Introduction
Pancreatic neuroendocrine tumors (pNETs) are a rare
clinical entity with an annual incidence of 1.09–5.25 cases per
million individuals (1), representing
a small percentage of all pancreatic neoplasms; however, their
incidence is rising (2,3). These tumors are generally slow-growing
and exhibit indolent behavior. However, distant metastasis is
possible, worsening the prognosis (4).
It is well recognized that compared with
non-neoplastic cells, malignant cells exhibit an accelerated
metabolism, a high glucose requirement and an increased uptake of
glucose. Glucose transporters (GLUTs) facilitate the entry of
glucose into cells. GLUTs are passive carriers that function as an
energy-independent system to transport glucose down a concentration
gradient (5). GLUT type 1 (GLUT-1) is
a high-affinity GLUT that is expressed in normal human tissues,
including red blood cells, the endothelium of the blood-brain
barrier and the placenta (6,7).
GLUT overexpression is frequently observed in
cancer, and it is associated with a high metabolism and the rapid
growth of cells in often-hypoxic tumor areas (8). Increased levels of GLUT-1 expression
have been demonstrated to be associated with a range of carcinomas,
including those of the breasts (9),
head and neck (10), bladder
(11), colorectum (12) and lungs (13), and pulmonary neuroendocrine carcinomas
(NECs) (14). GLUT-2 was previously
suggested to be overexpressed in hepatic tumors (15), and breast (16) and gastric cancers (17).
It is known that hypoxia-inducible factor 1 (HIF-1)
is a master regulator of the transcriptional responses of mammalian
cells to hypoxia. HIF-1 plays a critical role in the expression of
a number of genes that control angiogenesis, glucose metabolism,
cell proliferation, cell survival and metastasis in response to
hypoxia (18,19). Elevated expression of HIF-1α is
associated with a poor prognosis in numerous types of solid tumors,
including lung, breast, colorectal, brain, pancreatic, ovarian,
renal and bladder cancer (20).
Downstream HIF-1 targets, such as GLUT-1, play critical roles in
cellular metabolism and glucose transport, where enhanced glucose
metabolism is observed following the upregulation of their
respective genes by hypoxia (18).
Insulin-like growth factor II messenger RNA-binding
protein 3 (IMP3) plays an important role in RNA trafficking and
stabilization, cell growth and cell migration during the early
stages of embryogenesis (21,22). The expression of IMP3 is found in
malignant tumors as an oncofetal protein that promotes cell
proliferation, and the adhesion and invasion of malignant neoplasms
(23). IMP3 expression has been
studied in neuroendocrine tumors of the lung (24), but to the best of our knowledge, no
studies have examined IMP3 expression in pNETs.
It is difficult to evaluate the malignant potential
of pNETs, as recurrence or distant metastasis is occasionally
observed in the group of low-grade pNETs. The aim of the present
study was to clarify the usefulness of the expression of GLUT-1,
GLUT-2, HIF-1α and IMP3 in pNETs, and their clinicopathological
correlation for evaluating the malignant potential of pNETs.
Materials and methods
Case selection
The study used 70 formalin-fixed paraffin-embedded
tissue samples of pNETs that had been obtained from surgical
resection samples and diagnosed at the Department of Anatomical
Pathology (Pathological Science, Graduate School of Medical
Science, Kyushu University, Fukuoka, Japan) between June 1991 and
May 2011. All samples were classified into four groups according to
the World Health Organization classification (2010) (25): G1
(mitotic count of <2 and/or ≤2% Ki-67 index; n=47), G2 (mitotic
count of 2–20 and/or 3–20% Ki-67 index; n=18), NEC (large- or
small-cell type; n=4) and mixed adenoneuroendocrine carcinoma
(mixed type, n=1). Available clinical follow-up data were obtained
from 50 of the pNET patients. This study was approved by the
Institutional Review Board of Kyushu University and conformed to
the ethical guidelines of the 1975 Declaration of Helsinki.
Immunohistochemical staining and
evaluation
All specimens were fixed in 10% formalin and
processed routinely. Hematoxylin and eosin staining was also
performed on 4-µm thick sections of formalin-fixed
paraffin-embedded tissue. The sections were deparaffinized in
xylene and rehydrated in ethanol. Endogenous peroxidase activity
was blocked by incubation in methanol containing 0.3%
H2O2 for 30 min. Antigen retrieval was
achieved by microwave heating in 10 mM citrate buffer (pH 6.0) for
20 min (for GLUT-1 and GLUT-2) or in Target Retrieval Solution (pH
9.0; Dako, Carpinteria, CA) for 20 min (for IMP3), or through use
of BORG Decloaker solution (Biocare Medical, Walnut Creek, CA, USA)
and a Decloaking Chamber (Biocare Medical) for ~30 min (for
HIF-1α).
