Introduction
Approximately 15–20% of patients with left-sided
colorectal cancer (LSCC) present with symptoms of acute obstruction
(1–4).
Patients with obstructive LSCC require emergency surgery, and a
high rate of surgical complications and poor prognosis have been
reported in these cases (5,6). Emergency management of acute left-sided
colonic obstruction remains controversial and at present several
treatments options exist, including simple colostomy, primary
resection with end colostomy (Hartmann's procedure), one-stage
resection anastomosis (subtotal or segmental colectomy) with or
without intraoperative colonic irrigation and colonic stenting
(7). Emergency surgery for acute
colonic obstruction carries a significant risk of mortality and
morbidity: The 30-day postoperative mortality rate is 7.6%, with
anastomotic dehiscence occurring in 4.9% of cases (2). Thus, 46% of patients require stoma
treatment that is permanent (2). To
reduce the risk of complications, laparoscopic Hartmann procedure
reversal is commonly used for LSCC, since it is considered to
reduce the morbidity caused by anastomotic dehiscence (8). However, re-establishing intestinal
continuity during laparoscopic Hartmann procedure reversal for LSCC
remains a major problem and it carries a serious risk of
significant morbidity, with reported anastomotic leak rates of
4–16% and a mortality rate of up to 10% (9,10). Stoma
closure is difficult, as colorectal malignancies require segmental
colostomy with lymph node dissection, which results in a high rate
of dense adhesions (10).
Additionally, LSCC is associated with a poor prognosis due to the
large size and high frequency of gastrointestinal strictures
(11). In addition, certain cases
exhibit peritoneal dissemination, which exposes the cancer cells to
the serosa (12). Thus, it is
important to consider these surgical and oncological factors when
planning a laparoscopic Hartmann procedure reversal for LSCC, since
these difficulties may prevent the success of this surgical
procedure, which was previously reported to be ~20% for LSCC
(11).
In the present study, second-look surgery was
performed in patients with no signs of recurrence following six
months of surveillance subsequent to Hartmann's procedure. The
present study aimed to determine the efficacy of laparoscopic
Hartmann procedure reversal and to assess local recurrence. The aim
of the present study was to investigate the short- and long-term
outcomes of second-look surgery following Hartmann's procedure for
LSCC.
Materials and methods
Patients
The present study was retrospective, however,
patient information was collected prospectively. The study analyzed
patients that underwent laparoscopic Hartmann procedure reversal
for LSCC between January 2007 and December 2013, at Meiwa Hospital
(Nishinomiya, Japan). The inclusion criteria for patients were as
follows: i) Hartmann's procedure was performed within 48 h of
admission to hospital due to LSCC; ii) diagnosis of colorectal
carcinoma was confirmed upon pathological examination of the
surgical specimen; and iii) radical surgery was performed during
the initial surgery. Therefore, patients with benign disease, such
as diverticulosis, and patients with distant, liver or local
residual disease at the time of initial surgery were excluded from
the study.
A total of 43 patients underwent Hartmann's
procedure due to LSCC at Meiwa Hospital. A total of 25 patients
underwent radical surgery, and 18 patients exhibited residual
disease (local, distant or liver metastasis). All 25 patients who
underwent radical surgery were confirmed to exhibit no signs of
recurrence following 6 months of surveillance. However, 10 patients
were unsuitable for second-look surgery due to advanced age (>80
years) or high American Society of Anesthesiologists (ASA) score
(ASA>II) (13). Therefore, 15
patients were included in the present study. Written informed
consent was obtained from all patients and the study was approved
by the ethics committee of Meiwa Hospital.
