Introduction
Lung cancer is the most commonly diagnosed cancer
worldwide, contributing to 12.7% of the total incidence of cancers,
and also the leading cause of mortality among all tumors (18.2% of
the total) (1). Multidrug resistance
(MDR) is a phenomenon whereby cancer cells exhibit simultaneous
resistance to anti-cancer drugs, with various structures and
mechanisms of action (2,3). MDR results in poor therapeutic efficacy
in the later stage of cancer treatments, and it is also the main
obstacle in obtaining a satisfactory therapeutic outcome in lung
cancer (4).
Extensive studies have revealed that MDR cancer
cells are immune from anti-cancer drug-induced cell apoptosis
through the upregulation of survival signaling pathways, including
phosphatidyl-3-phosphate kinase (PI3K) and extracellular-regulated
kinase-1 (ERK1) (5), or the
suppression of anti-proliferative signaling pathways, including p38
mitogen-activated protein kinases (MAPKs) (6). Bcl-2 is an oncogene which contributes to
tumor occurrence mainly due to the inhibition of apoptosis, and the
overexpression of Bcl-2 usually results in the resistance of cancer
cells to anti-cancer agents associated with abnormal changes of the
pathways discussed above (7,8).
Preliminary observations by the present authors
demonstrated that the pro-apoptotic and cell cycle arrest
activities of triptolide (TPL) largely contribute to its antitumor
effect, and were direct outcomes of the modulation of various
important upstream pathways, including PI3K/Akt, MAPK, JAK/STAT and
nuclear factor-κB, which subsequently modulated the expression of
apoptosis-related proteins such as those of the Bcl-2 family
(9–11). In our preliminary observations, we
demonstrated that TPL inhibited the proliferation of MDR A549/Taxol
cells in vitro mainly through selective modulation of MAPK
signaling (9–11). However, until now, our understanding
of the association between the inhibitory activity of TPL in MDR
cells and the MAPK pathway has been limited. Hence, in the present
study, we investigated the modulatory effect of TPL on the MAPK
pathway in A549/Taxol cells along with its correlation with the
inhibitory effect on the proliferation.
Materials and methods
Drugs and reagents
TPL (≥98%, L-004-130304) was purchased from Chengdu
Herb Purity Co., Ltd. (Chengdu, China). It was dissolved in
dimethyl sulfoxide (DMSO) at a concentration of 10 mmol/l to obtain
the stock solution and kept below −20°C, then diluted to various
concentrations with phosphate-buffered saline (PBS) prior to the
assays. The final concentration of DMSO was less than 0.1%.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
RPMI-1640, a bicinchoninic acid (BCA) protein assay kit, inhibitor
of ERK U0126, propidium iodide (PI) staining kit and Annexin
V-fluorescein isothiocyanate (FITC)/PI apoptosis detection kit were
purchased from Nanjing KeyGen Biotechnology Co. Ltd. (Nanjing,
China). Anti-p-p38, anti-p-JNK, anti-p-ERK antibodies, inhibitor of
p38 SB202190 and inhibitor of JNK SP600125 were purchased from Cell
Signaling Technology (Beverly, MA, USA). Anti-p-Akt (Ser473),
anti-p-GSK-3β (Ser9), cleaved caspase (c-caspase)-3 (1:200),
c-caspase-9 (1:1,000), Bcl-2 (1:500) and COX IV (1:1,000) were
supplied by Bioworld Technology (Nanjing, China). An enhanced
chemiluminescence kit was purchased from Thermo Fisher (Shanghai,
China). Bovine calf serum was purchased from Wisent corporation
(Nanjing, China). All other chemicals and reagents used were of
analytical grade.
Cell culture
The human lung cancer cell line A549 and the
corresponding MDR cell line A549/Taxol were purchased from Nanjing
KeyGen Biotechnology Co. Ltd., and cultured in RPMI-1640 medium
supplemented with 10% bovine calf serum, 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2. Paclitaxel (Taxol, 200 ng/ml) was added to the
medium in order to maintain MDR in the A549/Taxol cells, and
removed two weeks before the experiments. Cells were passaged every
2–3 days.
Cell viability
For the cell viability assay, A549 and A549/Taxol
cells were seeded onto a 96-well plate at a density of
8×103 cells per well. Following overnight incubation,
the culture medium was aspirated, and the cells were incubated with
various concentrations of TPL (the final concentrations were 0.01,
0.02, 0.04, 0.06 and 0.08 µmol/l) or co-treated with MAPK
inhibitors in complete culture medium for 48 h. The same volume of
complete culture medium served as the negative control. Then 20 µl
MTT solution (5 mg/ml) was added to each well, and the plates were
further incubated for 4 h. The medium was removed and 150 µl DMSO
was added to solubilize the MTT formazan salt. The absorbance of
the solution was measured on a microplate reader (Spectra MAX190,
Molecular Devices LLC, Sunnyvale, CA, USA) at 490 nm and the
results were expressed as a percentage of the control cells.