The sections were incubated overnight at 4°C with
the following primary antibodies: Rabbit polyclonal anti-GLUT-1
(1:300 dilution; cat. no. ab15309; Abcam, Cambridge, UK), mouse
monoclonal anti-GLUT-2 (1:1,000 dilution; cat. no. ab85715; Abcam),
mouse monoclonal anti-HIF-1α (1:500 dilution; cat. no. NB100-105;
Novus Biologicals, Littleton, CO, USA), mouse monoclonal anti-IMP3
(1:100 dilution; cat. no. M3626; Dako) and mouse monoclonal
anti-insulin (1:1 dilution; cat. no. ab6995; Abcam). The labeled
antigens were detected with an EnVision+ system -Horseradish
Peroxidase-Labeled Polymer system (Dako) and visualized using
3,3′-diaminobenzidine tetrahydrochloride as a chromogen.
Counterstaining was then performed with hematoxylin.
Samples of clear cell renal cell carcinoma, normal
liver tissue, colon cancer and normal tonsil tissue were used as
the positive controls of GLUT-1, GLUT-2, HIF-1α and IMP3,
respectively. Immunoreactivities were assessed in the membranous
staining for GLUT-1, the cytoplasmic staining for GLUT-2, IMP3 and
insulin, and the nuclear staining for HIF-1α, and were defined as
positive for any extent of expression. Islets of Langerhans or red
blood cells were used as internal controls of GLUT-1 and GLUT-2,
respectively. All stained slides were reviewed independently by two
pathologists.
Statistical analysis
All statistical analyses were performed using JMP
9.0.2 software (SAS Institute, Cary, NC, USA). Clinicopathological
comparisons were conducted using the Pearson, χ2 and
Fisher's exact tests. Survival curves were calculated by the
Kaplan-Meier method, and the survival data were examined by the
log-rank test. P<0.05 was considered to indicate a significant
difference.
Results
GLUT-1, GLUT-2, HIF-1α and IMP3