Initial surgery
During Hartmann's procedure, radical surgery with
lymph node dissection was conducted, and the vascular pedicle was
transected at the inferior mesenteric artery, followed by high-tie
ligation. Resected specimens of colorectal malignancies were
removed to leave the proximal segment of the bowel as an end
colostomy, while the remaining distal bowel was oversewn as a
rectal stump. The resection line of the distal bowel was generally
located at the level of the peritoneal reflection with
consideration of the blood supply, and the distal rectal stump was
marked with non-absorbable PROLENE® sutures (Ethicon,
Inc., Summerville, NJ, USA). Following surgery, the administration
of adjuvant oral 5-fluorouracil (5-FU)-based chemotherapy
(TS-1®; Taiho Pharmaceutical Co, Ltd., Tokyo, Japan; 80
mg/m2/day) was recommended for all patients, and
patients with N2 stage or a positive pathological P stage also
received oxaliplatin (Elplat®, Yakult Pharmaceutical Co,
Ltd., Tokyo, Japan; 85 mg/m2/day) intravenously as
additional adjuvant chemotherapy for a total of 6 months.
Second-look surgery
Following six months of surveillance, second-look
surgery was considered in cases that exhibited no signs of
recurrence during chest and abdominal computed tomography (CT;
SOMATOM Definition Flash; Siemens AG, Munich, Germany)
examinations. During the second-look surgery, patients were
assessed for local recurrence, and the intestinal continuity, which
was re-established during laparoscopic Hartmann procedure reversal,
was reviewed. If localized recurrence or peritoneal dissemination
was observed, the carcinomatous lesion was resected and anastomosis
was then performed.
Patient surveillance
Following second-look surgery, patient surveillance
was performed as follows: Chest and abdominal CT examinations were
conducted every six months; colonoscopy was performed annually; and
blood tests, which included measurement of carcinoembryonic antigen
and cancer antigen 19-9 levels, were performed every three months.
Local recurrence was defined as the identification of a recurrent
tumor within the pelvis, while systemic recurrence was defined as
the presence of recurrent disease outside the pelvis.
The pathological findings of the resected specimens
were assessed according to the Japanese General Rules for Clinical
and Pathological Studies on Cancer of the Colon, Rectum and Anus
(7th edition) (14).
Statistical analysis
Time to recurrence was measured from the date of
second-look surgery to the date of recurrence, and the long-term
outcomes were assessed to determine the 3-year local relapse-free
survival (LFS) and recurrence-free survival (RFS) rates, which were
estimated using the Kaplan-Meier method. All data analysis was
performed using JMP 10.0 statistical software (SAS Institute Inc.,
Cary, NC, USA).
Results
Patients' characteristics at the time
of initial surgery
The patient cohort included 10 females (66.7%) and 5
males (33.3%), with a median age of 61 years (range, 32–75 years)
(Table I). All patients exhibited an
ASA score of I or II, and the median body mass index was 21
kg/m2 (range, 16–31 kg/m2). The most common
type of tumor was rectosigmoid carcinoma (10 patients; 66.7%). A
total of 14 patients exhibited pathological stage T3 disease,
whereas 1 patient exhibited stage T4 (sigmoid) disease. In
addition, 8 patients exhibited lymph node metastasis (Table I). At laparotomy, 3 patients were
observed to exhibit peritoneal dissemination (Table I); however, all lesions were localized
and were radically resected. Following surgery, all patients
received adjuvant chemotherapy, and 4 patients received oxaliplatin
as additional adjuvant chemotherapy.
 | Table I.Patients' characteristics at the time
of initial surgery. |
Table I.
Patients' characteristics at the time
of initial surgery.
