Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
fifth most common malignancy worldwide (1). Despite advances in the diagnosis and
treatment of HNSCC, the 5-year survival rates for patients with
HNSCC have remain unchanged at at 30–40% for the last 30 years.
Furthermore, local and distant metastases remain significant
barriers to disease eradication (1).
Nasopharyngeal carcinoma (NPC) is a prevalent head and neck cancer
in Southeast Asia, with an annual incidence rate of 54.7 cases per
100,000 individuals (2). NPC is
unique among head and neck cancers due to its epidemiology,
carcinogenic risk factors, biological markers, prognostic factors,
clinical presentation, therapeutic strategies and outcome, as well
as its close association with the Epstein-Barr virus and its high
incidence of metastasis (3).
An increasing number of studies have suggested that
the majority of human cancers exhibit dysregulated expression
and/or functioning of one or more receptor tyrosine kinases (RTKs)
(4). The protein tyrosine kinases
belong to a large, multigene family involved in the regulation of
cell-to-cell signaling that is associated with cell growth,
differentiation, adhesion, motility and apoptosis (4).
Discoidin domain receptors (DDRs) are a unique set
of RTKs that exhibit an important function in cancer progression by
regulating the interaction of tumor cells with their surrounding
collagen matrix. DDRs, which are distinguished from other RTKs by
the discoidin motif in their extracellular domain (5), are upregulated in a number of solid
tumors, including head and neck cancer, and nasopharyngeal, breast,
ovarian and esophageal carcinomas (6–18).
According to the homology of the C-terminal region, DDRs may be
divided into two categories: DDR1 and DDR2. The two categories of
DDRs exhibit different tissue-specific expression patterns: DDR1 is
predominantly expressed in epithelial cells and DDR2 is
predominantly expressed in mesenchymal cells (19,20).
Previous studies have demonstrated that DDR1 and DDR2 exhibit vital
functions in the regulation of fundamental cellular processes,
including proliferation, survival, differentiation, adhesion and
matrix remodeling (21,22). In addition, the dysregulation of DDR1
and DDR2 has been associated with a number of human diseases,
including fibrotic disorders, atherosclerosis and cancer (5,23). In a
previous study, inhibition of DDR1 using small interfering RNA
(siRNA) was demonstrated to suppress tumorigenicity, inhibit lung
cancer bone metastasis and increase cancer cell chemosensitivity
(24). Therefore, DDR1 is considered
a potential molecular target for cancer therapy.
SRC family kinases (SFKs), which are a family of
non-RTKs, exhibit elevated protein expression levels in NPC cells
(25). Activated SRC may in turn
activate AKT, a key effector of the phosphoinositide 3-kinase
(PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway (26). In addition, AKT is an important
component of signaling pathways that regulate proliferation,
survival, metastasis and angiogenesis (27). Thus, due to its involvement in these
oncogenic processes, AKT presents a potential therapeutic target
for the treatment of cancer.
To the best of our knowledge, no previous studies
have evaluated the effect of the DDR1 inhibitor in NPC cells. Since
high levels of DDR1 protein have been identified in HNSCC tissues
(6) and DDR1 transcripts are
upregulated in NPC, NPC metastasis, and head and neck tumor tissues
(7), we hypothesize that inhibition
of DDR1 may be useful for the treatment of patients with NPC.
Therefore, the present study aimed to investigate the effects of a
(3-(2-(pyrazolo(1,5-a)pyrimidin-6-yl)-ethynyl)benzamide compound,
7RH (Fig. 1A), which is a potent and
reversible small molecule inhibitor of DDR1 (28), on the proliferation and apoptosis of
NPC cells, as well as the underlying mechanisms of such. In
addition, the ability of 7RH to act synergistically with the SFK
inhibitor, dasatinib, was evaluated.
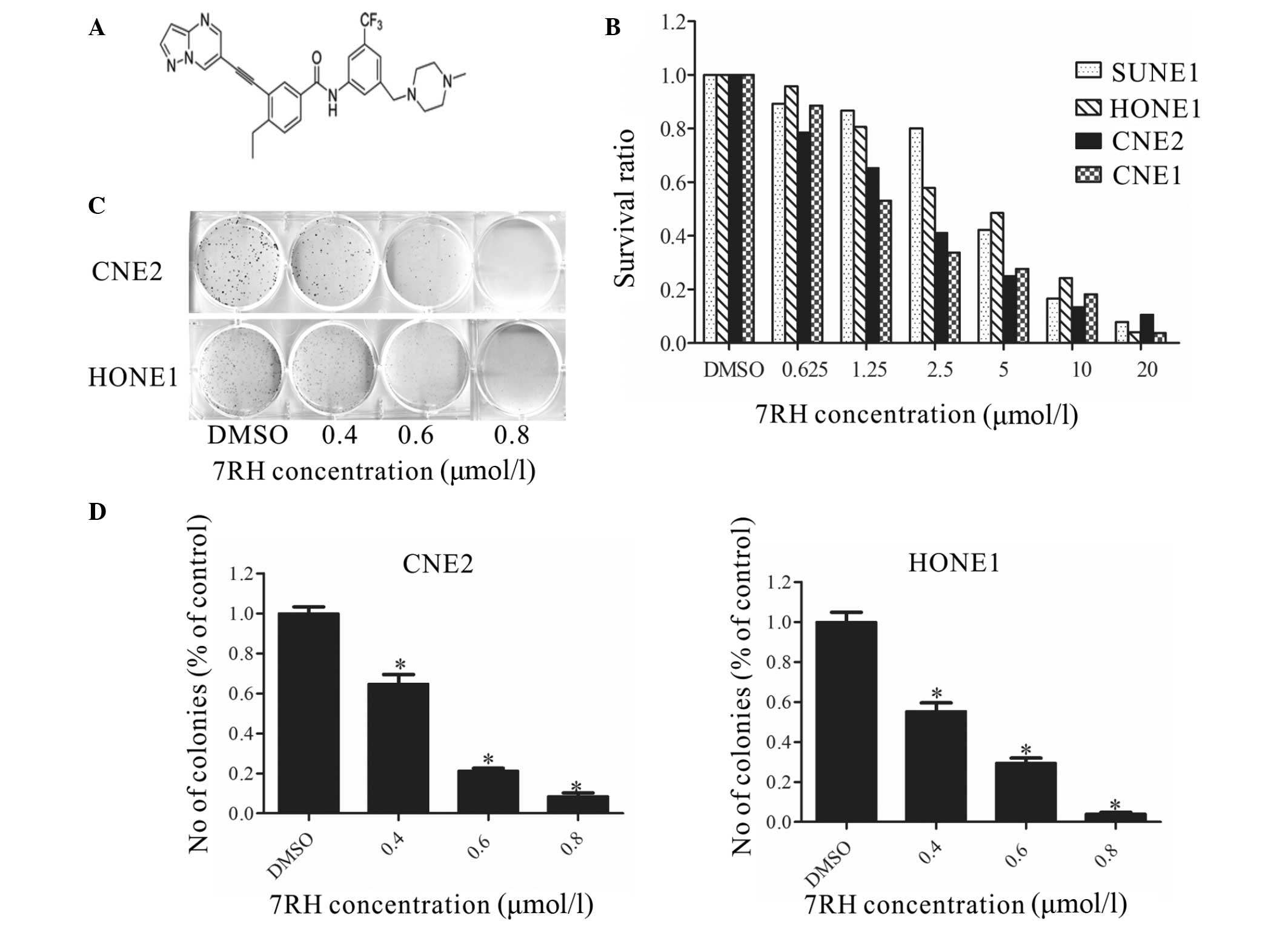 | Figure 1.7RH inhibited the proliferation and
colony formation of nasopharyngeal carcinoma cells. (A) Structure
of the (3-(2-(pyrazolo(1,5-a)pyrimidin-6-yl)-ethynyl)benzamide
compound, 7RH. (B) SUNE1, HONE1, CNE2 and CNE1 cells were treated
with 0, 0.625, 1.25, 2.5, 5, 10 or 20µmol/l 7RH for 72 h, after
which the antiproliferative effect of 7RH was measured using MTT
assays. Cell proliferation was decreased in a dose-dependent manner
following treatment with 7RH in all cell lines. (C) The colony
forming abilities of CNE2 and HONE1 cells were decreased in a
dose-dependent manner following treatment with 0.4, 0.6 and 0.8
µmol/l 7RH and (D) quantified using ImageJ software. Data are
presented as the mean ± standard deviation of triplicate
experiments. *P<0.05 vs. DMSO. DMSO, dimethyl sulfoxide. |
Materials and methods
Materials and reagents
Human NPC cell lines (CNE1, CNE2, HONE1 and SUNE1)
were obtained from the Cancer Center of Sun-Yat-sen University
(Guangzhou, China) and cultured in RPMI-1640 medium supplemented
with 5% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/l penicillin and 100 mg/ml
streptomycin at 37°C in 5% CO2. All experiments were
performed using cells in the logarithmic phase. The DDR1 inhibitor,
7RH (Guangzhou Institutes of Biomedicine and Health, Chinese
Academy of Sciences, Guangzhou, China) was dissolved in 50 mmol/l
dimethyl sulfoxide (DMSO) and stored at −20°C. Dasatinib
(Selleckchem, Co., Ltd., Houston, TX, USA) was dissolved in 50
mmol/l DMSO prior to storage at −20°C. Primary antibodies against
DDR1 (catalog no. 3917; 1:1,000; rabbit), phosphorylated (p)-SRC
(catalog no. 2105; 1:1,000; rabbit), SRC (catalog no. 2108;
1:1,000; rabbit), p-Janus kinase 1 (JAK1; catalog no. 3331;
1:1,000; rabbit), JAK1 (catalog no. 3332; 1:1,000; rabbit), p-AKT
(Ser473; catalog no. 4060; 1:2,000; rabbit), p-glycogen synthase
kinase 3β (GSK3β; catalog no. 5558; 1:1,000; rabbit), p-MEK
(catalog no. 3958; 1:1,000; rabbit), MEK (catalog no. 9126;
1:1,000; rabbit), p-eukaryotic translation initiation factor 4E
binding protein 1 (p-4EBP1; catalog no. 9456; 1:1,000; rabbit),
4EBP1 (catalog no. 9452; 1:1,000; rabbit), poly(ADP-ribose)
polymerase 1 (PARP; catalog no. 9544; 1:1,000; rabbit), E-cadherin
(catalog no. 14472; 1:1,000; mouse), p-Pyk2 (catalog no. 3291;
1:1,000; rabbit), Pyk2 (catalog no. 3292; 1:1,000; rabbit), p-focal
adhesion kinase (FAK; catalog no. 3284; 1:1,000; rabbit) and FAK