Apoptosis and cell cycle analyses
A549/Taxol cells were seeded onto six-well plates at
a density of 3×105 cells per well. Following overnight
incubation, cells were treated with TPL at concentrations of 0.025
and 0.05 µM for 24 h. For the purpose of apoptosis analysis,
following treatment with TPL, the cells were harvested and washed
with PBS, and then stained with Annexin V/PI according to the
manufacturer's recommendations. Stained samples were analyzed by
flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA).
For analysis of the cell cycle distribution, the supernatant was
discarded, and attached cells were harvested and fixed in cold 70%
ethanol overnight at −20°C. Cell cycle distribution was analyzed
using a flow cytometer according to the manufacturer's instructions
for the PI staining kit.
Protein extraction and western
blot
A549/Taxol cells (3×105 cells/well) were
treated with TPL at concentrations of 0.025 and 0.05 µM, or
co-treated with MAPK inhibitors, for 24 h. Following the
treatments, cells were collected, washed twice with pre-chilled
PBS, lysed, and then centrifuged at 12,000 rpm for 10 min at 4°C.
The supernatant of the lysate was boiled, and total protein was
measured using the BCA protein assay kit. Proteins were separated
by SDS-PAGE, and then transferred onto polyvinylidene fluoride
(PVDF) membranes (0.45 µm; Millipore). Non-specific protein binding
sites were blocked with 5% bovine serum albumin diluted in
Tris-buffered saline buffer containing 0.1% Tween-20 (pH 7.4) for 2
h at room temperature, followed by incubation with the appropriate
primary and secondary antibodies. The expression of proteins was
detected using a Chemi Doc XRS+ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The grey level ratio of the target protein to
COX IV was used to represent the relative protein expression
level.
Statistical analysis
Results are expressed as the means ± standard
deviation. Statistical differences among groups were evaluated by
the t-test using GraphPad Prism 6. P<0.05 and P<0.01 were
considered to represent different levels of statistical
significance.
Results
Resistance fold of A549/Taxol
cells
A549 and A549/Taxol cells were treated with Taxol
for 48 h and the cytotoxicity was assessed using MTT assay. The
IC50 of Taxol against A549 and A549/Taxol cells was
3.52±0.47 and 71.31±7.95 µM, respectively. A549/Taxol cells
exhibited more than a 20-fold resistance to Taxol in comparison
with the drug-sensitive A549 cell line.
TPL inhibits proliferation of
A549/Taxol cells
Various doses (0.01, 0.02, 0.04, 0.06, 0.08 µM) of
TPL were used to treat A549/Taxol cells for 48 h. The
anti-proliferative effect of TPL on A549/Taxol was assessed by MTT
assay, and the results revealed that TPL inhibited the
proliferation of A549/Taxol cells efficiently in a dose-dependent
manner, with an IC50 value of 46.47±0.31 nM. It was far
more efficient than the positive drug cisplatin, which demonstrated
an IC50 value of 8.87±0.98 uM. TPL exhibits potent
cytotoxicity in vitro in A549/Taxol cells. Based on the
primary findings, we used TPL at concentrations of 0.025 and 0.05
µM in subsequent experiments.
