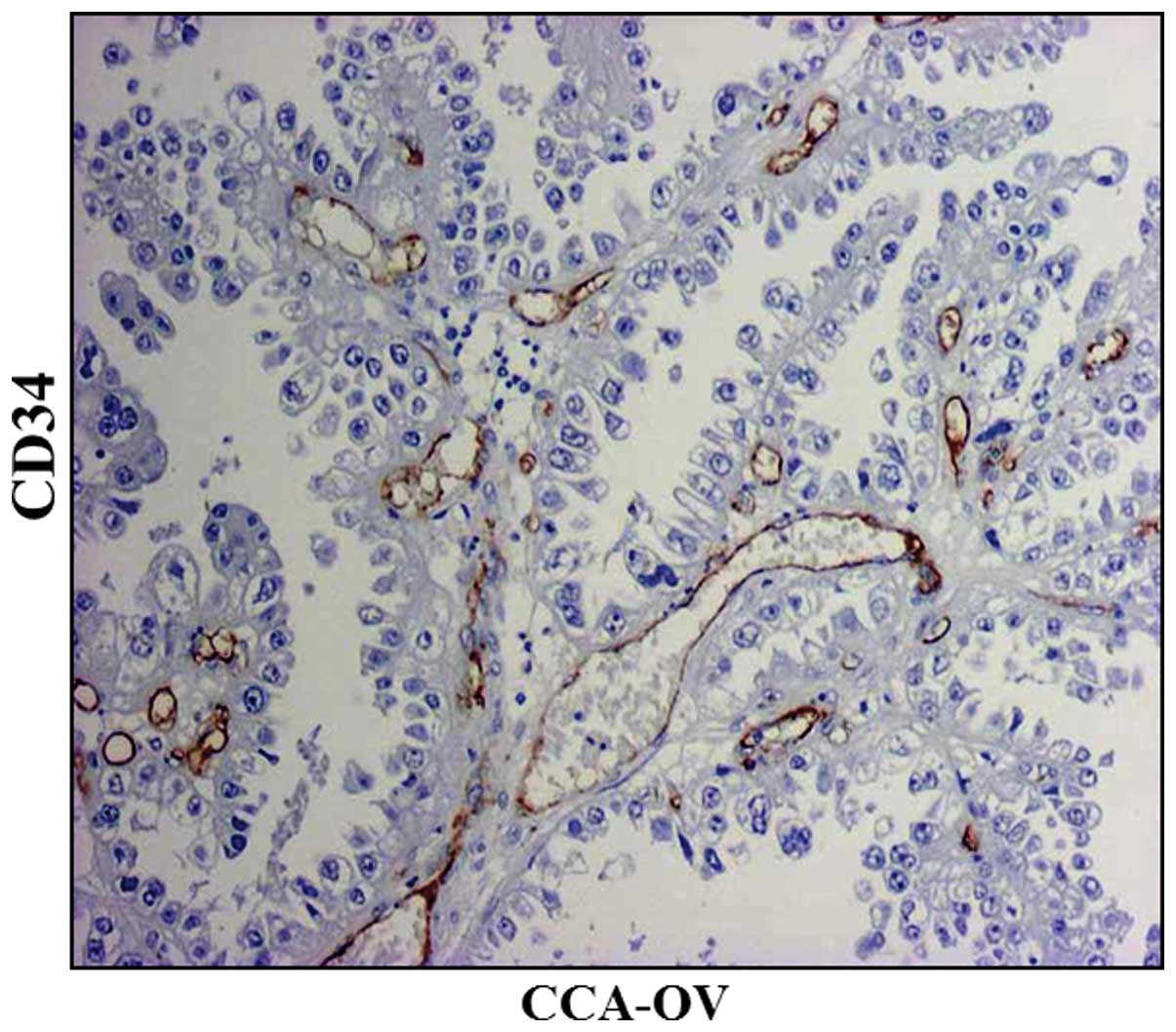
When the area occupied by microvessels in a section
was measured in each type of ovarian cancer, the part of the
vasculature defined as microvessels had an area of 116.6
µm2 in CCA-OV, 71.8 µm2 in SEA-OV and 56.8
µm2 in EMA-OV (Table II
and Fig. 6). There were no
significant differences among uterine cervical tumors and uterine
body tumors (data not shown).
 | Table II.Analysis of vascularity on monochrome
images of ovarian carcinoma. |
Table II.