(catalog no. 3285; 1:1,000; rabbit) were obtained from Cell
Signaling Technology Inc. (Danvers, MA, USA). Primary antibodies
against AKT (catalog no. sc-5298; 1:1,000; mouse), GSK3β (catalog
no. sc-24501; 1:1,000; rabbit), p-extracellular signal-regulated
kinase (p-ERK; catalog no. sc-7976; 1:1,000; rabbit), ERK (catalog
no. sc-514302; 1:1,000; mouse), caspase-3 (catalog no. sc-65497;
1:1,000; mouse), myeloid cell leukemia-1 (MCL-1; catalog no.
sc-12756; 1:1,000; mouse), B-cell lymphoma-2 (BCL-2; catalog no.
sc-492; 1:1,000; rabbit), cyclin-dependent kinase 4 (CDK4; catalog
no. sc-260; 1:1,000; rabbit), cyclin D1 (catalog no. sc-450;
1:1,000; mouse), P21 (catalog no. sc-6246; 1:1,000; mouse), c-Myc
(catalog no. sc-789; 1:1,000; rabbit), signal transducer and
activator of transcription 3 (STAT3; catalog no. 12640; 1:1,000;
rabbit), p-STAT3 (catalog no. 9145; 1:2,000; rabbit) and
glyceraldehyde 3-phosphate dehydrogenase (catalog no. sc-365062;
1:2,000; mouse) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The secondary antibodies, anti-rabbit
immunoglobulin G (IgG), horseradish-peroxidase (HRP)-linked
antibody (catalog no. 7074; 1:2,000) and anti-mouse IgG, HRP-linked
antibody (catalog no. 7076; 1:2,000), were obtained from Cell
Signaling Technology, Inc.
MTT assay
NPC cells in the logarithmic phase were seeded into
96-well plates at a density of 1,500 cells per well in 195 µl
RPMI-1640 medium and cultured at 37°C overnight. Subsequently, the
cells were treated with various concentrations (0.625, 1.25, 2.5,
5, 10 and 20 µmol/l) of 7RH and/or dasatinib for 72 h at 37°C in 5%
CO2. Control cells were treated in the same manner as
the treatment group but without 7RH or dasatinib. Experiments were
terminated by adding 10 µl of 5 mg/ml MTT and incubated at 37°C for
4 h. Following complete removal of the medium, 100 µl DMSO was
added to each well to dissolve the purple formazan product. The
optical densities (ODs) of the resultant purple solutions were
measured at an absorbance wavelength of 570 nm. The half maximal
inhibitory concentration (IC50) was calculated using the
following equation: Inhibitory rate (%) = (1 - mean OD value of the
treatment group / mean OD value of the control group) × 100.
Western blot analysis
Prior to drug treatment, CNE2 cells were seeded into
6-well plates at a density of 3×105 cells per well and
incubated at 37°C in 5% CO2 overnight. Following
treatment, CNE2 cells were collected and washed three times with 1X
phosphate-buffered saline (PBS) and lysed using 100 µl lysis buffer
(Cell Signaling Technology, Inc.) per well. Cell lysates were
centrifuged at 13,439 × g for 15 min at 4°C and protein
concentrations were determined using the Bio-Rad Protein Assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). SDS-PAGE loading
buffer was added to the cell lysates, which were then heated at
100°C for 10 min. Equal quantities of protein (24 µg) were
separated by 10 and 15% SDS-PAGE and the resolved proteins were
transferred onto polyvinylidene difluoride membranes. After
blocking with 5% skimmed milk, the membranes were incubated
sequentially with the primary and secondary antibodies overnight at
4°C. After washing three times with Tris-buffered saline
supplemented with Tween-20 (pH 7.4; 10 mmol/l Tris-HCl, 150 mmol/l
NaCl, 0.1% Tween 20), the proteins were detected using enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA) and
BioMax® XAR film (Kodak, Rochester, NY, USA). Band
intensities were analyzed using ImageJ software (version 1.45;
National Institutes of Health, Bethesda, MA, USA).
Cell apoptosis analysis by flow
cytometry
Cell apoptosis was assessed by measuring the
membrane redistribution of phosphatidylserine using an Annexin
V-FITC Apoptosis Detection kit (Roche, Basel, Switzerland),
according to the manufacturer's instructions. Briefly, cells
(1×106/well) were seeded into 6-well plates and allowed
to attach overnight. Subsequently, the cells were treated with 2, 4
or 8 µmol/l 7RH for 48 h, washed twice with PBS, resuspended in 250
µl binding buffer and stained with Annexin V-FITC and propidium
iodide. Following incubation in the dark for 30 min, the cells were
analyzed using the BD FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Colony formation assays
To assess colony formation, 200 CNE2 or HONE1 cells
were seeded into each well of a 6-well plate in replicates. After
24 h, the cells were incubated with or without 7RH (0, 0.4, 0.6 and
0.8 µmol/l) in 5% FBS at 37°C and allowed to grow for 5 days. At
the end of the treatment period, the cells were fixed with 100%
ethanol, stained with 0.1% crystal violet and washed with distilled
water. Images of the colonies were captured with an inverted
microscope and quantification was performed using ImageJ software
(National Institutes of Health).
Cell adhesion assays
Cells in the logarithmic phase were seeded into
6-well plates at a density of 3×105 cells per well in
RPMI-1640 medium containing 5% FBS and cultured at 37°C overnight.
Subsequently, the cells were treated with various concentrations
(0, 2, 4 and 8 µmol/l) of 7RH and/or dasatinib for 24 h at 37°C in
5% CO2, while 96-well plates were precoated with BD
Matrigel (BD Biosciences) at 37°C for 2 h. Single cell suspensions
were prepared in RPMI-1640 medium containing 5% FBS, and seeded
into the 96-well plates with DMSO control or 7RH at a density of
1×105 cells/well. Following 2 h of incubation at 37°C,
the cells were washed with PBS to remove the nonadherent cells. To
obtain the adherent cell number, adherent cells were extracted
using 200 µl MTT and the absorbance at 570 nm was measured.
siRNA transfection
Prior to transfection, the cells were seeded into
6-well plates with antibiotic-free growth medium (Gibco; Thermo
Fisher Scientific, Inc.) at a density of 2.5×105 cells
per well and cultured overnight at 37°C. At ~50% confluence, the
cells were transfected with DDR1-specific siRNA (DDR1 #1 sense,
5′-CCACCAACUUCAGCAGCUUTT-3′ and antisense,
5′-AAGCUGCUGAAGUUGGUGGTT-3′; DDR1 #2 sense,
5′-GGUUACUCUUCAGCGAAAUTT-3′ and antisense,
5′-AUUUCGCUGAAGAGUAACCTT-3′; and control sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) in Opti-MEM® reduced serum
medium (Gibco; Thermo Fisher Scientific, Inc.) using
Lipofectamine® 2000 (Gibco; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. After 4–6 h,
the medium was replaced with fresh RPMI-1640 medium containing 5%
FBS and cells were cultured for another 24 h.