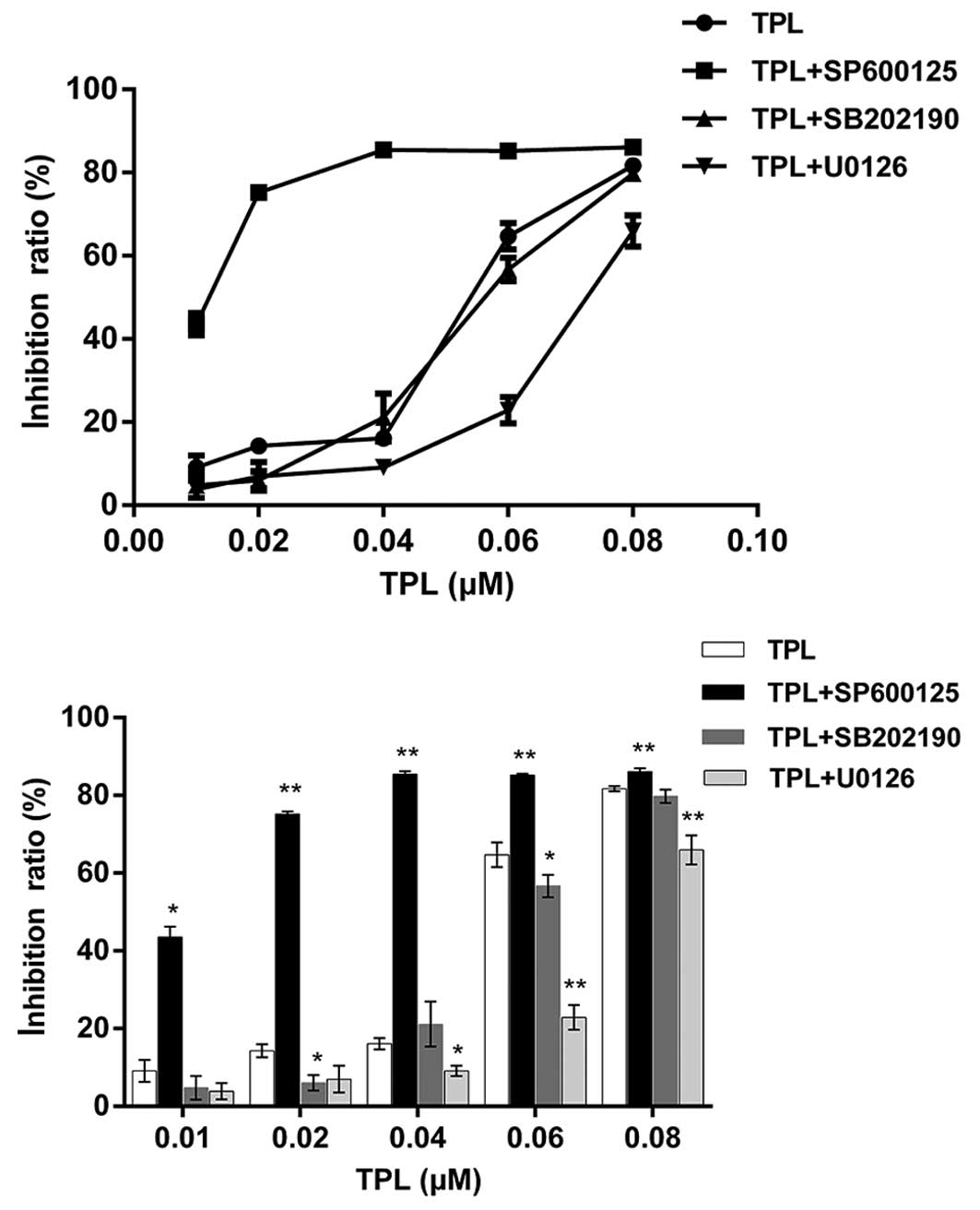
TPL modulates MAPKs in A549/Taxol
cells
To investigate the modulatory effects of TPL on
MAPKs in the course of inhibition of proliferation of A549/Taxol
cells, we performed MTT assay using MAPK inhibitors (SB202190,
SP600125 and U0126) coupled with TPL. SB202190 exerted little
effect on the result, while SP600125 and U0126 significantly
reinforced the inhibitory effects of TPL (Fig. 1). Since there was no significant
effect observed with the addition of SB202190, it was suggested
that the modulation of p38 contributed little to the inhibitory
effect exhibited by TPL on A549/Taxol cells. SP600125 exhibited
synergistic effects with TPL, while antagonism caused by U0126 was
also noted. These findings suggested that TPL may exert its
inhibitory effects by regulating the JNK and ERK signaling
pathways.