expression, and clinicopathological findings
The correlations between the immunohistochemical
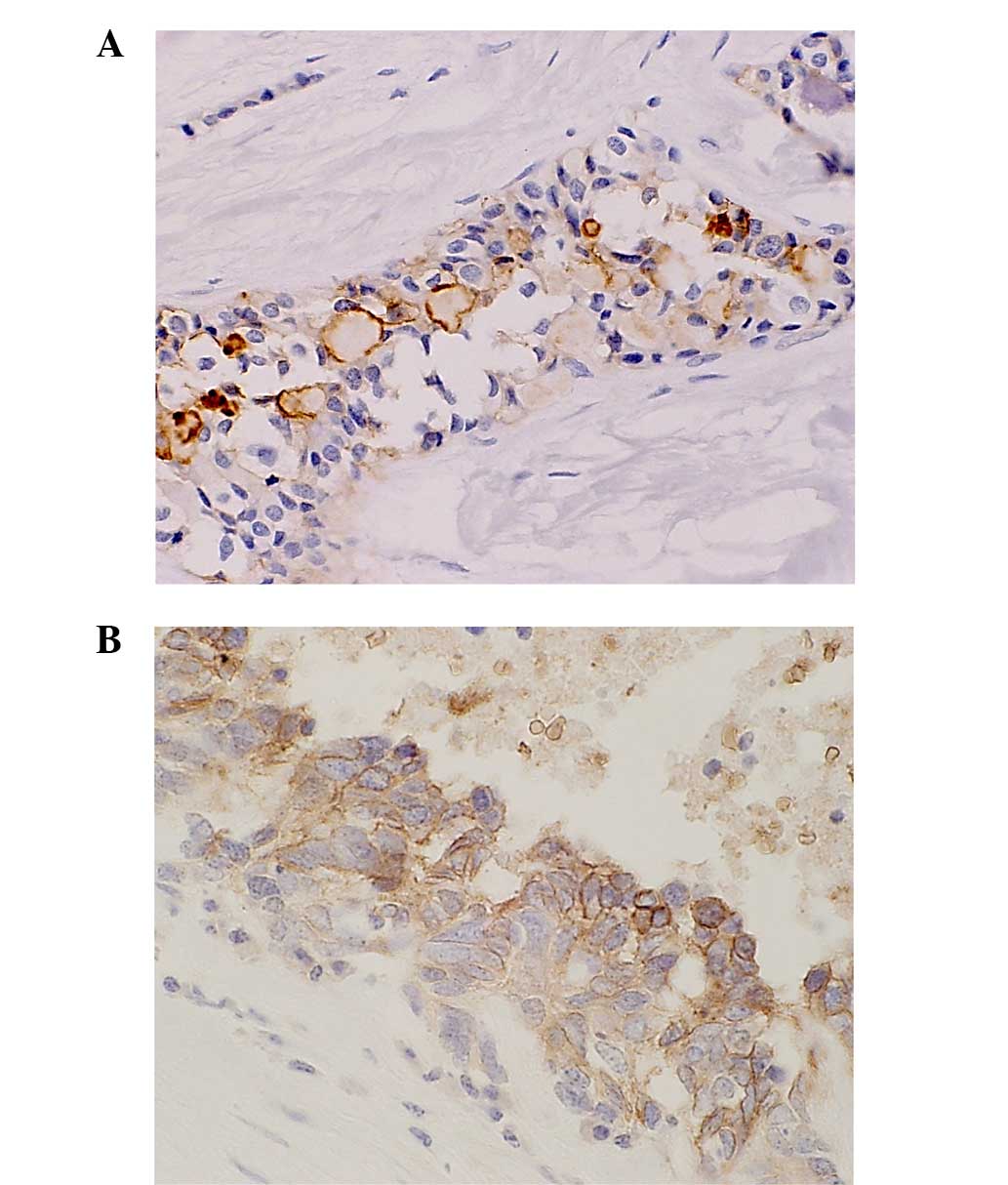
results and clinicopathological findings are summarized in Table I. In the GLUT-1-positive cases,
positive cells were distributed along the periphery to the center
of the tumor, accompanied by moderate to severe fibrosis or
necrosis. Membranous staining of GLUT-1 was found in 8 out of the
47 (17%) G1 tumors, in 8 out of the 18 (44%) G2 tumors, and in all
of the NEC and mixed-type tumors (100%) (Fig. 1). The expression of GLUT-1 was
significantly higher in the G2, NEC and mixed-type cases compared
with the G1 cases (P=0.0007). Vessel invasion (P=0.0007), lymph
node metastasis (P=0.026), a high Ki-67 labeling index (P=0.0019)
and high mitotic counts (P=0.0002) were significantly more frequent
in the GLUT-1-positive cases (Table
I).
 | Table I.Association of GLUT-1, GLUT-2, HIF-1α
and IMP3 expression with clinicopathological variables. |
Table I.
Association of GLUT-1, GLUT-2, HIF-1α
and IMP3 expression with clinicopathological variables.
|
|
| GLUT-1 | GLUT-2 | HIF-1α | IMP3 |
|---|
|
|
|
|
|
|
|
|---|
| Variables | n |
Positive/negative | P-value |
Positive/negative | P-value |
Positive/negative | P-value |
Positive/negative | P-value |
|---|
| Total cases | 70 | 21/49 |
| 8/62 |
| 10/60 |
| 10/60 |
|
| Mean age, years |
|
| 0.3208 |
| 0.8634 |
| 0.6250 |
| 0.6250 |
|
<55 | 33 | 8/25 |
| 4/29 |
| 4/29 |
| 4/29 |
|
| ≥55 | 37 | 13/24 |
| 4/33 |
| 6/31 |
| 6/31 |
|
| Gender |
|
| 0.6296 |
| 0.9473 |
| 0.5475 |
| 0.4226 |
|
Female | 43 | 12/31 |
| 5/38 |
| 7/36 |
| 5/38 |
|
| Male | 27 | 9/18 |
| 3/24 |
| 3/24 |
| 5/22 |
|
| Tumor size, cm |
|
| 0.1244 |
| 0.6228 |
| 0.4561 |
| 1.0000 |