Animals
A total of 24 male BALB/c nude mice (age, 6 weeks;
weight, 20±2 g) were obtained from the Guangdong Province Animal
Facility (Sun Yat-sen University) and provided with distilled water
and a commercial stock diet in an air-conditioned room at 22°C. All
animals were sacrificed by cervical dislocation following
anesthetization with ethyl ether (Sigma-Aldrich; EMD Millipore).
The present study was approved by the Institutional Animal Care and
Use Committee of Guangdong Medical Laboratory Animal Center
(Guangdong, China). All animal studies were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (29).
Mouse tumor xenograft model
CNE2 cells (1.3×106 cells per 0.2 ml PBS)
were injected subcutaneously into the right armpit of nude mice.
Tumor size was measured every 4 days in two dimensions using a
caliper and tumor volumes were calculated using the formula:
ab2/2, where b is the smaller dimension. When tumors
reached ~150 mm3 in size, the mice were randomized into
four groups (6 mice/group), as follows: i) Normal saline (NS)
group, intraperitoneally injected with NS for 20 days; ii) 7RH
group, intraperitoneally injected with 8 mg/kg 7RH for 20 days;
iii) dasatinib group, intraperitoneally injected with 10 mg/kg
dasatinib for 20 days; and iv) 7RH + dasatinib group,
intraperitoneally injected with 8 mg/kg 7RH and 10 mg/kg dasatinib
for 20 days.
Tumor inhibition rate
The inhibitory effect of 7RH and/or dasatinib on
tumor growth was evaluated. After 20 days of treatment, the mice
were anesthetized with ethyl ether and sacrificed via cervical
dislocation and the whole body and tumor mass were weighed
immediately. The inhibition rates of solid tumor growth were
calculated using the following formula: Inhibition rate (%) = (1 -
mean tumor volume of drug treated groups/mean tumor volume of NS
group) × 100. The IC50 and CI values were calculated
with CalcuSyn software (Biosoft, Cambridge, UK).
Statistical analysis
Data are presented as the mean ± standard deviation.
To determine significant differences between the treatment and
control groups, the Student's two-tailed t-test was used. SPSS 19.0
(IBM SPSS, Armonk, NY, USA) was used for the analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
7RH inhibits NPC cell proliferation
and colony formation in vitro
To investigate the cytotoxicity of the DDR1
inhibitor 7RH in cancer cells, MTT assays were performed (Fig. 1B). 7RH exhibited potent cytotoxicity
in NPC cell lines (CNE2, HONE1, CNE1 and SUNE1). Exposure to 7RH
(0.625–20 µmol/l) resulted in a dose-dependent inhibition of cell
viability. The IC50 values were 1.97, 3.71, 2.06 and
3.95 µmol/l in CNE2, HONE1, CNE1 and SUNE1 cells, respectively.
These results indicate that 7RH inhibits NPC cell proliferation in
a dose-dependent manner. In addition, it was observed that
treatment with 0.4, 0.6 and 0.8 µmol/l 7RH significantly inhibited
colony formation in CNE2 and HONE1 cells (P<0.05; Fig. 1C and D). Taken together, these results
suggest that treatment 7RH effectively inhibits the tumorigenicity
of NPC cells.
7RH induces cell cycle arrest and
apoptosis by inhibiting JAK/STAT signaling in NPC cells
A previous study demonstrated that the STAT family
(STAT1a/b, STAT3 and STAT5) directly bind to the DDR1 protein
(5). Therefore, the present study
aimed to investigate whether 7RH affects JAK/STAT signaling, which
is involved in cell cycle progression, by analyzing the protein
expression levels of key genes involved in this pathway. Following
treatment with 4 µmol/7RH for 12, 24 or 48 h, the protein
expression levels of JAK1, p-JAK1, STAT3, p-STAT3, BCL-2, MCL-1,
c-Myc, cyclin D1 and CDK4 were markedly reduced in a time-dependent
manner, whereas those of p21 were markedly increased in CNE2 cells
(Fig. 2A). These results indicate
that 7RH is able to effectively induce the cell cycle arrest of NPC
cells.
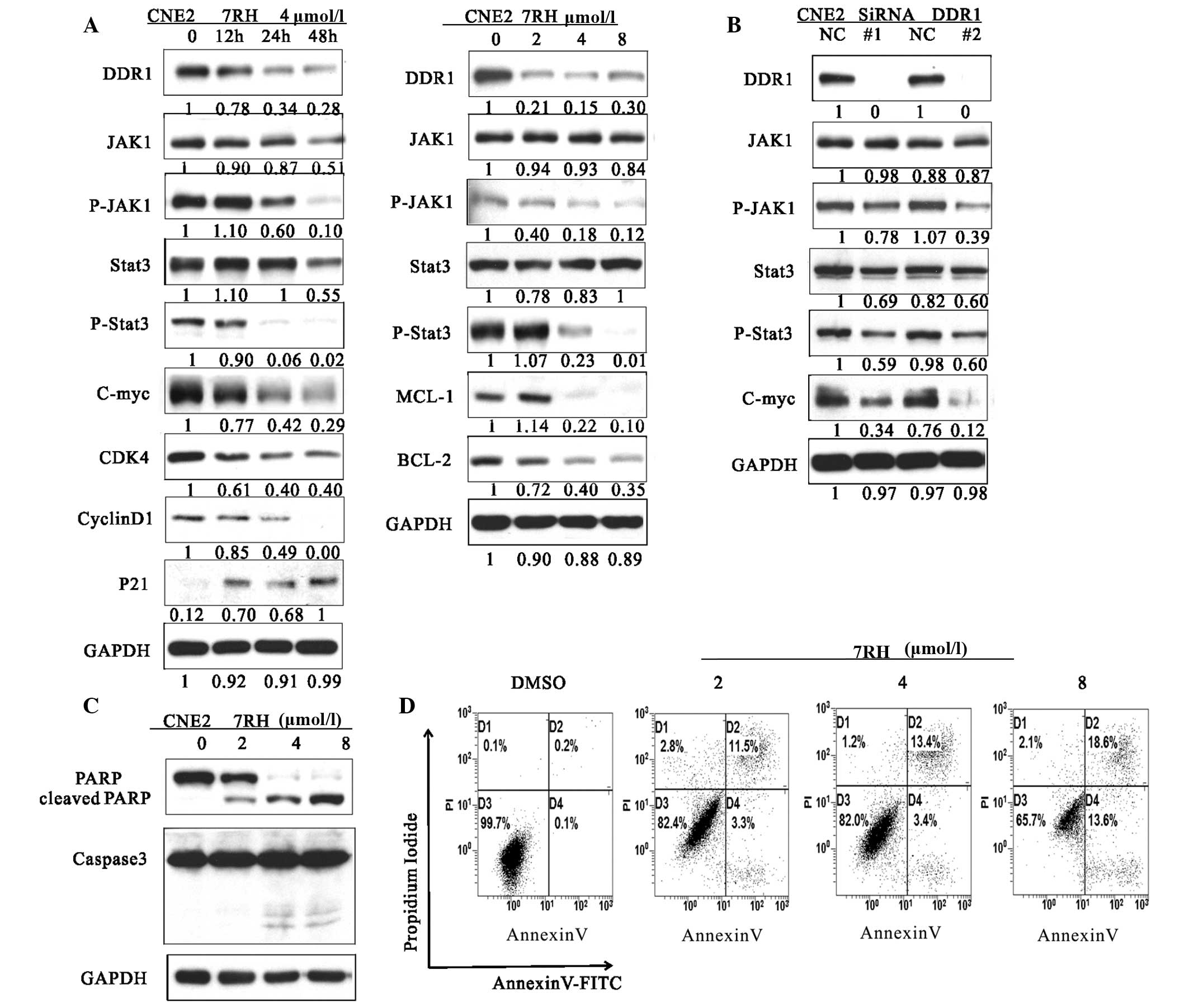 | Figure 2.7RH induced cell cycle arrest and
apoptosis of CNE2 cells by inhibiting the JAK1-STAT3 signaling
pathway. (A) The effect of 7RH on the cell cycle progression of
CNE2 cells. CNE2 cells were incubated for 12, 24 or 48 h with 4
µmol/l 7RH or treated with 2, 4 or 8 µmol/l 7RH for 24 h, after
which the cell cycle profile was analyzed by western blotting. (B)
Transfection of CNE2 cells with DDR1-specific siRNA was associated
with similar alterations to the JAK1/STAT3 signaling pathway as
observed following treatment of the cells with 7RH, as determined
by western blotting. (C) Western blot analysis of cleaved PARP and
caspase-3. CNE2 cells were treated with 2, 4 or 8 µmol/l 7RH for 24
(PARP) or 48 h (caspase-3). (D) Induction of apoptosis in cells
following 7RH treatment was assessed by flow cytometry using an
Apoptosis Detection kit. The apoptotic rates of the cell lines were
increased following treatment with 2, 4 or 8 µmol/l 7RH for 48 h.