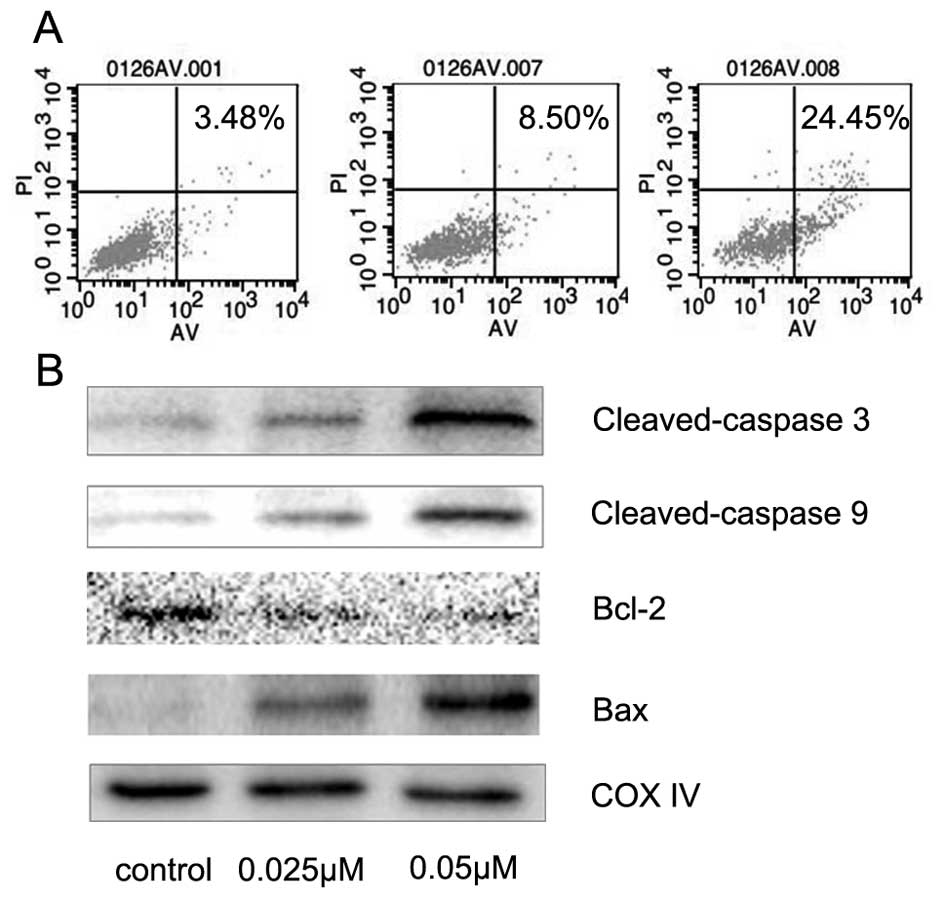
TPL exerts anti-proliferative effects
on A549/Taxol cells via pro-apoptosis
To investigate the mechanism of TPL's involvement in
the anti-proliferative activity of A549/Taxol cells, A549/Taxol
cells were treated with TPL at concentrations of 0.025 and 0.05 µM.
Twenty-four hours later, Annexin V-FITC/PI staining flow cytometry
was used to assess the rate of cell apoptosis. Compared with the
control group, we observed that the number of apoptotic cells
increased significantly in the TPL group (Fig. 2A). In addition, we noted that TPL
significantly increased the expression of caspase-3 and caspase-9
(Fig. 2B). These results indicated
that TPL induced caspase family-dependent apoptosis in A549/Taxol
cells. In addition, as shown in Fig.
2B, upregulation of the Bax/Bcl-2 ratio was observed following
TPL treatment, which supported our previous hypothesis concerning
the role of pro-apoptosis.
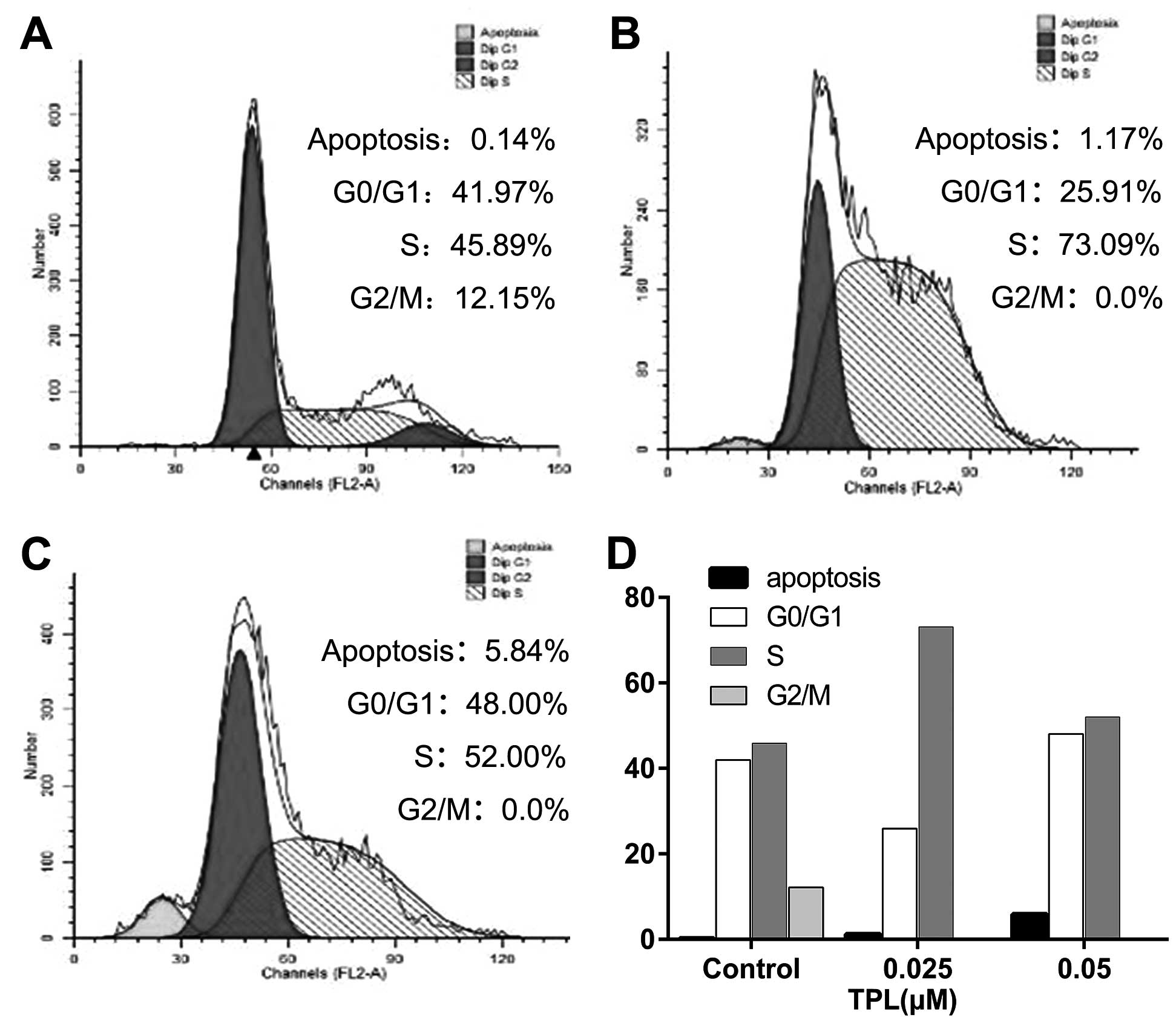
Induction of S-phase arrest in
A549/Taxol cells by TPL
To elucidate the mechanism of TPL-induced
proliferation inhibition, we examined the effect of TPL on cell
phase distribution by flow cytometry. As shown in Fig. 3, concomitant with the growth
inhibitory effect, treatment with TPL induced a significant S-phase