Analysis of vascularity on monochrome
images of ovarian carcinoma.
| Histology | Microvessel area,
µm2 |
|---|
| CCA-OV | 116.6 |
| SEA-OV |
71.8 |
| EMA-OV |
56.8 |
| MUA-OV |
43.6 |
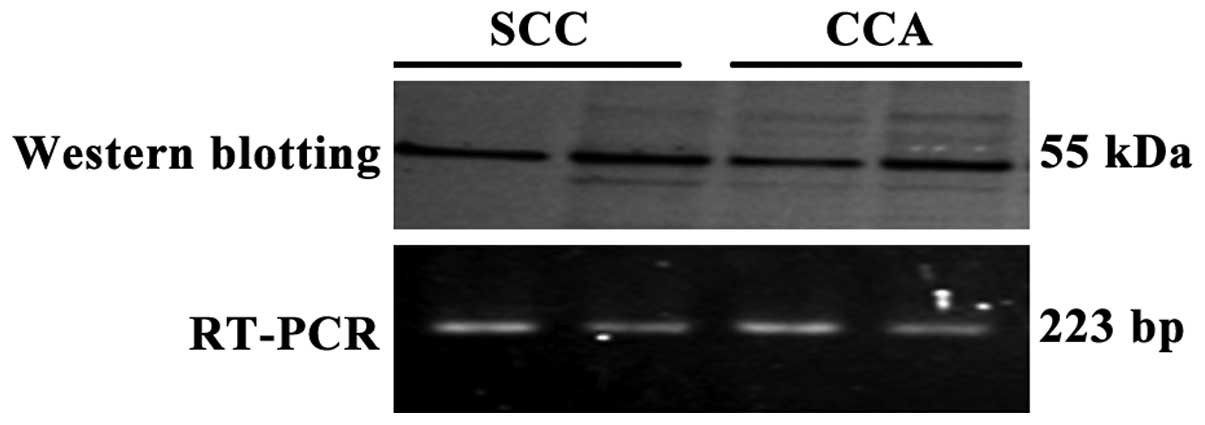
Western blotting analysis
Western blotting revealed dense bands for TP protein
expression in patients with SCC and CCA-OV, who exhibited high TP
activity and TP positive reaction on immunostaining (Fig. 7).
RT-PCR analysis
Analysis of TP mRNA expression in patients with
prominent TP protein bands in western blot analysis revealed that
TP mRNA expression was closely associated with TP protein
expression (Fig. 7).
Discussion
TP activity, which was the focus of the present
study, could be measured in fresh tissue specimens and serum, and
could also be evaluated by IHC. Therefore, the efficacy of 5-FU
prodrugs may be predicted if the activity and expression of TP are
investigated prior to initiation of the treatment. While TP
activity is known to be increased in cervical cancer (14), the present study demonstrated that TP
activity is also high in certain patients with CCA-OV and malignant
epithelial ovarian tumors.
DPD acts to degrade TP, and may weaken the
therapeutic effect of oral pyrimidine fluoride-based drugs
(5). UFT, which contains an oral
pyrimidine fluoride-based drug combined with uracil to inhibit DPD,
was designed to overcome this problem, even when both TP and DPD
activities are high in a tumor (15,16). UFT
is a 5-FU prodrug that was developed in Japan as a catabolic enzyme
for tegafur and 5-FU (16). Since the
response rate to this drug was high (16%) in a previous phase II
study performed in 25 patients with advanced/recurrent cervical
cancer, the use of UFT for the treatment of cervical cancer is
already covered by national health insurance in Japan (14). The usefulness of UFT as adjuvant
chemotherapy for various other types of cancer has also been
established (6–9). These findings suggest that UFT may be
useful as adjuvant chemotherapy for gynecological tumors. In a
previous phase III study comparing radiotherapy combined with low-
or high-dose Z-100 and maintenance therapy, oral pyrimidine
fluoride-based drugs such as 5-FU and UFT were used as adjuvant
chemotherapy, and comparison was performed with patients not
receiving adjuvant chemotherapy. In that study, the 3- and 5-year
survival rates were respectively 22.5 and 15.8% higher in the
adjuvant chemotherapy group than in the group without adjuvant
chemotherapy (17). Based on those
results, a phase III, randomized, comparative study of UFT in
patients with locally advanced cervical cancer receiving radical
radiotherapy (termed LUFT trial) is currently ongoing in Japan,
whereby radiotherapy and chemotherapy are administered
simultaneously to patients with stage IB2-IVA cervical cancer. By
July 2014, 180 subjects were enrolled in that study, whose results
have not been published thus far. However, exploration of
biomarkers was not performed during the LUFT trial (18).