| ≥3.0 | 21 | 9/12 |
| 3/18 |
| 4/17 |
| 3/18 |
|
|
<3.0 | 49 | 12/37 |
| 5/44 |
| 6/43 |
| 7/42 |
|
| Vessel
invasion |
|
| 0.0007a |
| 0.7664 |
| 0.0484a |
| 0.0965 |
| + | 23 | 13/10 |
| 3/20 |
| 6/17 |
| 1/22 |
|
| − | 47 | 8/39 |
| 5/42 |
| 4/43 |
| 9/38 |
|
| Lymph node
metastasis |
|
| 0.0261a |
| 0.5131 |
| 0.4755 |
| 0.0745 |
| + | 15 | 8/7 |
| 1/14 |
| 3/12 |
| 0/15 |
|
| − | 55 | 13/42 |
| 7/48 |
| 7/48 |
| 10/45 |
|
| Necrosis |
|
| 0.6903 |
| 0.6228 |
| 0.4561 |
| 0.4561 |
| + | 21 | 7/14 |
| 3/18 |
| 2/19 |
| 2/19 |
|
| − | 49 | 14/35 |
| 5/44 |
| 8/41 |
| 8/41 |
|
| Functioning |
|
| 0.1307 |
| 0.4239 |
| 0.8399 |
| 0.8399 |
| + | 26 | 5/21 |
| 4/22 |
| 4/22 |
| 4/22 |
|
| − | 44 | 16/28 |
| 4/40 |
| 6/38 |
| 6/38 |
|
| Insulin |
|
| 0.8644 |
| 0.1896 |
| 0.4561 |
| 0.0253a |
| + | 21 | 6/15 |
| 4/17 |
| 2/19 |
| 6/15 |
|
| − | 49 | 15/34 |
| 4/45 |
| 8/41 |
| 4/45 |
|
| Ki-67 index, % |
|
| 0.0019a |
| 0.1224 |
| 0.0116a |
| 0.8263 |
|
>2 | 19 | 11/8 |
| 4/15 |
| 6/13 |
| 3/16 |
|
| ≤2 | 51 | 10/41 |
| 4/47 |
| 4/47 |
| 7/44 |
|
| Mitotic count |
|
| 0.0002a |
| 0.1045 |
| 0.0383a |
| 0.5174 |
| ≥2 | 12 | 9/3 |
| 3/9 |
| 4/8 |
| 1/11 |
|
|
<2 | 58 | 12/46 |
| 5/53 |
| 6/52 |
| 9/49 |
|
| WHO
classification |
|
| 0.0007a,b |
| 0.2727b |
| 0.0484a,b |
| 0.8354b |
| G1 | 47 | 8/39 |
| 4/43 |
| 4/43 |
| 7/40 |
|
| G2 | 18 | 8/10 |
| 2/16 |
| 4/14 |
| 2/16 |
|
|
NEC | 4 | 4/0 |
| 1/3 |
| 1/3 |
| 1/3 |
|
|
Mixed-type | 1 | 1/0 |
| 1/0 |
| 1/0 |
| 0/0 |
|
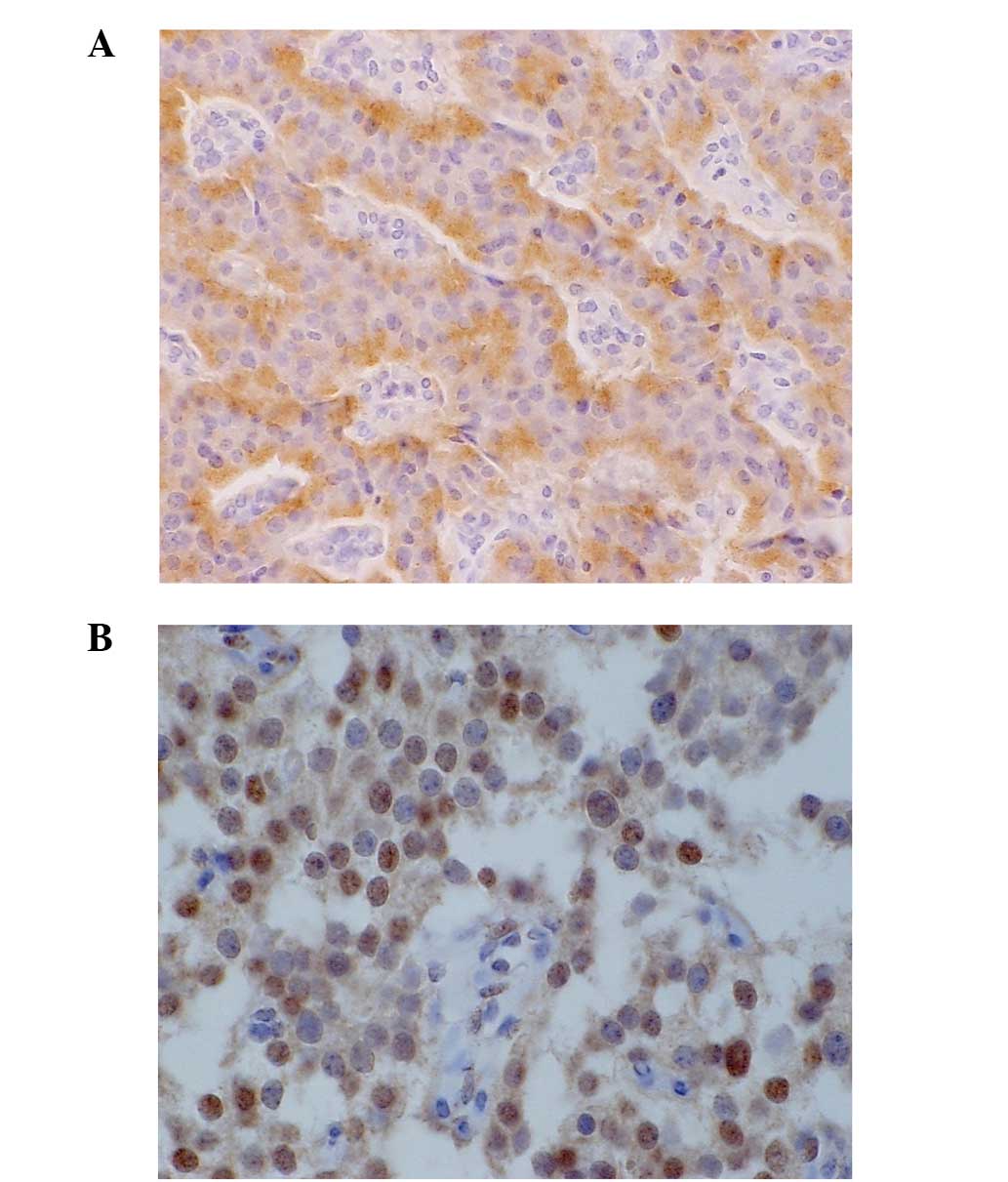
GLUT-2 staining was detected in the cytoplasm of the
tumor cells in 4 out of the 47 (8.5%) G1 cases, in 2 out of the 18
(11%) G2 cases, in 1 out of the 4 (25%) NEC cases and in the single
(100%) mixed-type case (Fig. 2A).