arrest. The cell populations in the G0/G1, S
and G2/M phases were 41.97, 45.89 and 12.15% in the
control group. However, after 24-h incubation with 0.025 and 0.05
µM TPL, the S-phase portion was notably enhanced by 27.2 and 6.11%,
respectively. Contrarily, the G2/M population of
A549/Taxol was markedly reduced following treatment with 0.025 and
0.05 µM TPL, indicating the blockage of the S-G2
transition in A549/Taxol cells. In addition, the sub-G1
population was increased by 1.03 and 5.70%, respectively.
Measurement of MAPKs and PI3K/Akt
signaling pathways
The inhibition of p38 usually promotes tumor
formation, but in certain cases, the tumor-forming ability is
suppressed by p38 activation. JNK plays two antagonistic roles:
oncogenic and and pro-apoptotic. The oncogenic function of JNK
pathways is highly expressed in cancer cells, which promotes cell
proliferation (12,13). The ERK1/2 cascades are mainly involved
in tumor cell survival and proliferation regulation, but increased
levels of the phosphorylated form of ERK1/2 also induce cell
apoptosis (14). The aberrant
activation of Akt is one of the most frequent alterations observed
in most types of cancer cells, and it has been observed to
correlate with poor prognosis and resistance to various anti-cancer
agents (15).