JAK1, Janus kinase 1; p-, phosphorylated; STAT3, signal transducer
and activator of transcription 3; DDR1, discoidin domain receptor
1; siRNA, small interfering RNA; DMSO, dimethyl sulfoxide. |
Transfection of CNE2 cells with DDR1-specific siRNA
was performed to investigate the effect of DDR1 inhibition on the
activation of the JAK/STAT signaling pathway (Fig. 2B). When DDR1 was knocked out by siRNA,
the expressions of JAK1, p-JAK1, STAT3, p-STAT3 and c-Myc was
reduced. In addition, to further investigate the mechanism of
growth inhibition by 7RH, the effect of 7RH on the apoptosis of
CNE2 cells was evaluated by western blotting and flow cytometry
(Fig. 2C and D). 7RH induced the
apoptosis of CNE2 cells, as indicated by the increased levels of
PARP cleavage and caspase-3 activation observed following treatment
with 2, 4 or 8 µmol/l 7RH for 24 or 48 h (Fig. 2C). The western blot analysis results
were correlated with a higher fraction of Annexin-V-positive cells
at 48 h (Fig. 2D). These results
indicate that 7RH induces cell cycle arrest and apoptosis of NPC
cells via alteration of the JAK/STAT signaling pathways.
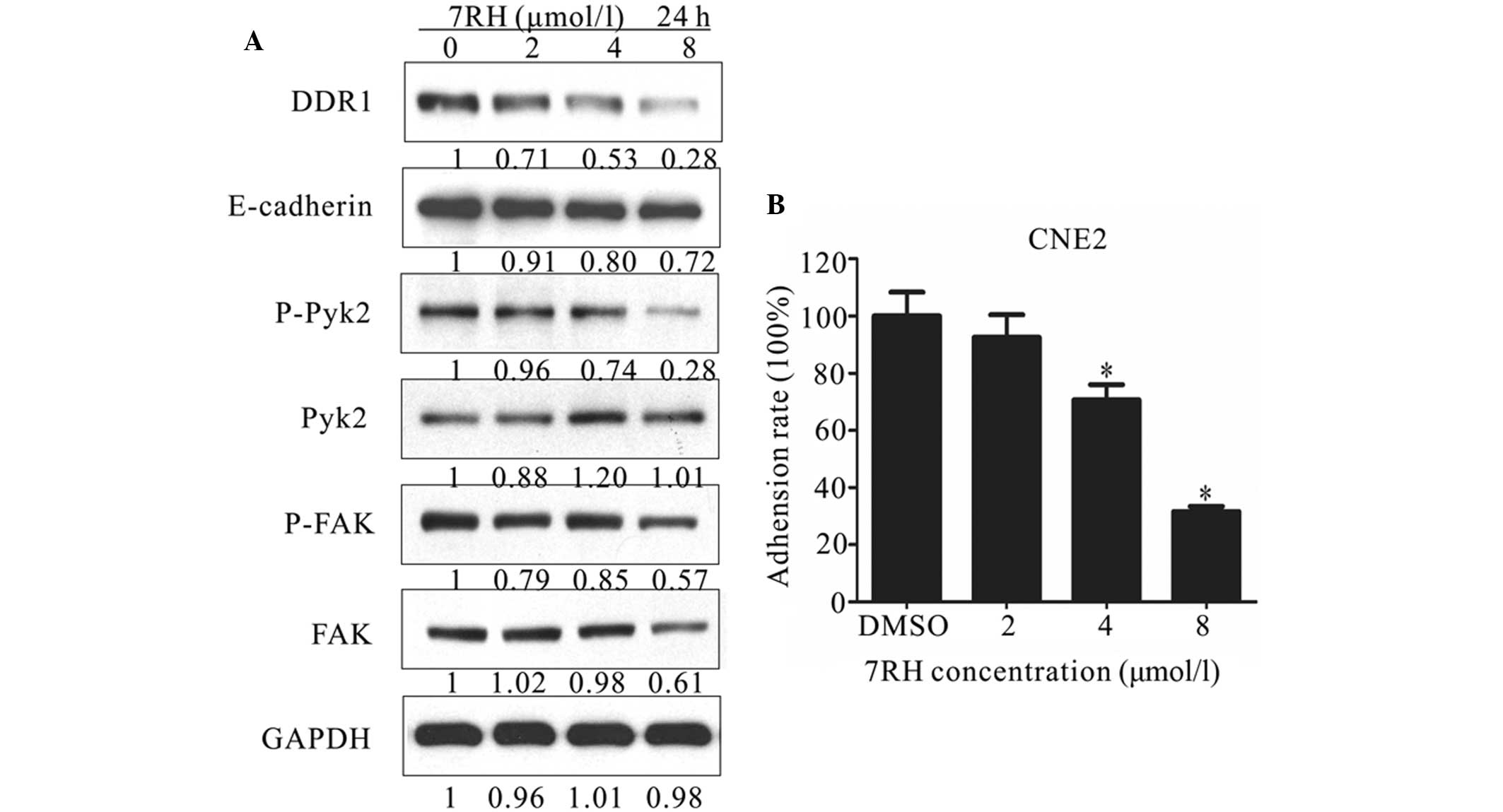
7RH inhibits NPC cell adhesion
Cell adhesion is involved in a number of regulatory
processes, including growth, differentiation, proliferation,
migration and regeneration (30). In
addition, adhesion exhibits a crucial function in the formation and
maintenance of coherent multicellular structures. DDR1 has
previously been demonstrated as one of the key regulators of cell
adhesion (31). Therefore, the
present study investigated the effect of 7RH on cell-matrix
adhesion by performing western blot analysis of cell
adhesion-associated proteins and adhesion assays. As shown in
Figure 3A, the protein expression
levels of E-cadherin, p-Pyk2 and p-FAK were markedly decreased in
CNE2 cells following treatment with 2, 4 or 8 µmol/l 7RH for 24 h.
For adhesion assays, CNE2 cells adhering to BD Matrigel-coated
wells were treated with 2, 4 or 8 µmol/l 7RH for 2 h and the number
of adherent cells was determined by measuring the absorbance at 570
nm. Notably, 7RH inhibited cell-matrix adhesion in a dose-dependent
manner (Fig. 3B) and this inhibition
was significant following treatment with 4 or 8 µmol/l 7RH
(P<0.05). Treatment with 2, 4 or 8 µmol/l 7RH for 2 h inhibited
cell-matrix adhesion by 7.4, 29.3 and 68.3%, respectively, in CNE2
cells, compared with DMSO-treated controls (Fig. 3B). These results indicate that 7RH
inhibits NPC cell adhesion.
7RH enhances the PI3K/AKT signaling
pathway via activation of SRC in NPC cells
To further investigate the mechanism of growth
inhibition by 7RH, the effect of 7RH on the PI3K/AKT and
mitogen-activated protein kinase (MAPK) signaling pathways were
investigated. Western blot analysis indicated that the expression
levels of p-AKT (Ser473), p-GSK3β and p-MEK were significantly
upregulated in CNE2 cells following treatment with 2, 4 or 8 µmol/l
7RH for 24 h (Fig. 4A). Activated SRC
is a potent activator of the PI3K/AKT signaling pathway (27). Therefore, the present study aimed to
elucidate whether PI3K/AKT signaling was upregulated by SRC
signaling. Notably, the protein expression levels of p-SRC were
markedly upregulated following the inhibition of DDR1 with both 7RH
and DDR1-specific siRNA (Fig. 4B and
C). These results indicate that 7RH enhances the PI3K/AKT
signaling pathway in NPC cells via the activation of SRC.