GLUT-2 expression exhibited no correlation with any
clinicopathological factors (Table
I).
HIF-1α expression was detected in the nucleus of the
tumor cells in 4 out of the 47 (8.5%) G1 cases, in 4 out of the 18
(22%) G2 cases, in 1 out of the 4 (25%) NEC cases and in the single
(100%) mixed-type case (Fig. 2B).
HIF-1α expression was also significantly higher in the G2, NEC and
mixed-type groups compared with the G1 group (P=0.048). The vessel
invasion (P=0.048), high Ki-67 labeling index (P=0.012) and high
mitotic counts (P=0.038) were each significantly correlated with
HIF-1α expression (Table I). There
was a significant correlation between GLUT-1 expression and HIF-1α
expression (P=0.025) (Table II).
 | Table II.Association between HIF-1α and
GLUT-1/GLUT-2. |
Table II.
Association between HIF-1α and
GLUT-1/GLUT-2.
|
| HIF-1α |
|---|
|
|
|
|---|
| Expression | Positive
(n=10) | Negative
(n=60) | P-value |
|---|
| GLUT-1 |
|
|
|
|
Positive/negative | 6/4 | 15/45 | 0.025 |
| GLUT-2 |
|
|
|
|
Positive/negative | 2/8 | 6/54 | 0.357 |
IMP3 expression was recognized in the cytoplasm of
the tumor cells. IMP3 was expressed in 7 out of the 47 (15%) G1
cases, in 2 of the 18 (11%) G2 cases, and in 1 out of the 4 (25%)
NEC cases, but not in the single (0%) mixed type case. Insulin
expression was significantly more frequently observed in the
IMP3-positive cases compared with the IMP3-negative cases (P=0.025)
(Table I).
Survival analysis
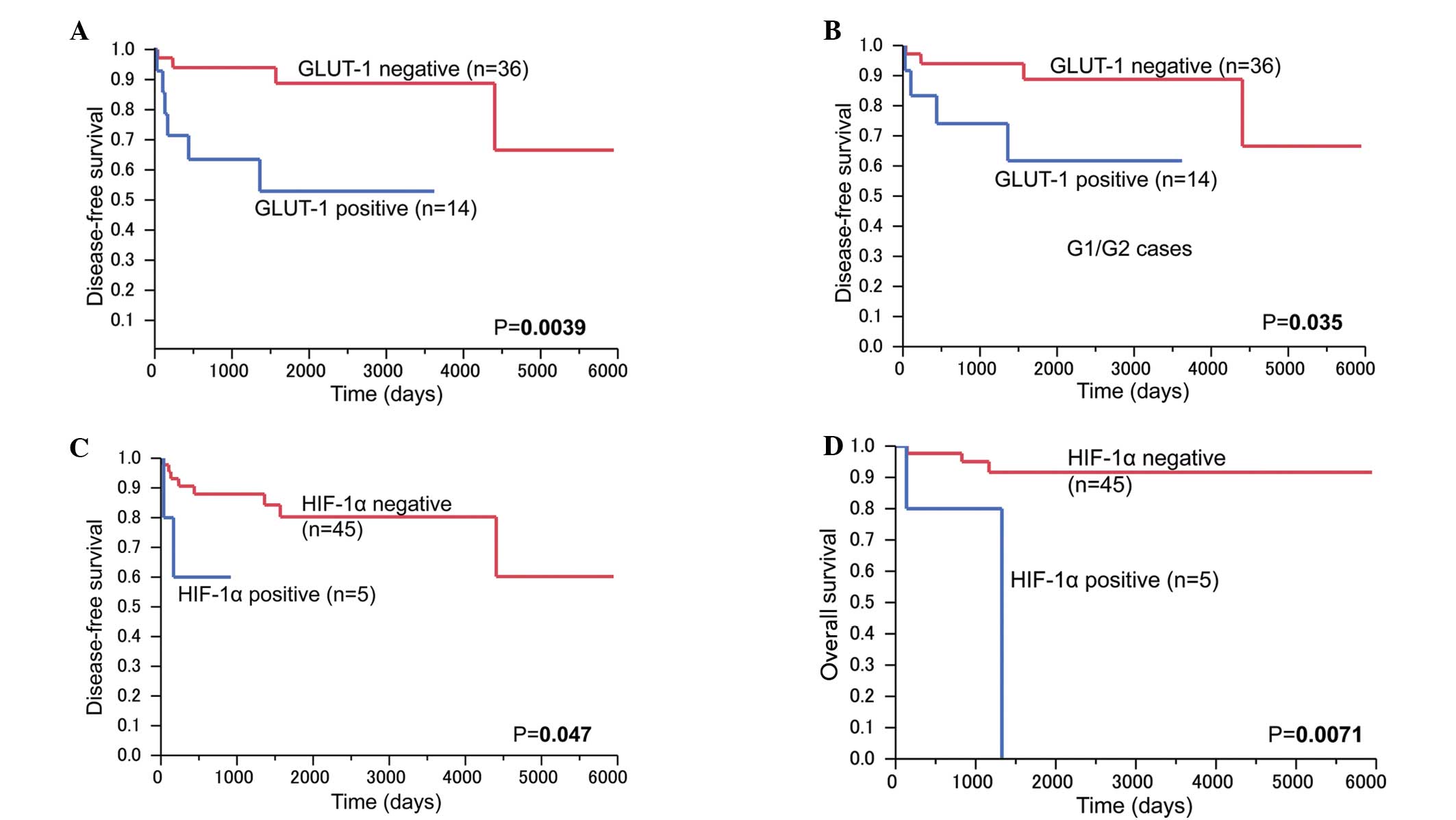
Patients in the positive GLUT-1 expression group
showed significantly poor disease-free survival rates compared with
those in the negative group (P=0.0039; Fig. 3A). Among the G1/G2 tumors, the
patients with positive GLUT-1 expression (n=12) showed
significantly poor disease-free survival rates compared with those
in the negative GLUT-1 expression group (n=36) (P=0.035; Fig. 3B). The patients with HIF-1α expression
(n=5) showed significantly poor disease-free survival and overall