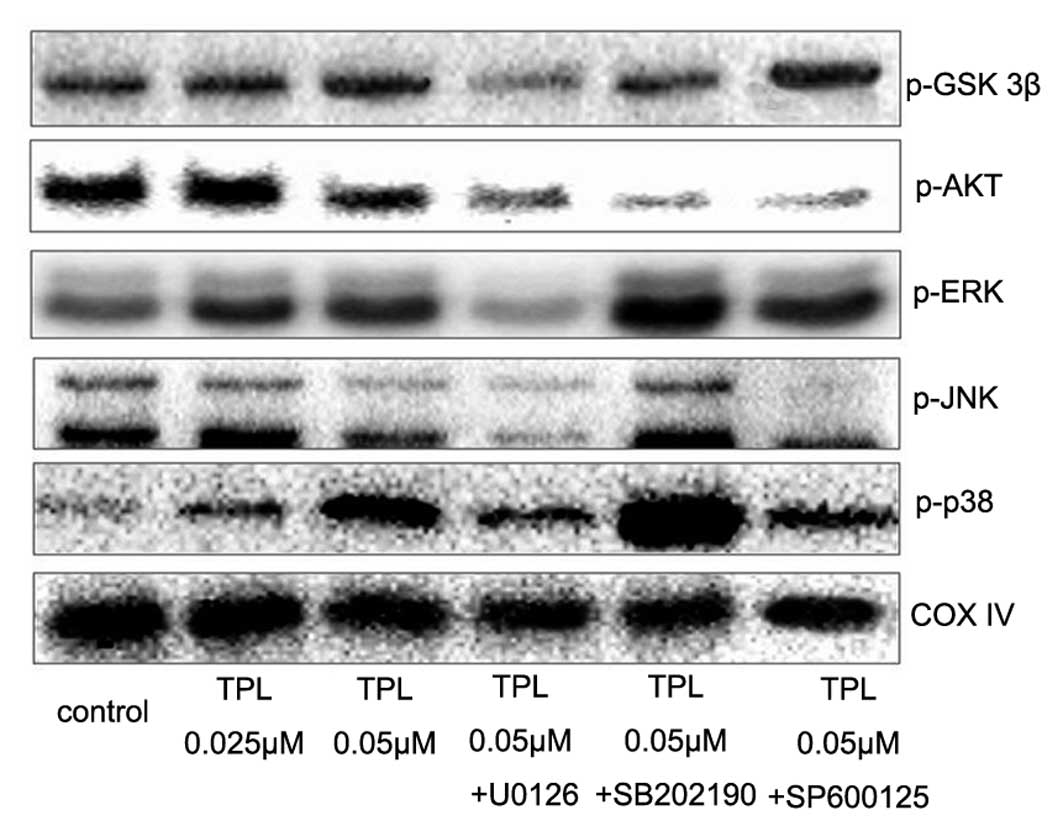
Preliminary observations in the current study shed
light on the mechanism of action of TPL on the inhibition of
proliferation of A549/Taxol cells. Based on the findings obtained,
we proposed that modulation of JNK and ERK signaling by TPL
contributed in a great manner to the anti-proliferative activity
observed. To validate the conclusion, the expression of three MAPKs
(p-p38, p-JNK and p-ERK) and PI3K/Akt (p-Akt and p-GSK-3β) was
analyzed by western blot analysis. As shown in Fig. 4, TPL increased the expressions of
p-p38, p-ERK and p-GSK-3β, but exerted the opposite effect on p-JNK
and p-Akt. Compared with the TPL group, the expression of p-ERK,
p-JNK and p-Akt was notably downregulated by MAPK inhibitors, while
the p-GSK-3β expression level was significantly upregulated by
SP600125. These results were consistent with conclusions deduced
from the results of MTT assay.
Discussion
MDR is a major obstacle in effective cancer
chemotherapy. Following cycles of chemotherapy, cancer cells have
been demonstrated to form simultaneous resistance to anti-cancer
drugs with various structures and mechanisms of action (2,3).
Traditional Chinese medicines have numerous advantages over
chemical agents, including low cost and lower toxicity, and have
long been used in the clinical treatment of cancer. In previous
studies, researchers have paid increasing attention to Chinese
medicines in their search for new potential MDR reversal agents
(16).
TPL is a diterpenoid triepoxide isolated from the
traditional Chinese herb Tripterygium wilfordii Hook. f. It
possesses efficient antitumor activity against a range of cancer
cells. TPL has been demonstrated to exert its reversal activity on
various MDR cancer cells, mainly through the downregulation of MDR
proteins, including P-glycoprotein (17–19). Our
primary study revealed that TPL could notably inhibit the
proliferation of a panel of MDR cancer cell lines, including
A549/Taxol, MCF-7/ADR and Bel7402/5-Fu (20). Significantly, we observed that the
activity was mainly associated with the modulation of MAPKs and the
downstream pathways. Although previous studies provided useful
clues for a better understanding of the mechanism concerning the
inhibition of proliferation of MDR cells mediated by TPL, there is
insufficient evidence to characterize the exact role of MAPKs in
this process. Hence, we designed and carried out the present
study.
MAPKs play significant roles in regulating an array
of cellular responses, particularly those involving cell
proliferation and apoptosis, and the modulation of these kinases
may bring promising benefits to the therapeutic treatment of tumors
(21–23). The development of MDR is often
accompanied by the modulation of MAPKs (24–26). ERK1
and 2 are known to act as anti-apoptotic cascades by transducing
survival signals, whereas JNK or p38 phosphorylation results in the
promotion of apoptosis (27).
However, the roles of these pathways are sophisticated. For
example, suppression of the MEK-ERK signaling pathway by PD98059
increased rather than decreased cisplatin resistance (28). In addition, inhibition of p38 MAPK
significantly reduced gemcitabine sensitivity in NTUB1 cells, and
Calebin-A enhanced the cytotoxicity of vincristine in
SGC7901/vincristine cells through the inhibition of JNK (29,30). The
exact effect of the modulation of MAPKs depends on the cell types
and crosstalk among the various pathways. The results from this
study clearly demonstrate that TPL exerts its anti-proliferative
effect via downregulation of p-JNK and upregulation of the p-ERK
and p-p38 pathways. The selective modulation effects differed from
the results of other studies, and are worthy of further
investigation.