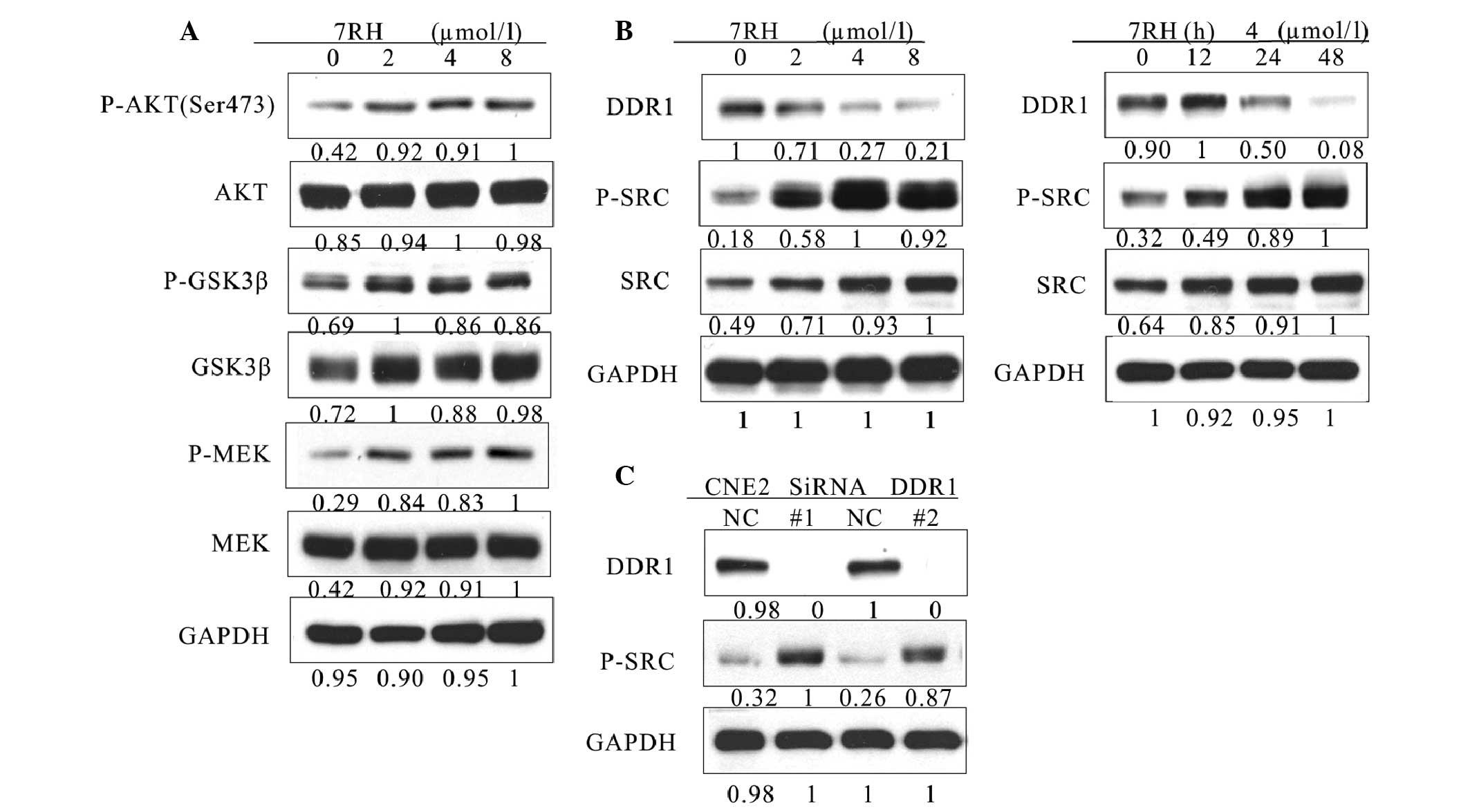 | Figure 4.7RH upregulated the PI3K/AKT pathway
by activating SRC in CNE2 cells. (A) 7RH enhanced the activity of
the PI3K/AKT signaling pathway. CNE2 cells were treated with 2, 4
or 8 µmol/l 7RH for 24 followed by western blotting of cell lysates
to analyze phosphorylation levels of AKT (Ser473), GSK3β and MEK,
relative to GAPDH. (B) 7RH treatment increased the phosphorylation
of SRC in a time- and dose-dependent manner. CNE2 cells were
treated with 2, 4 or 8 µmol/l 7RH for 24 h, or with 4 µmol/l 7RH
for 12, 24 or 48 h, followed by western blotting to assess protein
expression levels of DDR1, SRC and p-SRC. (C) CNE2 cells were
transfected with siRNA-specific DDR1 for 24 h followed by western
blotting to analyze the expression levels of DDR1 and p-SRC. PI3K,
phosphoinositide 3-kinase; GSK 3β, glycogen synthase kinase 3β;
p-phosphorylated; MEK, mitogen-activated protein kinase kinase;
DDR1, discoidin domain receptor 1. |
Dual inhibition of DDR1 and SFK
decreases the tumorigenicity of NPC cells in vitro
Subsequently, whether the combined inhibition of
DDR1 and SFKs exhibited a synergistic effect on cell tumorigenicity
was investigated. Firstly, the combined inhibitory effect of 7RH
and dasatinib on cell proliferation was examined using MTT assays.
The proliferation of CNE2 cells was significantly inhibited
following treatment with 7RH in combination with various
concentrations of dasatinib in a dose-dependent manner. The
corresponding combination indexes (CIs) were <1 for CNE2 cells,
which were determined to be statistically significant (Table I). These results suggest that combined
treatment with 7RH and dasatinib exhibits a synergistic effect on
the suppression of human NPC cell proliferation.
 | Table I.Dual inhibition of discoidin domain
receptor 1 and SRC family kinase decreased the tumorigenicity of
nasopharyngeal carcinoma cells in vitro. |
Table I.
Dual inhibition of discoidin domain
receptor 1 and SRC family kinase decreased the tumorigenicity of
nasopharyngeal carcinoma cells in vitro.
| 7RH (µmol/l) | Dasatinib
(µmol/l) | Fa | CI |
|---|
| 0.3125 | 1.25 | 0.377 | 0.289 |
| 0.625 |
2.5 | 0.486 | 0.378 |
| 1.25 |
5 | 0.738 | 0.268 |
| 2.5 | 10 | 0.827 | 0.326 |
| 5 | 20 | 0.840 | 0.596 |
| 10 | 40 | 0.895 | 0.755 |
To investigate the combined effect of 7RH and
dasatinib on the PI3K/AKT signaling pathway, the protein expression
levels of key proteins in this signaling pathway in CNE2 cells were
evaluated in CNE2 cells by western blotting. Notably, dual
inhibition of DDR1 and SFK following treatment with 4 µM 7RH and 5
µM dasatinib markedly decreased the protein expression of p-AKT
(Ser473) compared with the groups of 7RH or dasatinib alone
(Fig. 5A); however, no evident
alterations in the expression levels of p-GSK3β and p-MEK were
observed between the dual treatment of 7RH + dasatinib and 7RH or
dasatinib alone (data not shown). In addition, the phosphorylation
levels of one of the essential substrates of mTORC1, 4EBP1, as well
as p-ERK, were significantly suppressed following combined
treatment with 4 µM 7RH and 5 µM dasatinib (Fig. 5A). These results indicated that the
mTOR/4EBP1 and MAPK signaling pathways were significantly
suppressed following treatment with 7RH and dasatinib in CNE2
cells. Furthermore, the induction of PARP and caspase-3 cleavage
and the decrease in the expression levels of MCL-1 and BCL-2
observed (Fig. 5A), correlated with a
higher fraction of Annexin-V-positive cells following 48 h of
treatment (4 µM 7RH and/or 5µM dasatinib; Fig. 5B), and indicated the effective
induction of apoptosis in NPC cells. Furthermore, the dual
inhibition of DDR1 and SFK significantly decreased NPC cell
adhesion rate (P<0.05; Fig. 5C).
Together, these results suggest that combined treatment with 7RH
and dasatinib more effectively inhibits NPC cell tumorigenicity
when compared with either drug alone.