survival rates (P=0.047 and P=0.0071, respectively) (Fig. 3C and D). GLUT-2 and IMP3 expression
did not affect disease-free survival or overall survival rates.
Multivariate analysis
The significant factors revealed by univariate
analysis were assessed using a Cox proportional hazard model. A
multivariate analysis revealed that lymph node metastasis was an
independent risk factor for disease-free survival in all cases
(P=0.0107) (Table III). In the
G1/G2 group, tumor size (P=0.0479) and lymph node metastasis
(P=0.0214) were shown to be independent risk factors for
disease-free survival (Table
IV).
 | Table III.Univariate and multivariate analysis
of disease-free survival in all cases. |
Table III.
Univariate and multivariate analysis
of disease-free survival in all cases.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age (≥55
years) | 0.0175 | 0.291 | 0.014–1.822 | 0.2099 |
| Tumor size (≥3.0
cm) | 0.0048 | 3.959 | 0.682–33.63 | 0.1267 |
| Vassel invasion
(+) | 0.0012 | 0.381 | 0.020–5.720 | 0.4891 |
| Lymph node
metastasis (+) | <0.0001 | 18.591 | 1.797–414.0 | 0.0107 |
| HIF-1α (+) | 0.047 | 1.998 | 0.257–11.68 | 0.4679 |
| GLUT-1 (+) | 0.0078 | 3.081 | 0.458–24.39 | 0.2490 |
 | Table IV.Univariate and multivariate analysis
of disease-free survival in the G1/G2 group. |
Table IV.
Univariate and multivariate analysis
of disease-free survival in the G1/G2 group.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Gender
(male/female) | 0.0377 | 1.515 | 0.169–16.02 | 0.7035 |
| Tumor size (≥3.0
cm) | 0.0302 | 8.328 | 1.018–182.3 | 0.0479 |
| Vassel invasion
(+) | 0.0085 | 0.261 | 0.004–6.712 | 0.4448 |
| Lymph nodes
metastasis (+) | 0.0003 | 32.486 | 1.522–2667 | 0.0214 |
| HIF-1α (+) | 0.3360 | 12.327 | 0.386–459.9 | 0.1363 |
| GLUT-1 (+) | 0.0509 | 2.436 | 0.354–19.75 | 0.3654 |
Discussion
Various studies have shown a close association
between GLUT-1 expression and tumor aggressiveness and poor
prognosis in a number of carcinomas (9–14).
However, little is known about GLUT-1 expression in pNETs.