PI3K/Akt has emerged as an essential pathway in
regulating cell proliferation, survival and apoptosis, and cell
migration. Accumulating evidence reveals that Akt activation plays
a significant role in the chemoresistance of cancer cells (12,31).
Activation of the Akt signaling pathway mediates acquired
resistance to sorafenib in hepatocellular carcinoma cells (32), and confers resistance to gefitinib in
lung cancer cells (33). Akt
phosphorylation entails inhibition of a highly conserved GSK-3, and
GSK-3β inactivation leads to β-catenin accumulation, which entails
drug resistance (34). Consistent
with these studies, downregulation of p-Akt and further regulation
of the downstream signal p-GSK-3β results in anti-proliferation of
TPL in A549/Taxol cells. SP600125 increased the expression of
p-GSK-3β and reinforced the effect of TPL, whereas U0126
downregulates the expression of p-GSK-3β. These findings support
the hypothesis that modulation of TPL in the PI3K/Akt signaling
pathway contributed to the pro-apoptosis and the subsequent
anti-proliferation of A549/Taxol cells, and that the effect was
regulated by the modulation effect of TPL on ERK. MAPKs and
PI3K/Akt signaling pathways are potential targets of TPL against
MDR cancers.
The pro-apoptotic activity of TPL is associated with
the modulation of MAPKs, but fundamentally via the modulation of
apoptosis-related proteins. The Bcl-2 family plays a key role in
regulating the intrinsic apoptotic pathway, and is modulated by
MAPKs (35). Bax is a pro-apoptotic
protein, while Bcl-2 possesses anti-apoptotic properties through
stabilizing mitochondrial membrane and suppressing the release of
cytochrome C. The balance between the proteins is crucial in the
progression of apoptosis (36).
Caspases are proteases with a well-defined role in apoptosis, and
caspase-3 and −9 activation eventually results in cell apoptosis.
In our study, we observed significant upregulation of Bax and
c-caspase-3 and −9, and downregulation of Bcl-2, along with
simultaneous modulation of MAPKs following treatment of TPL in
A549/Taxol cells. These findings indicate a close association
between modulations of Bcl and MAPKs.
The anti-proliferative activity exerted by TPL on
A549/Taxol cells may be due to the modulation of MAPKs and PI3K/Akt
pathways, which subsequently led to the activation or suppression
of the expression of apoptosis-related proteins, apoptosis and cell
cycle arrest. This study provides useful indications for a better
understanding of the mechanisms of TPL-induced apoptosis against
A549/Taxol cells.
Acknowledgements
This study was supported by grants from the National
Natural Science Fund (81274057 and 81573577) and the Natural
Science for Youth Foundation (81403082).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ling V, Gerlach J and Kartner N: Multidrug
resistance. Breast Cancer Res Treat. 4:89–94. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luqmani YA: Mechanisms of drug resistance
in cancer chemotherapy. Med Prin Pract. 14(Suppl 1): S35–S48.
2005.
|
|
5
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
6
|
Olson JM and Hallahan AR: p38 MAP kinase:
a convergence point in cancer therapy. Trends Mol Med. 10:125–129.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dive C: Avoidance of apoptosis as a
mechanism of drug resistance. J Intern Med Suppl. 740:139–145.
1997.PubMed/NCBI
|
|
9
|
Yang M, Huang J, Pan HZ and Jin J:
Triptolide overcomes dexamethasone resistance and enhanced
PS-341-induced apoptosis via PI3k/Akt/NF-kappaB pathways in human
multiple myeloma cells. Int J Mol Med. 22:489–496. 2008.PubMed/NCBI
|
|
10
|
Meng GM, Wang W, Chai KQ, Yang SW, Li FQ
and Jiang K: Combination treatment with triptolide and
hydroxycamptothecin synergistically enhances apoptosis in A549 lung
adenocarcinoma cells through PP 2A-regulated ERK, p38 MAPKs and Akt
signaling pathways. Int J Oncol. 46:1007–1017. 2015.PubMed/NCBI
|
|
11
|
Wang ZP, Jin HF, Xu RD, Mei QB and Fan DM:
Triptolide downregulates Rac1 and the JAK/STAT3 pathway and
inhibits colitis-related colon cancer progression. Exp Mol Med.