| Parameter | n |
|---|
| Gender |
|
| Male | 5 |
|
Female | 10 |
| Age, years |
|
| Median
(range) | 61 (32–75) |
| BMI,
kg/m2 |
|
| Median
(range) | 21 (16–31) |
| ASA score |
|
| I | 13 |
| II | 2 |
| III | 0 |
| IV | 0 |
| Tumor location |
|
|
Descending | 2 |
|
Sigmoid | 3 |
|
Rectosigmoid | 10 |
| Pathological T
stagea |
|
| T0-2 | 0 |
| T3 | 14 |
| T4 |
1c |
| Pathological N
stagea |
|
|
N− | 7 |
| N1 |
6 |
| N2 | 2 |
| Pathological P
stagea |
|
|
P− | 12 |
|
P+ | 3 |
| TNM
stageb |
|
| II | 5 |
| III | 7 |
| IV | 3 |
| Adjuvant
chemotherapy |
|
| Oral
intake only | 11 |
|
Oxaliplatin added | 4 |
Patients' characteristics at the time
of second-look surgery
Following six months of surveillance, none of the
patients exhibited signs of recurrence on physical or radiological
examination. The median interval between initial and second-look
surgery was 273 days (range, 212–592 days; data not shown). During
the second-look surgery, 11 patients underwent colorectal
anastomosis (CRA), 2 patients underwent colo-anal anastomosis
(CAA), 1 patient underwent ceco-rectal anastomosis (Ce-RA) and 1
patient underwent ileo-anal canal anastomosis (IACA) (Table II). CRA has been proposed as the
standard procedure to re-establish intestinal continuity (10–12).
However, 2 patients underwent CAA due to the formation of dense
adhesions between the rectal stump and bladder, small bowel and
vagina. In addition, 2 patients with short-reconstructed colons
underwent further mobilization of the splenic flexure to achieve
sufficient mobility of the descending colon, and therefore, were
reconstructed with the right-side colon or ileum. A total of 5
patients required a diverting ileostomy due to incomplete
anastomosis.
 | Table II.Patients' characteristics at the time
of second-look surgery. |
Table II.
Patients' characteristics at the time
of second-look surgery.
| Parameter | n |
|---|
| Surgical
procedure |
|
|
CRA | 11 |
|
CAA | 2 |
|
Ce-RA | 1 |
|
IACA | 1 |
| Diverting
ileostomy |
|
|
Yes | 5 |
| No | 10 |
| Length of surgery,
min |
|
| Median
(range) | 257 (189–600) |
| Amount of blood
lost, ml |
|
| Median
(range) | 405
(290–3,340) |
| Local
recurrence |
|
|
Yes |
2a |
| No | 13 |
| Distant
recurrence |
|
|
Yes | 0 |
| No | 15 |
| TNM residual tumor
classificationb |
|
| R0 | 15 |
|
R1-2 | 0 |
| Complications |
|
| Wound
infection | 2 |
|
Ileus | 2 |
| Pelvic
infection | 1 |
|
Abdominal hernia | 1 |
|
Intraoperative injury
(bladder) | 1 |
| Wound
infection and ileus | 1 |
| Wound
infection and pelvic infection | 1 |
| Wound
infection and cholecystitis | 1 |
|
None | 5 |
Second-look surgery revealed that 2/3 patients with
peritoneal dissemination at the time of initial surgery exhibited
localized peritoneal dissemination at the time of second-look
surgery. However, these patients underwent radical surgery with
partial peritonectomy. The other patient did not exhibit peritoneal
dissemination at the time of second-look surgery, and exhibited no
signs of local or distant recurrence following 37 months of
follow-up. In certain cases, postoperative complications developed,
including wound infections (n=5), ileus (n=3), pelvic infection
(n=2), cholecystitis (n=1) abdominal hernia (n=1) and
intraoperative bladder injury (n=1; Table II); however, all patients were
successfully treated conservatively using infusion fluids and/or
antibiotics, with the exception of the abdominal hernia patient,
who remains under observation.