It is known that TP activates 5-FU prodrugs and is
also structurally similar to PD-ECGF, an angiogenic factor that
enhances the migration of vascular endothelial cells (19). In the present study, IHC demonstrated
that TP was strongly positive in three types of epithelial ovarian
cancer, including CCA-OV, SEA-OV and EMA-OV (Figs. 4 and 5).
Suzuki et al (20) reported
that TP was probably closely associated with the mechanism of
angiogenesis in CCA-OV, unlike MUA-OV, since TP expression was
higher in this type of adenocarcinoma than in other histological
types, and was significantly lower in MUA-OV than in other
histological types. However, TP expression in different types of
cancer remains a matter of controversy. For example, TP expression
was reported to be low in CCA-OV, but high in MUA-OV (19), while another study demonstrated high
TP expression in SEA-OV and EMA-OV, but not in CCA-OV (21). It was observed in the present study
that TP activity could vary considerably within each tumor, and TP
expression may depend on the local growth pattern characterized by
the histological structure, including papillary, tubular and solid
nests. At present, there is little information concerning the
association between TP expression and prognosis, due to the
insufficient number of patients and duration of observations.
However, Fujimoto et al (21)
reported that the treatment outcome was significantly worse for
ovarian cancer patients with high TP expression than for those with
low TP expression. In the present study, the ovarian tumors with a
TP/DPD ratio >1.0 presented poor prognosis (Fig. 3).
The present study also revealed that TP expression
was increased compared with other histological types, and the
number of microvessels was larger than other histological types, in
CCA-OV and SEA-OV patients, presumably because papillary
proliferation is characteristic of these two tumors (22). Ogawa et al (23) also reported that the prognosis was
better for CCA-OV patients with a high microvessel density than
those with a low microvessel density. Since the present study also
demonstrated that CCA-OV tumors exhibited the largest vascular area
(Fig. 6) of all the gynecological
tumors analyzed, the association between TP expression and
angiogenesis or long-term prognosis will be further analyzed in
future studies.
The localization of TP was investigated by IHC in
the present study, and TP expression was detected in both tumor
cells and stromal cells. It has been previously reported that the
production of TP by colorectal cancer cells is limited, with the
majority of TP being produced by stromal cells around the tumor,
particularly activated macrophages (24). It has also been reported that the
number of TP+ activated macrophages is positively
correlated with the number of stromal microvessels, and that
activated macrophages producing TP are involved in angiogenesis in
colorectal cancer (24). In addition,
Konishi (25) reported that TP
expression was more common in the stroma of ovarian cancer than in
tumor cells, and was closely correlated with the presence of
CD68+ cells and microvessels, particularly at sites of
cancer cell infiltration or metastasis associated with a stromal
reaction and angiogenesis (25).
These findings suggest that macrophages may be activated by the
tumor cells via certain mechanism to increase TP expression and
promote angiogenesis. Thus, the significance of TP expression may
depend on its location (in the tumor cells or in the stroma,
particularly at sites of infiltration). Accordingly, the
significance of TP localization in patients with gynecological
tumors requires further investigation.
In recent years, attempts have been made to
transiently increase TP activity by administration of cytokines or
other anti-neoplastic drugs, so that pyrimidine fluoride-based
drugs could also be used in patients with low TP activity (26). As a result, drugs that inhibit
angiogenesis by directly blocking TP activity have been developed
(27,28). Phase I clinical studies of these drugs
in patients with colorectal and breast cancer have already
commenced in the USA (29). While
awaiting the results of these studies, research on the development
of novel treatments must continue, focusing on TP activity, the
significance of TP as a biomarker and the utility of UFT
maintenance therapy for gynecological cancer.