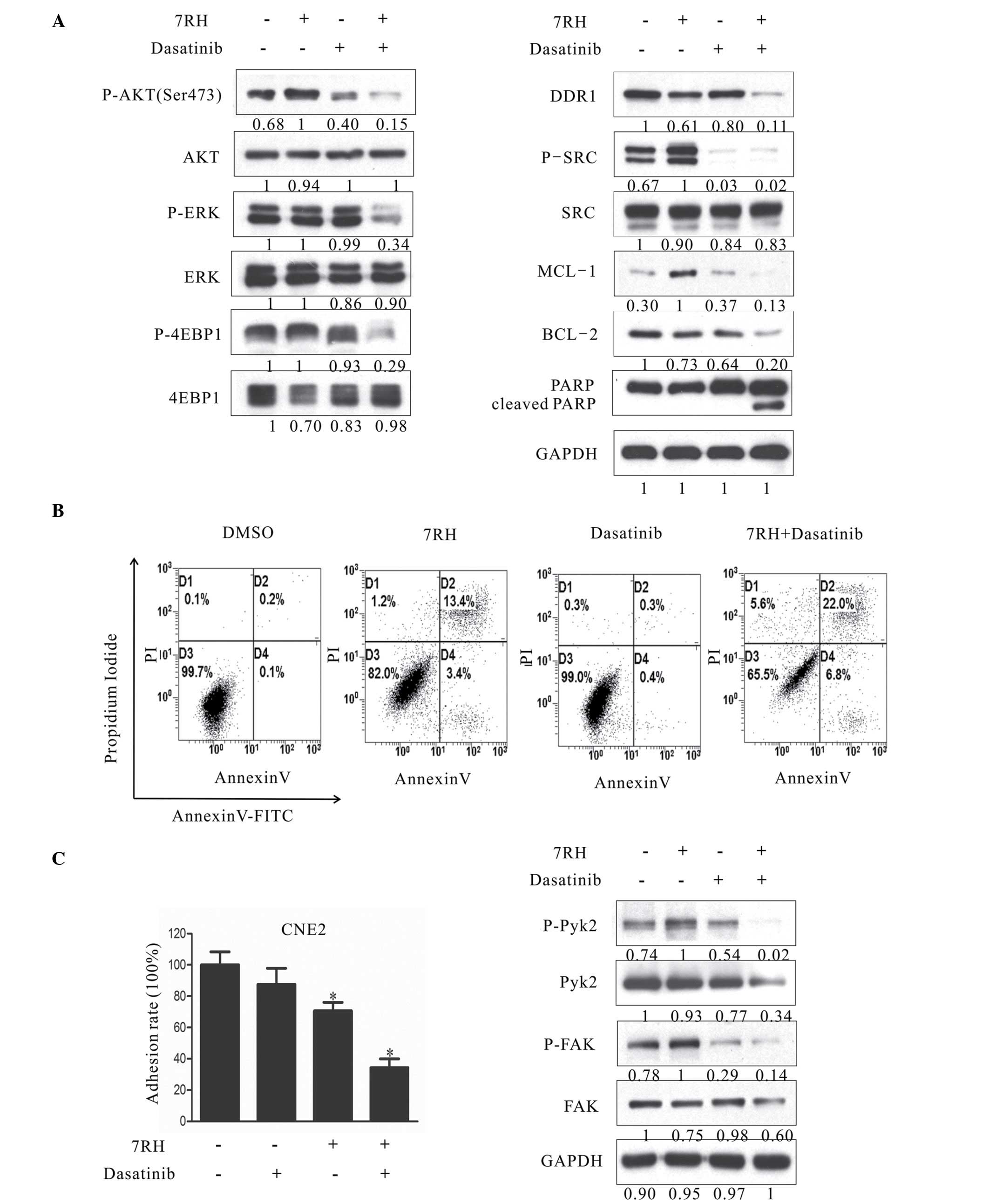 | Figure 5.Dual inhibition of DDR1 and SFK
decreased the tumorigenecity of NPC cells in vitro. (A)
Effect of combined 7RH and dasatinib treatment on the survival,
progression and apoptosis of CNE2 cells. (A) CNE2 cells were
treated with 4 µM 7RH alone, 5 µM dasatinib alone or 7RH ±
dasatinib and the expression levels of cell cycle-associated
proteins were analyzed by western blotting. (B) The apoptosis of
NPC cells treated with 4 µM 7RH and/or 5 µM dasatinib for 48 h and
stained with Annexin V and propidium iodide was analyzed using flow
cytometry. The apoptosis rates of the cells treated with 7RH ±
dasatinib were increased when compared with cells treated with
either agent alone. (C) The cell adhesion profile was analyzed by
adhesion assays and western blot analysis of p-Pyk2 and p-FAK.
Decreased cell adhesion ratios were observed for cells treated with
7RH and 7RH + dasatinib, as quantified using ImageJ software. Data
are presented as the mean ± standard deviation of triplicate
experiments *P<0.05 vs. control experiments within the same
group. NPC, nasopharyngeal carcinoma; DDR1, discoidin domain
receptor 1; SFK, SRC family kinase; p-, phosphorylated; ERK,
extracellular signal-regulated kinase; 4EBP1, eukaryotic
translation initiation factor 4E binding protein 1; MCL-1, myeloid
cell leukemia-1; BCL-2, B-cell lymphoma-2; PARP, poly(ADP-ribose)
polymerase 1; FAK, focal adhesion kinase; DMSO, dimethyl sulfoxide;
FITC, fluorescein isothiocyanate. |
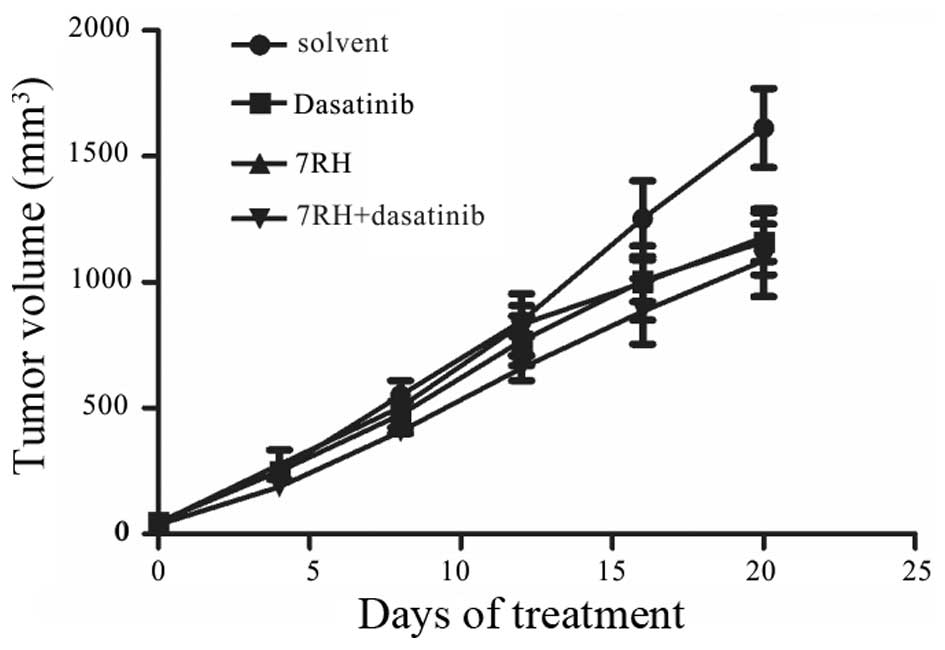
Dual inhibition of DDR1 and SFK
suppresses tumor growth in vivo
To determine whether treatment with 7RH and
dasatinib exhibits synergistic effects in vivo, CNE2 cells
were implanted into the right armpit of mice, which were divided
into NC, 7RH, dasatinib or 7RH + dasatinib treatment groups.
Following 20 days of treatment, the mice were sacrificed and the
mean tumor volumes were measured. The mean tumor volumes were
significantly decreased in the treatment groups when compared with
the NC group (P<0.05; Fig. 6). In
the 7RH, dasatinib and 7RH + dasatinib groups, the growth
inhibitory rates were 27, 28 and 33%, respectively (Fig. 6).
Discussion
The present study demonstrated that 7RH, a highly
potent inhibitor of DDR1, effectively suppresses the tumorigenicity
of NPC cells. This is consistent with the observation that DDR1
transcripts are upregulated in NPC, NPC metastasis, and head and
neck tumor tissues (7). Furthermore,
these results are consistent with a previous study that reported
that inhibition of DDR1 by siRNA suppressed tumorigenicity,
inhibited lung cancer bone metastasis and increased cancer cell
chemosensitivity (24).