41:717–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Modi PK, Komaravelli N, Singh N and Sharma
P: Interplay between MEK-ERK signaling, cyclin D1 and
cyclin-dependent kinase 5 regulates cell cycle reentry and
apoptosis of neurons. Mol Biol Cell. 23:3722–3730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang WC and Hung MC: Induction of Akt
activity by chemotherapy confers acquired resistance. J Formos Med
Assoc. 108:180–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Sun BQ and Gai XD: Compounds from
Chinese herbal medicines as reversal agents for
P-glycoprotein-mediated multidrug resistance in tumours. Clin
Transl Oncol. 16:593–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Q, Nan XX, Yang JR, Yi L, Liang BL,
Wei YB, Zhu N, Hu SB, Zhang H, Luo Y and Xu YF: Triptolide inhibits
the multidrug resistance in prostate cancer cells via the
downregulation of MDR1 expression. Neoplasma. 60:598–604. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Hui L, Xu W, Shen H, Chen Q, Long L
and Zhu X: Modulation of P-glycoprotein expression by triptolide in
adriamycin-resistant K562/A02 cells. Oncol Lett. 3:485–489.
2012.PubMed/NCBI
|
|
19
|
Chen YW, Lin GJ, Chuang YP, Chia WT, Hueng
DY, Lin CK, Nieh S and Sytwu HK: Triptolide circumvents
drug-resistant effect and enhances 5-fluorouracil antitumor effect
on KB cells. Anti-Cancer Drugs. 21:502–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie CQ, Zhou P, Yuan F, Li X and Chen JW:
Screening out potential agents from traditional Chinese medicine
with anti-multidrug resistance tumor cells in vitro. Lishizhen Med
Mater Med Res. 7:1572–1574. 2015.(In Chinese).
|
|
21
|
Thompson N and Lyons J: Recent progress in
targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug
discovery. Curr Opin Pharmacol. 5:350–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J and Sun P: The pathways to tumor
suppression via route p38. Trends Biochem Sci. 32:364–371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY
and Ng DC: c-Jun N-terminal kinase (JNK) signaling: recent advances
and challenges. Biochim Biophys Acta. 1804:463–475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katayama K, Yoshioka S, Tsukahara S,
Mitsuhashi J and Sugimoto Y: Inhibition of the mitogen-activated
protein kinase pathway results in the down-regulation of
P-glycoprotein. Mol Cancer Ther. 6:2092–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barancik M, Bohácová V, Kvackajová J,
Hudecová S, Krizanová O and Breier A: SB203580, a specific
inhibitor of p38-MAPK pathway, is a new reversal agent of
P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci.
14:29–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo X, Ma N, Wang J, Song J, Bu X, Cheng
Y, Sun K, Xiong H, Jiang G, Zhang B, et al: Increased p38-MAPK is
responsible for chemotherapy resistance in human gastric cancer
cells. BMC Cancer. 8:3752008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishna M and Narang H: The complexity of
mitogen-activated protein kinases (MAPKs) made simple. Cell Mol
Life Sci. 65:3525–3544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeh PY, Chuang SE, Yeh KH, Song YC, Ea CK
and Cheng AL: Increase of the resistance of human cervical
carcinoma cells to cisplatin by inhibition of the MEK to ERK
signaling pathway partly via enhancement of anticancer drug-induced
NF kappa B activation. Biochem Pharmacol. 63:1423–1430. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kao YT, Hsu WC, Hu HT, Hsu SH, Lin CS,
Chiu CC, Lu CY, Hour TC, Pu YS and Huang AM: Involvement of p38
mitogen-activated protein kinase in acquired gemcitabine-resistant
human urothelial carcinoma sublines. Kaohsiung J Med Sci.
30:323–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Li S, Han Y, Liu J, Zhang J, Li F,
Wang Y, Liu X and Yao L: Calebin-A induces apoptosis and modulates
MAPK family activity in drug resistant human gastric cancer cells.
Eur J Pharmacol. 591:252–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH,
Chen PJ and Cheng AL: Activation of phosphatidylinositol
3-kinase/Akt signaling pathway mediates acquired resistance to
sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther.
337:155–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu BN, Yan HQ, Wu X, Pan ZH, Zhu Y, Meng
ZW, Zhou QH and Xu K: Apoptosis induced by benzyl isothiocyanate in
gefitinib-resistant lung cancer cells is associated with Akt/MAPK
pathways and generation of reactive oxygen species. Cell Biochem
Biophys. 66:81–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galoian K, Temple HT and Galoyan A: mTORC1
inhibition and ECM-cell adhesion-independent drug resistance via
PI3K-AKT and PI3K-RAS-MAPK feedback loops. Tumour Biol. 33:885–890.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|