References
|
1
|
Haraguchi M, Miyadera K, Uemura K,
Sumizawa T, Furukawa T, Yamada K, Akiyama S and Yamada Y:
Angiogenic activity of enzymes. Nature. 368:1981994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miwa M, Ura M, Nishida M, Sawada N,
Ishikawa T, Mori K, Shimma N, Umeda I and Ishitsuka H: Design of a
novel oral fluoropyrimidine carbamate, capecitabine, which
generates 5-fluorouracil selectively in tumours by enzymes
concentrated in human liver and cancer tissue. Eur J Cancer.
34:1274–1281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furukawa T, Yoshimura A, Sumizawa T,
Haraguchi M, Akiyama S, Fukui K, Ishizawa M and Yamada Y:
Angiogenic factor. Nature. 356:6681992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dushinsky R, Pleven E and Heidelberger C:
The synthesis of 5-fluoropyrimidines. J Am Chem Soc. 79:4559–4560.
1957. View Article : Google Scholar
|
|
5
|
Heidelberger C, Chaudhuri NK, Danneberg P,
Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E and
Scheiner J: Fluorinated pyrimidines, a new class of
tumour-inhibitory compounds. Nature. 179:663–666. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakajima T, Kinoshita T, Nashimoto A,
Sairenji M, Yamaguchi T, Sakamoto J, Fujiya T, Inada T, Sasako M
and Ohashi Y: National Surgical Adjuvant Study of Gastric Cancer
Group: Randomized controlled trial of adjuvant uracil-tegafur
versus surgery alone for serosa-negative, locally advancer gastric
cancer. Br J Surg. 94:1468–1476. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato H, Ichinose Y and Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N and
Ohta M: Japan Lung Cancer Research Group on Postsurgical Adjuvant
Chemotherapy: A randomized trial of adjuvant chemotherapy with
uracil-tegafur for adenocarcinoma of the lung. N Engl J Med.
350:1713–1721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akasu T, Moriya Y, Ohashi Y, Yoshida S,
Shirao K and Kodaira S: National Surgical Adjuvant Study of
Colorectal Cancer: Adjuvant chemotherapy with uracil-tegafur for
pathological stage III rectal cancer after mesorectal excision with
selective lateral pelvic lymphadenectomy: A multicenter randomized
controlled trial. Jpn J Clin Oncol. 36:237–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kato T, Ohashi Y, Nakazato H, Koike A,
Saji S, Suzuki H, Takagi H, Nimura Y, Hasumi A, Baba S, et al:
Efficacy of oral UFT as adjuvant chemotherapy to curative resection
of colorectal cancer: Multicenter prospective randomized trial.
Langenbecks Arch Surg. 386:575–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasumi F, Yoshimoto M, Uchino J, Abe R,
Nomura Y, Sugimachi K, Nakazato H and Abe O: Meta-analysis of five
studies on tegafur plus uracil (UFT) as post-operative adjuvant
chemotherapy for breast cancer. Oncology. 64:146–153. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirashima Y and Shirao K: Predicting drug
efficacy-fluorinated pyrimidines (fluorouracil, S-1 and
capecitabine). Gan To Kagaku Ryoho. 39:1603–1607. 2012.(In
Japanese). PubMed/NCBI
|
|
12
|
Yamamoto K, Noda K, Hatae M, Kudo T,
Hasegawa K, Nishimura R, Honjo H, Yajima A, Sato S, Mizutani K, et
al: Effects of concomitant use of doxifluridine, radiotherapy and
immunotherapy in patients with advanced cervical cancer. Oncol Rep.
8:273–277. 2001.PubMed/NCBI
|
|
13
|
Piver MS, Rose PG and Freedman MF: Change
in International Federation of Gynecology and Obstetrics staging.
Am J Obstet Gynecol. 1988 Mar;158(3)1678–679. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noda K, Teshima K, Ikeda M, Sugawa T,
Yamagata S, Sekiba K, Kohno I, Kaneshige E, Sawaragi I, Matsuoka I,
et al: Phase II study of UFT in cancer of the uterine cervix. Gan
To Kagaku Ryoho. 12:900–906. 1985.(In Japanese). PubMed/NCBI
|
|
15
|
Fujii S, Kitano S, Ikenaka K and Shirasaka
T: Effect of coadministration of uracil or cytosine on the
anti-tumor activity of clinical doses of
1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in
rodents. Gan. 70:209–214. 1979.PubMed/NCBI
|
|
16
|
Ikenaka K, Shirasaka T, Kitano S and Fujii
S: Effect of uracil on metabolism of 5-fluorouracil in vitro. Gan.