The results of the present study indicated that
treatment with 7RH alone inhibited the proliferation of NPC cells
in a dose-dependent manner. However, the exact mechanism underlying
the effects of 7RH on cancer cells remains unclear. In human breast
and colon carcinoma cell lines, DDR1 activation triggers the
pro-survival Ras/Raf/ERK and PI3K/AKT signaling pathways, resulting
in the upregulation of anti-apoptotic Bcl-xL and the promotion of
survival under conditions of genotoxic stress (32). In colon cancer cells, DDR1 forms a
complex with Notch1; DDR1 activation triggers Notch1 cleavage by
γ-secretase, generating the Notch1 intracellular domain, which
translocates into the nucleus and upregulates the expression of
pro-survival genes, including Hes1 and Hey2 (33). The results of the present study
indicated that 7RH-mediated inhibition of NPC cell growth may be
due to inhibition of the JAK/STAT3 signaling pathway. This
hypothesis was based on the observation that 7RH inhibited JAK and
STAT3 phosphorylation in a time- and dose-dependent manner, which
was also observed following treatment of NPC cells with
DDR1-specific siRNA. Activation of the JAK-cytokine receptor
complex leads to the recruitment and phosphorylation of specific
cytoplasmic STAT proteins, which subsequently results in the
dimerization of STAT proteins and their subsequent translocation
into the nucleus where they interact with various regulatory gene
expression elements, including c-Myc, CDK4, cyclin D1, p21 and the
BCL-2 family (34). The present study
demonstrated that the protein expression levels of c-Myc, CDK4,
cyclin D1, P21 and BCL-2 family members in NPC cells were altered
following treatment with 7RH, indicating that 7RH affects the
JAK/STAT3 signaling pathway.
The present study demonstrated that the PI3K/AKT
signaling pathway and levels of p-SRC tyrosine kinase in NPC cells
were increased following 7RH treatment. Previous studies have
reported that the SRC tyrosine kinase is overexpressed in variety
of solid tumors, and is associated with the promotion of cell
growth and proliferation via activation of the RAS/RAF/MEK/ERK and
PI3K/AKT signaling pathways, as well as the promotion of metastasis
by enhancing FAK activity (35,36). In
the majority of human cancers, including NPC, the PI3K/AKT/mTOR
signaling pathway is involved in cancer progression (26). It has been demonstrated that
constitutively-activated AKT regulates cancer processes such as
cell growth, cell cycle progression, invasion, migration,
epithelial-mesenchymal transition and angiogenesis, as well as
promoting resistance to chemotherapy and radiotherapy (37,38).
Similarly, SRC has been associated with cancer chemoresistance; SRC
is involved in the development of resistance to trastuzumab, an
anti-human epidermal growth factor receptor 2 (HER2) monoclonal
antibody, used in breast cancer therapy (39). Consistent with this, activated forms
of SRC and HER kinases are frequently identified in cancer, with
HER kinases and SRC exhibiting hyperfunctionality in ~70% of breast
cancer cases (39). Furthermore, SRC
is involved in the signal transduction mediated by HER kinases
(40). In prostate cancer, SRC family
kinases and FAK signaling are frequently hyperfunctional and have
been associated with poor patient outcome, as well as resistance to
antihormonal therapies (41). In
addition, previous studies have demonstrated that SRC is involved
in resistance to epidermal growth factor receptor inhibitors, which
has been associated with poor outcomes in NPC patients treated with
lapatinib and cetuximab (42,43). The present study demonstrated that 7RH
enhanced the activation of the PI3K/AKT signaling pathway, which
was accompanied by increased protein expression levels of p-SRC.
Similarly, knockdown of DDR1 by siRNA led to SRC activation. These
results suggested that SRC exhibits a vital function in 7RH
resistance by activating the PI3K/AKT signaling pathway.
Dasatinib is a small molecule tyrosine kinase
inhibitor, initially isolated as a dual SRC/Abelson murine leukemia
viral oncogene homolog 1 inhibitor (44). Dasatinib inhibits SFKs, including LCK,
HCK, FYN, YES, FGR, BLK, LYN, and FRK (45). Furthermore, dasatinib blocks the
kinase activities of certain RTKs with high potency, including
c-KIT, c-FMS, platelet-derived growth factor receptors A and B,
DDR1 and Ephrin receptors (46).
Several preclinical studies have demonstrated that dasatinib
potentiates the antitumor effects of various drugs used clinically:
Trials using combinations of dasatinib with ErbB/HER antagonists
have been performed for breast (47),
head and neck (48) and colorectal
cancer (49). The present study
demonstrated that dasatinib used in combination with agents
targeting DDR1 presents a potential therapeutic strategy for
NPC.
Notably, in the present study, combined treatment of
NPC cells with dasatinib and 7RH decreased the expression levels of
p-AKT when compared with either drug treatment alone. It has been
demonstrated that AKT exerts its cellular effects via the
phosphorylation of >20 substrate proteins, including members of
the forkhead protein family (FKHR, FKHRL1 and AFX), mTOR, MDM2 and
Bad (50). In the present study, the
phosphorylation levels of downstream targets of mTORC1, such as
4EBP-1, were decreased following the combined drug treatment when
compared with single treatments. In addition, the MAPK signaling
pathway was altered in response to combined dasatinib and 7RH
treatment. The inhibition of multiple signaling pathways indicated
that the synergistic antitumor effects of the combined drug
treatment may have resulted from the inhibition of multiple
signaling pathways involved in the control of cell proliferation
and survival.
In the present study, 7RH and dasatinib exhibited
antitumor effects in vivo. These results suggested that DDR1
may be considered a potential molecular target for NPC
treatment.
In conclusion, the present study demonstrated that
the DDR1 inhibitor 7RH was able to suppress NPC cells in
vitro, via the inhibition of JAK1/STAT3 signaling pathway
activation. SRC was demonstrated to exhibit a vital function in the
resistance of NPC cells to 7RH via activation of the PI3K/AKT
signaling pathway. Notably, the present study formed a conceptual
basis for the application of combination chemotherapy targeting
both DDR1 and SFK for the treatment of patients with NPC in the
future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30873009). The
authors would like to thank Dr Ding Ke for providing 7RH.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
DDRs
|
discoidin domain receptors
|
|
RTK
|
receptor tyrosine kinase
|
|
siRNA
|
small interfering RNA
|
|
SFK
|
Src family kinase
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
References
|
1
|
Chin D, Boyle GM, Porceddu S, Theile DR,
Parsons PG and Coman WB: Head and neck cancer: Past, present and
future. Expert Rev Anticancer Ther. 6:1111–1118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: Molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robinson DR, Wu YM and Lin SF: The protein
tyrosine kinase family of the human genome. Oncogene. 19:5548–5557.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valiathan RR, Marco M, Leitinger B, Kleer
CG and Fridman R: Discoidin domain receptor tyrosine kinases: New
players in cancer progression. Cancer Metastasis Rev. 31:295–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hidalgo-Carcedo C, Hooper S, Chaudhry SI,
Williamson P, Harrington K, Leitinger B and Sahai E: Collective
cell migration requires suppression of actomyosin at cell-cell
contacts mediated by DDR1 and the cell polarity regulators Par3 and
Par6. Nat Cell Biol. 13:49–58. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chua HH, Yeh TH, Wang YP, Huang YT, Sheen
TS, Lo YC, Chou YC and Tsai CH: Upregulation of discoidin domain
receptor 2 in nasopharyngeal carcinoma. Head Neck. 30:427–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laval S, Butler R, Shelling AN, Hanby AM,
Poulsom R and Ganesan TS: Isolation and characterization of an
epithelial-specific receptor tyrosine kinase from an ovarian cancer
cell line. Cell Growth Differ. 5:1173–1183. 1994.PubMed/NCBI
|
|
9
|
Shen Q, Cicinnati VR, Zhang X, Iacob S,
Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G and
Beckebaum S: Role of microRNA-199a-5p and discoidin domain receptor
1 in human hepatocellular carcinoma invasion. Mol Cancer.
9:2272010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao
MS and Vogel WF: Expression and mutation analysis of the discoidin
domain receptors 1 and 2 in non-small cell lung carcinoma. Br J
Cancer. 96:808–814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barker KT, Martindale JE, Mitchell PJ,
Kamalati T, Page MJ, Phippard DJ, Dale TC, Gusterson BA and
Crompton MR: Expression patterns of the novel receptor-like
tyrosine kinase, DDR, in human breast tumours. Oncogene.
10:569–575. 1995.PubMed/NCBI
|
|
12
|
Weiner HL, Rothman M, Miller DC and Ziff
EB: Pediatric brain tumors express multiple receptor tyrosine
kinases including novel cell adhesion kinases. Pediatr Neurosurg.