Ozbudak et al demonstrated that GLUT-1
expression was associated with an increased risk of mortality among
patients with pulmonary NECs (14),
and that GLUT-1 expression was strongly correlated with
neuroendocrine differentiation/grade, but not with other
clinicopathological variables. In the present study, GLUT-1
expression was similarly significantly increased in the high-grade
pNET group. Unlike in the pulmonary NECs, the present pNET cases
with GLUT-1 expression were correlated with markers of tumor
aggressiveness, including vessel invasion, lymph node metastasis, a
high Ki-67 labeling index and a high mitotic count.
These findings indicate that GLUT-1 expression in
pNETs is a more useful marker of malignant potential than that in
pulmonary NECs. Moderate to severe fibrosis and/or necrosis is
observed in pNETs, suggesting the presence of a hypoxic area in
pNETs (17). In response to hypoxia,
HIF-1 plays a critical role in the expression of a number of genes
that control angiogenesis, cell proliferation, cell survival,
metastasis and glucose metabolism. GLUT-1 plays critical roles in
cellular metabolism and glucose transport, where enhanced glucose
metabolism is observed following the upregulation of their
respective genes by hypoxia (17).
The present study found that the expression of GLUT-1 was
significantly correlated with HIF1-α expression (P=0.025) in pNETs,
indicating the possibility of the induction of GLUT-1 by HIF1-α in
the hypoxic condition.
The patients in the present positive GLUT-1
expression group showed significantly poor disease-free survival
rates compared with those in the negative GLUT-1 expression group.
In addition, the GLUT-1-positive cases in the G1/G2 group showed
significantly poor disease-free survival rates (P=0.035) compared
with the negative GLUT-1 expression group. These findings suggest
that the expression of GLUT-1 is one of the factors that can be
used for the prognostic assessment of pNETs.
Frendrich et al reported that GLUT-2
expression was detectable in normal islet cells and pancreatic
intraepithelial neoplasia 1B lesions or higher grade lesions
(26). The present study observed the
expression of GLUT-2 in normal islet cells, but only in 8 out of
the 70 (11%) cases of pNET. These data suggest that elevated
glucose metabolism occurs in pNETs via GLUT-1, but not GLUT-2.
IMP3 is expressed in malignant neoplasms, including
intraductal papillary mucinous neoplasms (27), pancreatic adenocarcinoma (28,29) and
hepatocellular carcinoma (30). In
these tumors, IMP3 expression is a useful diagnostic marker for
distinguishing the malignant phenotype from benign lesions and is a
prognostic biomarker associated with poor survival. The present
study showed no association between IMP3 expression and malignant
characteristics or prognosis in pNET. However, it was found that
GLUT-1 expression is associated with a poor disease-free survival
rate, indicating that GLUT-1 is a useful biomarker rather than IMP3
in pNET. Among the 18 cases of insulinoma (18/70; 26%), 15 cases
were positive for insulin; IMP3 expression exhibited a close
association with insulin expression, but the mechanism underlying
this association is not yet known.
In conclusion, the findings of the present study
indicated that GLUT-1 expression is correlated with malignant
potential and that its overexpression is a prognostic biomarker for
pNET.
Acknowledgements
The language editing of the original manuscript was
performed by KN International (http://www.kninter.com/).
References
|
1
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘Carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao JC, Eisner MP, Leary C, Dagohoy C,
Phan A, Rashid A, Hassan M and Evans DB: Population-based study of
islet cell carcinoma. Ann Surg Oncol. 14:3492–3500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fitzgerald TL, Hickner ZJ, Schmitz M and
Kort EJ: Changing incidence of pancreatic neoplasms: A 16-year
review of statewide tumor registry. Pancreas. 37:134–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panzuto F, Boninsegna L, Fazio N, Campana
D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L,
Tomassetti P, et al: Metastatic and locally advances pancreatic
endocrine carcinomas: Analysis of factors associated with disease
progression. J Clin Oncol. 29:2372–2377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farrell CL, Yang J and Pardridge WM:
GLUT-1 glucose transporter is present within apical and basolateral
membranes of brain epithelial interfaces and in microvascular
endothelia with and without tight junctions. J Histochem Cytochem.
40:193–199. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueckler M: Facilitative glucose
transporters. Eur J Biochem. 219:713–725. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clavo AC, Brown RS and Wahl RL:
Fluorodeoxyglucose uptake in human cancer cell lines is increased
by hypoxia. J Nucl Med. 36:1625–1632. 1995.PubMed/NCBI
|
|
9
|
Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ,
Hong SR, Lee JH, Lee SG and Park YK: Clinical significance of
glucose transporter 1 (GLUT1) expression in human breast carcinoma.
Jpn J Cancer Res. 93:1123–1128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Schutter H, Landuyt W, Verbeken E,
Goethals L, Hermans R and Nuyts S: The prognostic value of the
hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in
head and neck squamous cell carcinoma treated by radiotherapy +/-
chemotherapy. BMC Cancer. 5:422005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reis H, Tschirdewahn S, Szarvas T, Rübben
H, Schmid KW and Grabellus F: Expression of GLUT1 is associated
with increasing grade of malignancy in non-invasive and invasive
urothelial carcinomas of the bladder. Oncol Lett. 2:1149–1153.