70:353–359. 1979.PubMed/NCBI
|
|
17
|
Noda K, Ohashi Y, Sugimori H, Ozaki M,
Niibe H, Ogita S, Kohno I, Hasegawa K, Kikuchi Y, Takegawa Y, et
al: Phase III double-blind randomized trial of radiation therapy
for stage IIIb cervical cancer in combination with low- or
high-dose Z-100: Treatment with immunomodulator, more is not
better. Gynecol Oncol. 101:455–463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gynecologic Oncology Trial and
Investigation Consortium, . LUFT Trial: A randomized phase III
trial of long UFT administration following curative radiation
therapy for locally advanced cervical cancer. Version 1.20. 2010,
http://www.gotic.jpApril 4–2016
|
|
19
|
Nakanishi Y, Kodama J, Tokumo K, Seki N,
Miyagi Y, Yoshinouchi M, Okuda H and Kudo T: The expression of
platelet-derived endothelial cell growth factor/thymidine
phosphorylase associates with angiogenesis in epithelial ovarian
cancer. Int J Clin Oncol. 2:19–23. 1997. View Article : Google Scholar
|
|
20
|
Suzuki M, Usui N, Furugen Y and Mitsuhasi
N: Pyrimidine nucleoside phosphorylase activity in normal tissues
of the uterus and ovary and in benign and malignant lesions of
these organs. Int J Clin Oncol. 6:19–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujimoto J, Ichigo S, Sakaguchi H, Hirose
R and Tamaya T: Expression of platelet-derived endothelial cell
growth factor (PD-ECGF) and its mRNA in ovarian cancers. Cancer
Lett. 126:83–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seidman JD, Cho KR, Ronnett BM and Kurman
RJ: Surface epithelial tumors of the ovaryBlaustein's Pathology of
the Female Genital Tract. Kurman RJ, Ellenson LH and Ronnett BM:
6th. Springer US; New York, NY: pp. 701–758. 2011
|
|
23
|
Ogawa S, Kaku T, Kobayashi H, Hirakawa T,
Ohishi Y, Kinukawa N and Nakano H: Prognostic significance of
microvessel density, vascular cuffing and vascular endothelial
growth factor expression in ovarian carcinoma: A special review for
clear cell adenocarcinoma. Cancer Lett. 176:111–118. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satou S, Nakajima A and Koyanagi Y:
PhNPase-expressing macrophage and angiogenesis in colorectal
cancer. Nippon Daicho Komonbyo Gakkai Zasshi. 51:267–275. 1998.
View Article : Google Scholar
|
|
25
|
Konishi I: Angiogenic factors in
gynecological cancer. Acta Obstet Gynaecol Jpn. 52:1222–1227.
2000.
|
|
26
|
Ogawa K, Katsube T, Konno S, Miura K,
Wakasugi S, Watanabe T, Shimakawa T, Ishikawa S, Naritaka Y, Yagawa
H, et al: Influence of intratumor administration of OK-432 on the
tumor selectivity of 5′-DFUR. Gan To Kagaku Ryoho. 22:2095–2100.
1995.(In Japanese). PubMed/NCBI
|
|
27
|
Takao S, Akiyama SI, Nakajo A, Yoh H,
Kitazono M, Natsugoe S, Miyadera K, Fukushima M, Yamada Y and Aikou
T: Suppression of metastasis by thymidine phosphorylase inhibitor.
Cancer Res. 60:5345–5348. 2000.PubMed/NCBI
|
|
28
|
Mori S, Takao S, Ikeda R, Noma H, Mataki
Y, Wang X, Akiyama S and Aikou T: Thymidine phosphorylase
suppresses Fas-induced apoptotic signal transduction independent of
its enzymatic activity. Biochem Biophys Res Commun. 295:300–305.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Overman MJ, Varadhachary G, Kopetz S,
Thomas MB, Fukushima M, Kuwata K, Mita A, Wolff RA, Hoff PM, Xiong
H and Abbruzzese JL: Phase 1 study of TAS-102 administered once
daily on a 5-day-per-week schedule in patients with solid tumors.
Invest New Drugs. 26:445–454. 2008. View Article : Google Scholar : PubMed/NCBI
|