25:64–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nemoto T, Ohashi K, Akashi T, Johnson JD
and Hirokawa K: Overexpression of protein tyrosine kinases in human
esophageal cancer. Pathobiology. 65:195–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Squire JA, Bayani J, Luk C, Unwin L,
Tokunaga J, MacMillan C, Irish J, Brown D, Gullane P and Kamel-Reid
S: Molecular cytogenetic analysis of head and neck squamous cell
carcinoma: By comparative genomic hybridization, spectral
karyotyping, and expression array analysis. Head Neck. 24:874–887.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada K, Nakamura M, Ishida E, Higuchi
T, Yamamoto H, Tsujikawa K and Konishi N: Prostate cancer antigen-1
contributes to cell survival and invasion though discoidin receptor
1 in human prostate cancer. Cancer Sci. 99:39–45. 2008.PubMed/NCBI
|
|
16
|
Rodrigues R, Roque L, Espadinha C, Pinto
A, Domingues R, Dinis J, Catarino A, Pereira T and Leite V:
Comparative genomic hybridization, BRAF, RAS, RET, and oligo-array
analysis in aneuploid papillary thyroid carcinomas. Oncol Rep.
18:917–926. 2007.PubMed/NCBI
|
|
17
|
Hajdu M, Singer S, Maki RG, Schwartz GK,
Keohan ML and Antonescu CR: IGF2 over-expression in solitary
fibrous tumours is independent of anatomical location and is
related to loss of imprinting. J Pathol. 221:300–307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiaretti S, Li X, Gentleman R, Vitale A,
Wang KS, Mandelli F, Foà R and Ritz J: Gene expression profiles of
B-lineage adult acute lymphocytic leukemia reveal genetic patterns
that identify lineage derivation and distinct mechanisms of
transformation. Clin Cancer Res. 11:7209–7219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdulhussein R, Koo DH and Vogel WF:
Identification of disulfide-linked dimers of the receptor tyrosine
kinase DDR1. J Biol Chem. 283:12026–12033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konitsiotis AD, Raynal N, Bihan D,
Hohenester E, Farndale RW and Leitinger B: Characterization of high
affinity binding motifs for the discoidin domain receptor DDR2 in
collagen. J Biol Chem. 283:6861–6868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou G, Vogel WF and Bendeck MP: Tyrosine
kinase activity of discoidin domain receptor 1 is necessary for
smooth muscle cell migration and matrix metalloproteinase
expression. Circ Res. 90:1147–1149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agarwal G, Mihai C and Iscru DF:
Interaction of discoidin domain receptor 1 with collagen type 1. J
Mol Biol. 367:443–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogel WF, Abdulhussein R and Ford CE:
Sensing extracellular matrix: An update on discoidin domain
receptor function. Cell Signal. 18:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valencia K, Ormazábal C, Zandueta C,
Luis-Ravelo D, Antón I, Pajares MJ, Agorreta J, Montuenga LM,
Martínez-Canarias S, Leitinger B and Lecanda F: Inhibition of
collagen receptor discoidin domain receptor-1 (DDR1) reduces cell
survival, homing, and colonization in lung cancer bone metastasis.
Clin Cancer Res. 18:969–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koon HK, Chan PS, Wong RN, Wu ZG, Lung ML,
Chang CK and Mak NK: Targeted inhibition of the EGFR pathways
enhances Zn-BC-AM PDT-induced apoptosis in well-differentiated
nasopharyngeal carcinoma cells. J Cell Biochem. 108:1356–1363.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ligresti G, Militello L, Steelman LS,
Cavallaro A, Basile F, Nicoletti F, Stivala F, McCubrey JA and
Libra M: PIK3CA mutations in human solid tumors: Role in
sensitivity to various therapeutic approaches. Cell Cycle.
8:1352–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao M, Duan L, Luo J, Zhang L, Lu X, Zhang
Y, Zhang Z, Tu Z, Xu Y, Ren X and Ding K: Discovery and
optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)
benzamides as novel selective and orally bioavailable discoidin
domain receptor 1 (DDR1) inhibitors. J Med Chem. 56:3281–3295.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Care NRCU and Animals AUOL: Guide for the
Care and Use of Laboratory Animals. National Academies Press (US);
Washington DC: 2011, PubMed/NCBI
|
|
30
|
Eswaramoorthy R, Wang CK, Chen WC, Tang
MJ, Ho ML, Hwang CC, Wang HM and Wang CZ: DDR1 regulates the
stabilization of cell surface E-cadherin and E-cadherin- mediated
cell aggregation. J Cell Physiol. 224:387–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeh YC, Wu CC, Wang YK and Tang MJ: DDR1
triggers epithelial cell differentiation by promoting cell adhesion
through stabilization of E-cadherin. Mol Biol Cell. 22:940–953.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ongusaha PP, Kim JI, Fang L, Wong TW,
Yancopoulos GD, Aaronson SA and Lee SW: p53 induction and
activation of DDR1 kinase counteract p53-mediated apoptosis and
influence p53 regulation through a positive feedback loop. EMBO J.
22:1289–1301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HG, Hwang SY, Aaronson SA, Mandinova A
and Lee SW: DDR1 receptor tyrosine kinase promotes prosurvival
pathway through Notch1 activation. J Biol Chem. 286:17672–17681.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vainchenker W and Constantinescu SN:
JAK/STAT signaling in hematological malignancies. Oncogene.
32:2601–2613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konig H, Copland M, Chu S, Jove R,
Holyoake TL and Bhatia R: Effects of dasatinib on SRC kinase
activity and downstream intracellular signaling in primitive
chronic myelogenous leukemia hematopoietic cells. Cancer Res.
68:9624–9633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thamilselvan V, Craig DH and Basson MD:
FAK association with multiple signal proteins mediates
pressure-induced colon cancer cell adhesion via a Src-dependent
PI3K/Akt pathway. FASEB J. 21:1730–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chin YR and Toker A: Function of Akt/PKB
signaling to cell motility, invasion and the tumor stroma in
cancer. Cell Signal. 21:470–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Huang WC, Li P, Guo H, Poh SB,
Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishizawar RC, Miyake T and Parsons SJ:
c-Src modulates ErbB2 and ErbB3 heterocomplex formation and
function. Oncogene. 26:3503–3510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tatarov O, Mitchell TJ, Seywright M, Leung
HY, Brunton VG and Edwards J: SRC family kinase activity is
up-regulated in hormone-refractory prostate cancer. Clin Cancer
Res. 15:3540–3549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lui VW, Lau CP, Ho K, Ng MH, Cheng SH,
Tsao SW, Tsang CM, Lei KI, Chan AT and Mok TS: Anti-invasion,
anti-proliferation and anoikis-sensitization activities of
lapatinib in nasopharyngeal carcinoma cells. Invest New Drugs.
29:1241–1252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sung FL, Pang RT, Ma BB, Lee MM, Chow SM,
Poon TC and Chan AT: Pharmacoproteomics study of cetuximab in
nasopharyngeal carcinoma. J Proteome Res. 5:3260–3267. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lombardo LJ, Lee FY, Chen P, Norris D,
Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM,
et al: Discovery of N-(2-chloro-6-methyl-
phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
(BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor
activity in preclinical assays. J Med Chem. 47:6658–6661. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karaman MW, Herrgard S, Treiber DK,
Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI,
Edeen PT, et al: A quantitative analysis of kinase inhibitor
selectivity. Nat Biotechnol. 26:127–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rix U, Hantschel O, Dürnberger G, Rix LL
Remsing, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P,
Colinge J, et al: Chemical proteomic profiles of the BCR-ABL
inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase
and nonkinase targets. Blood. 110:4055–4063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seoane S, Montero JC, Ocaña A and
Pandiella A: Effect of multikinase inhibitors on
caspase-independent cell death and DNA damage in
HER2-overexpressing breast cancer cells. J Natl Cancer Inst.
102:1432–1446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Johnson FM, Saigal B, Talpaz M and Donato
NJ: Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses
invasion and induces cell cycle arrest and apoptosis of head and
neck squamous cell carcinoma and non-small cell lung cancer cells.
Clin Cancer Res. 11:6924–6932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koppikar P, Choi SH, Egloff AM, Cai Q,
Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM
and Grandis JR: Combined inhibition of c-Src and epidermal growth
factor receptor abrogates growth and invasion of head and neck
squamous cell carcinoma. Clin Cancer Res. 14:4284–4291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|