2011.PubMed/NCBI
|
|
12
|
Wincewicz A, Sulkowska M, Koda M and
Sulkowski S: Clinicopathological significance and linkage of the
distribution of HIF-1alpha and GLUT-1 in human primary colorectal
cancer. Pathol Oncol Res. 13:15–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Younes M, Brown RW, Stephenson M, Gondo M
and Cagle PT: Overexpression of GLUT1 and GLUT3 in stage I nonsmall
cell lung carcinoma is associated with poor survival. Cancer.
80:1046–1051. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozbudak IH, Shilo K, Tavora F, Rassaei N,
Chu WS, Fukuoka J, Jen J, Travis WD and Franks TJ: Glucose
tranporter-1 in pulmonary neuroendocrine carcinomas: Expression and
survival analysis. Mod Pathol. 22:633–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H,
Wei PL, Lee CH, Liu RS and Lin SY: In vitro and in vivo study of
phloretin-induced apoptosis in human liver cancer cells involving
inhibition of type II glucose transporter. Int J Cancer.
124:2210–2219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown RS and Wahl RL: Overexpression of
GLUT-1 glucose transporter in human breast cancer. An
immunohistochemical study. Cancer. 72:2979–2985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung JH, Im S, Jung ES and Kang CS:
Clinicopathological implications of the expression of
hypoxia-related proteins in gastric cancer. Int J Med Sci.
10:1217–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Hypoxia, clonal selection and
the role of HIF-1 in tumor progression. Biochm Mol Biol. 35:71–103.
2000.
|
|
19
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Cancer. 3:721–732. 2003.PubMed/NCBI
|
|
20
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
21
|
Mueller-Pillasch F, Pohl B, Wilda M,
Lacher U, Beil M, Wallrapp C, Hameister H, Knöchel W, Adler G and
Gress TM: Expression of the highly conserved RNA binding protein
KOC in embryogenesis. Mech Dev. 88:95–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nielsen J, Christiansen J, Lykke-Andersen
J, Johnsen AH, Wewer UM and Nielsen FC: A family of insulin-like
growth factor II mRNA binding proteins represses translation in
late development. Mol Cell Biol. 19:1262–1270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao B, Hu Y, Herrick DJ and Brewer G: The
RNA-binding protein IMP-3 is a translational activator of
insulin-like growth factor II leader-3 mRNA during proliferation of
human K562 leukemia cells. J Biol Chem. 280:18517–18524. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Burne PA, Spaulding BO and Wang HL:
High-grade neuroendocrine carcinomas of the lung express K homology
domain containing protein overexpressed in cancer but carcinoid
tumors do not. Hum Pathol. 38:555–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klimstra DS, Arnold R, Capella C, Hruban
RH, Klöppel G, Komminoth P, Solcia E and Rindi G: Neuroendocrine
neoplasms of the pancreas. WHO Classification of Tumors of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
IARC Press. (Lyon). 322–326. 2010.
|
|
26
|
Frendrich V, Schneider R, Maitra A,
Jacobsen ID, Opfermann T and Bartsch DK: Detection of precursor
lesions of pancreatic adenocarcinoma in PET-CT in a genetically
engineered mouse model or pancreatic cancer. Neoplasisa.
13:180–186. 2011. View Article : Google Scholar
|
|
27
|
Morimatsu K, Aishima S, Yamamoto H,
Hayashi A, Nakata K and Oda Y, Shindo K, Fujino M, Tanaka M and Oda
Y: Insulin-like growth factor II messenger RNA-binding protein-3 is
a valuable diagnostic and prognostic marker of intraductal
papillary mucinous neoplasm. Hum Pathol. 44:1714–1721. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaeffer DF, Owen DR, Lim HJ, Buczkowski
AK, Chung SW, Scudamore CH, Huntsman DG, Ng SS and Owen DA:
Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3)
overexpression in pancreatic ductal adenocarcinoma correlates with
poor survival. BMC Cancer. 10:592010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wachter DL, Schlabrakowski A, Hoegel J,
Kristiansen G, Hartmann A and Riener MO: Diagnostic value of
immunohistochemical IMP3 expression in core needle biopsies of
pancreatic ductal adenocarinoma. Am J Surg Patol. 35:873–877. 2011.
View Article : Google Scholar
|
|
30
|
Jeng YM, Chang CC, Hu FC, Chou HE, Kao HL,
Wang TH and Hsu HC: RNA-binding protein insulin-like growth factor
II mRNA-binding protein 3 expression promotes tumor invasion and
predicts early recurrence and poor prognosis in hepatocellular
carcinoma. Hepatology. 48:1118–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|