Long-term outcomes following
second-look surgery
The median follow-up period subsequent to
second-look surgery was 35.4 months (range, 9.0–64.0 months; data
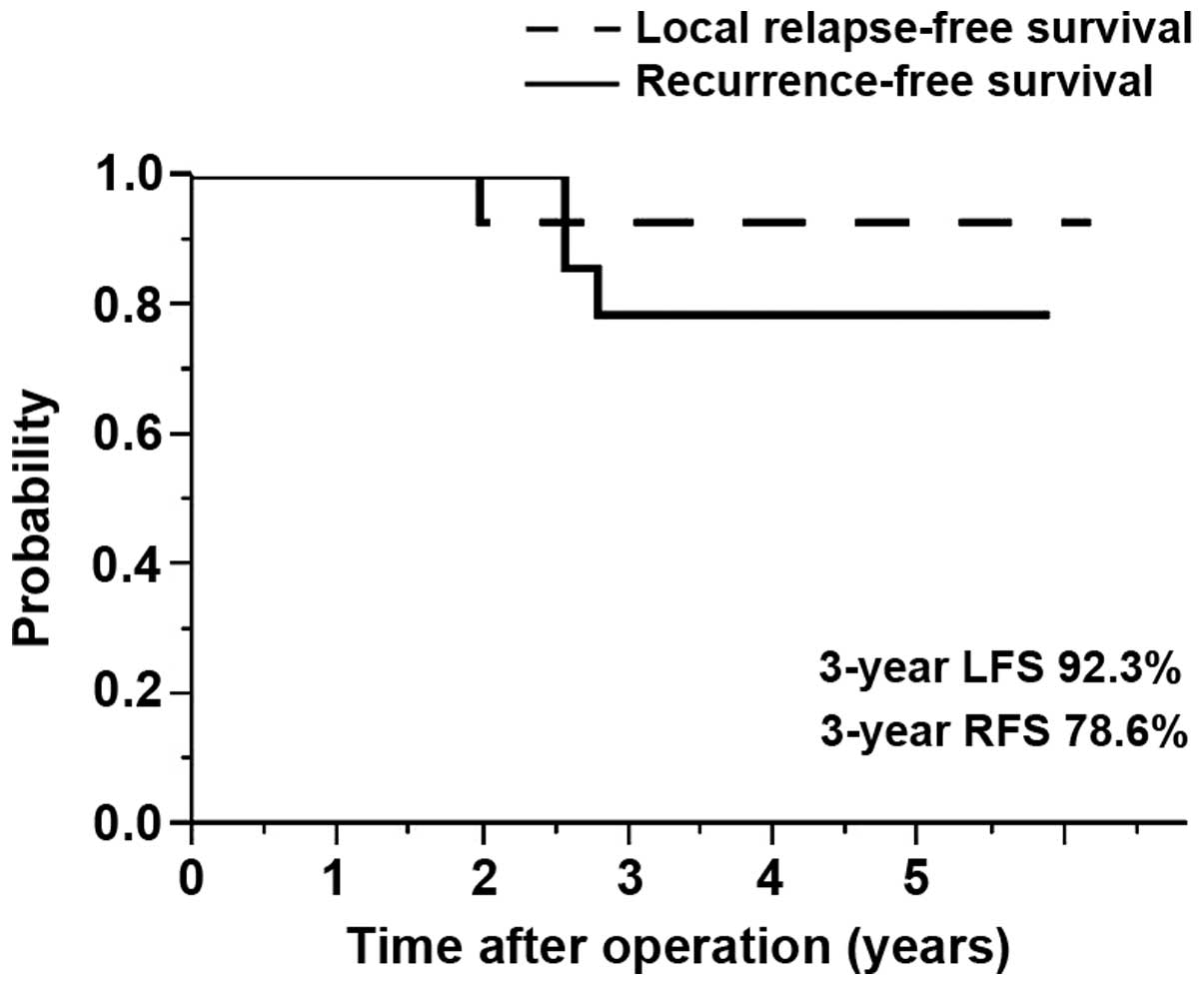
not shown). The 3-year LFS and RFS rates were 92.3 and 78.6%,
respectively (Fig. 1). Of the 2
patients who exhibited peritoneal dissemination at the time of
second-look surgery, 1 patient was recurrence-free following 27
months of follow-up, and 1 patient exhibited local recurrence in
the peritoneum after 30 months. Of the 5 patients who required
diverting ileostomy, the stoma in 4 patients had already closed,
and the stoma in the remaining patient was planned to be closed.
Therefore, intestinal continuity was considered to have been
maintained in all patients 8 months subsequent to the performance
of second-look surgery.
Discussion
Laparoscopic Hartmann procedure reversal for LSCC is
associated with a substantial risk of surgical complications, as
the surgery may result in a short rectal stump, dense adhesions and
a short left-side reconstructed colon (10–12). In
addition, local recurrence, in particular peritoneal dissemination,
disrupts intestinal continuity (10–12).
However, patients who are able to undergo stoma closure experience
an improved quality of life following surgery (10–12).
Two major problems are associated with the technique
used for laparoscopic Hartmann procedure reversal exist: As a
result of the procedure, dense adhesions develop between the rectal
stump and other tissues, and the left-side reconstructed colon may
be of an inadequate length to reach to the rectal stump (16,17). In
the present study, a high-tie ligation was routinely performed, and
consequently, the distal rectal stump was transected at the level
of the peritoneal reflection following consideration of the blood
supply. Previous studies have reported that the length of the
distal rectal stump is associated with postoperative complications,
and that a short distal rectal stump contributes to a longer
duration of surgery and increased postoperative complications
(16,17). Although the distal rectal stump may be
marked with non-absorbable sutures during the initial surgery, it
may be difficult to identify the rectal stump due to the
development of dense adhesions with the bladder or vagina, in males
and females, respectively (18). In
certain cases, marking sutures cannot be identified and thus,
rectal dilators or a sigmoidoscope may be used, as well as
insufflating the bladder, which enables the safe dissection and
localization of the rectal stump (10,19).
However, despite using these approaches in the present study,
intraoperative complications occurred in 2 patients due to dense
adhesions. In the present study, intestinal continuity was
maintained in all patients following CAA. However, the adhesions
led to an inability to mobilize the rectum, and the CAA technique
was a useful method to prevent a failed attempt of maintaining
intestinal continuity in cases with severe adhesions.
Additionally, surgery may result in the left-sided
reconstructed colon being of inadequate length, leading to a
subsequent inability to reach to the rectal stump (16,17). In
the present study, this occurred in 2 patients, and sub-total or
total colectomy with Ce-RA or IACA were selected to use the
right-sided colon or ileum, which released the excessive tension on
the anastomosis, thus avoiding associated complications.
With regard to the timing of the laparoscopic
Hartmann procedure reversal, Pearce et al (20) reported that postoperative
complications were significantly greater in patients who underwent
surgery within six months of the prodedure compared with those who
underwent surgery more than six months subsequent to the procedure.
By contrast, Fleming et al (21) reported that a longer interval between
initial surgery and laparoscopic Hartmann procedure reversal (>9
months) was associated with a higher risk of postoperative
complications. However, these reports were assessed according to
the risk of postoperative morbidity, and analyzed only in patients
with benign disease (21,22). By contrast, the present study only
analyzed patients with colorectal malignancies.
Time to progression (TTP) is defined as the time
period between the start of disease treatment and disease
progression (23). Previous studies
have demonstrated that chemotherapy reduces the postoperative
recurrence rate, and TTP is one of the factors used to evaluate the
effects of chemotherapy (24,25). The TTP following treatment with oral
tegafur/uracil with leucovorin (LV), 5-FU/LV and capecitabine has
been reported to be 3.5, 3.8 and 4.3 months, respectively (24,25).
Therefore, exceeding these periods may aid early detection of tumor
recurrence (22,24). Therefore, in the present study,
second-look surgery was performed six months subsequent to the
initial surgery, and aimed to assess the patients for local
recurrence and to review the efficacy of the laparoscopic Hartmann
procedure reversal.
In the current study, 3 patients exhibited
peritoneal dissemination at the time of initial surgery, 2 of which
appear to have been in remission at present. In ~10% of colorectal
cancer cases, patients present with peritoneal dissemination at the
time of diagnosis, and 25% of patients develop peritoneal
dissemination at recurrence (25–28). At
present, no evidence suggests that performing peritonectomy for
peritoneal dissemination of colorectal malignancies improves
patients' outcomes; however, partial peritonectomy for localized
peritoneal dissemination may improve patients' survival (29). Therefore, the early detection of
peritoneal dissemination and prompt treatment may have contributed
to the favorable outcomes observed in the present cases.
The current study presents certain limitations.
Firstly, the present study is a single arm of a retrospective
study, and the patient cohort was small. Secondly, the indications
for Hartmann's procedure have decreased in the past years, as other
surgical procedures for LSCC are currently available, including
primary anastomosis with ileostomy or elective surgery using
self-expanding metallic stents (30).
However, perforated or pre-perforated LSCC remain reasonable
indications for Hartmann's procedure (30).
In conclusion, hand-sewn CAA and reconstruction with
right-sided colon or ileum may be useful to prevent failed attempts
to maintain intestinal continuity in patients with LSCC. With
regard to the oncological outcomes, TTP following oral adjuvant
chemotherapy is generally <6 months; therefore, any time
subsequent to this period would be reasonable to conduct a
second-look surgery to confirm whether recurrence has occurred.
Furthermore, early detection and intensive treatment for localized
peritoneal dissemination may lead to advances in colorectal cancer
treatment in certain cases.
Acknowledgements
The authors would like to thank Dr Kakuno
(Department of Pathology, Meiwa Hospital, Nishinomiya, Japan) for
performing the pathological assessments.
References
|
1
|
Phillips RK, Hittinger R, Fry JS and
Fielding LP: Malignant large bowel obstruction. Br J Surg.
72:296–302. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mella J, Biffin A, Radcliffe AG,
Stamatakis JD and Steele RJ: Population-based audit of colorectal
cancer management in two UK health regions. Colorectal Cancer
Working Group, Royal College of Surgeons of England Clinical
Epidemiology and Audit Unit. Br J Surg. 84:1731–1736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serpell JW, McDermott FT, Katrivessis H
and Hughes ES: Obstructing carcinomas of the colon. Br J Surg.
76:965–969. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Umpleby HC and Williamson RC: Survival in
acute obstructing colorectal carcinoma. Dis Colon Rectum.
27:299–304. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finan PJ, Campbell S, Verma R, MacFie J,
Gatt M, Parker MC, Bhardwaj R and Hall NR: The management of
malignant large bowel obstruction: ACPGBI position statement.
Colorectal Dis. 9(Suppl 4): 1–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McArdle CS and Hole DJ: Emergency
presentation of colorectal cancer is associated with poor 5-year
survival. Br J Surg. 91:605–609. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trompetas V: Emergency management of
malignant acute left-sided colonic obstruction. Ann R Coll Surg
Engl. 90:181–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borowski DW, Kanakala V, Agarwal AK,
Tabaqchali MA, Garg DK and Gill TS: Single-port access laparoscopic
reversal of Hartmann operation. Dis Colon Rectum. 54:1053–1056.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breitenstein S, Rickenbacher A, Berdajs D,
Puhan M, Clavien PA and Demartines N: Systematic evaluation of
surgical strategies for acute malignant left-sided colonic
obstruction. Br J Surg. 94:1451–1460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen MJ, Cobb WS, Kercher KW, Sing RF and
Heniford BT: Laparoscopic restoration of intestinal continuity
after Hartmann's procedure. Am J Surg. 189:670–674. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kristiansen VB, Lausen IM, Frederiksen HJ
and Kjaergaard J: Hartmann's procedure in the treatment of acute
obstructive left-sided colonic cancer. Ugeskr Laeger.
155:3816–3818. 1993.(In Danish). PubMed/NCBI
|
|
12
|
Canney AL, Kevans D, Wang LM, Hyland JM,
Mulcahy HE, O'Donoghue DP, O'Sullivan J, Geraghty R and Sheahan K:
Stage II colonic adenocarcinoma: A detailed study of pT4N0 with
emphasis on peritoneal involvement and the role of tumour budding.
Histopathology. 61:488–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saklad M: Grading of patients for surgical
procedures. Anesthesiology. 2:281–284. 1941. View Article : Google Scholar
|
|
14
|
Japanese Society for Cancer of the Colon
and Rectum, . General rules for clinical and pathological studies
on cancer of the colon, rectum, and anus 7th. Kanehara Shuppan.
Tokyo, Japan: 2006.
|
|
15
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Rectal cancer. J Natl
Compr Canc Netw. 7:838–881. 2009.PubMed/NCBI
|
|
16
|
Alberts JC, Parvaiz A and Moran BJ:
Predicting risk and diminishing the consequences of anastomotic
dehiscence following rectal resection. Colorectal Dis. 5:478–482.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mäkelä JT, Kiviniemi H and Laitinen S:
Risk factors for anastomotic leakage after left-sided colorectal
resection with rectal anastomosis. Dis Colon Rectum. 46:653–660.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oomen JL, Cuesta MA and Engel AF: Reversal
of Hartmann's procedure after surgery for complications of
diverticular disease of the sigmoid colon is safe and possible in
most patients. Dig Surg. 22:419–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson CA, Fowler DL, White S and Wintz
N: Laparoscopic colostomy closure. Surg Laparosc Endosc. 3:69–72.
1993.PubMed/NCBI
|
|
20
|
Pearce NW, Scott SD and Karran SJ: Timing
and method of reversal of Hartmann's procedure. Br J Surg.
79:839–841. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fleming FJ and Gillen P: Reversal of
Hartmann's procedure following acute diverticulitis: Is timing
everything? Int J Colorectal Dis. 24:1219–1225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Douillard JY, Hoff PM, Skillings JR,
Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC,
Thompson S, Maniero A and Benner SE: Multicenter phase III study of
uracil/tegafur and oral leucovorin versus fluorouracil and
leucovorin in patients with previously untreated metastatic
colorectal cancer. J Clin Oncol. 20:3605–3616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Middleton G, Brown S, Lowe C, Maughan T,
Gwyther S, Oliver A, Richman S, Blake D, Napp V, Marshall H, et al:
A randomised phase III trial of the pharmacokinetic biomodulation
of irinotecan using oral ciclosporin in advanced colorectal cancer:
results of the Panitumumab, Irinotecan & Ciclosporin in
COLOrectal cancer therapy trial (PICCOLO). Eur J Cancer.
49:3507–3516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoff PM, Ansari R, Batist G, Cox J, Kocha
W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, et al:
Comparison of oral capecitabine versus intravenous fluorouracil
plus leucovorin as first-line treatment in 605 patients with
metastatic colorectal cancer: Results of a randomized phase III
study. J Clin Oncol. 19:2282–2292. 2001.PubMed/NCBI
|
|
25
|
Verwaal VJ, van Ruth S, Witkamp A, Boot H,
van Slooten G and Zoetmulder FA: Long-term survival of peritoneal
carcinomatosis of colorectal origin. Ann Surg Oncol. 12:65–71.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carraro PG, Segala M, Cesana BM and
Tiberio G: Obstructing colonic cancer: Failure and survival
patterns over a ten-year follow-up after one-stage curative
surgery. Dis Colon Rectum. 44:243–250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jayne DG, Fook S, Loi C and Seow-Choen F:
Peritoneal carcinomatosis from colorectal cancer. Br J Surg.
89:1545–1550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Russell AH, Pelton J, Reheis CE, Wisbeck
WM, Tong DY and Dawson LE: Adenocarcinoma of the colon: An autopsy
study with implications for new therapeutic strategies. Cancer.
56:1446–1451. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pestieau SR and Sugarbaker PH: Treatment
of primary colon cancer with peritoneal carcinomatosis: Comparison
of concomitant vs. delayed management. Dis Colon Rectum.
43:1341–1348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garber A, Hyman N and Osler T:
Complications of Hartmann takedown in a decade of preferred primary
anastomosis. Am J Surg. 207:60–64. 2014. View Article : Google Scholar : PubMed/NCBI
|