Introduction
Hepatocellular carcinoma (HCC), the most frequent
primary tumor of the liver, is one of the leading causes of
cancer-associated mortality worldwide (1). Regarding treatment options for HCC,
immunotherapy remains an important adjuvant treatment, despite
advances in surgery, chemotherapy and radiotherapy (2). Cytokine-induced killer (CIK) cell
therapy is currently one of the most commonly used immunotherapies
(3). In order to improve the efficacy
of CIK cell therapy, numerous strategies, including gene
transfection (4), stimulation with
certain growth factors (5),
pretreatment with antitumor drugs (6), or combination with genetically modified
dendritic cells (7), have been
attempted in practice. However, a number of clinical trials of CIK
cell therapies failed to demonstrate any noticeable improvement
over other therapeutic regimens, neither in terms of HCC control
rates nor long-term survival rates (8), suggesting that other factors, such as
the environment where CIK cells are functioning, should also be
considered for immunotherapies.
It has been demonstrated that the high metabolic
demand of rapidly proliferating cancer cells, in conjunction with a
shift toward glycolytic metabolism reflecting both tumor hypoxia
and oncogene-induced changes in gene expression, leads to an often
greatly increased production and extrusion of acid by cancer cells
(9,10). Therefore, the extracellular pH (pHe)
of malignant solid tumors is usually acidic, ranging from 6.5 to
6.9, whereas the pHe of normal tissues is significantly more
alkaline, ranging from 7.2 to 7.5 (11). This disturbance of the acid-base
balance is able to remodel various physiological functions, and
results in solid tumors becoming more invasive and metastatic
(12). In addition, it may also
induce undesired resistance to immunotherapy (13). By contrast, inhibition of the acid
extrusion of tumor cells may alleviate their resistance to
immunotherapy (14). However, none of
these speculations has ever been confirmed for CIK cell
immunotherapy in vitro or in vivo. In the present
study, the antitumor activities of CIK cells against HepG2 cells
were evaluated both in vitro and in vivo under
environments with acidic and alkaline pH.
Materials and methods
Cell culture
HepG2 cells (ATCC, Manassas, VA, USA) or HepG2-luc
cells (HepG2 cells stably transfected with a firefly luciferase
gene), were cultured in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) in an incubator at 37°C with humidified
atmosphere and 5% CO2 in air. Cells were adapted in
acidic (pH 6.5) or alkaline (pH 7.4) environments for three
passages prior to be used for experiments. The pH values of the
medium were accordingly adjusted with lactic acid,
NaHCO3 and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid.
Preparation of CIK cells
Peripheral blood mononuclear cells (PBMCs) were
isolated from peripheral blood by standard Ficoll separation, as
previously described (5). Human
peripheral blood samples were obtained with full informed consent
from patients with HCC. In total, six samples were collected
between January 2014 and July 2014 at the Department of
Gastroenterology of Renmin Hospital, Hubei University of Medicine
(Shiyan, China). The isolated cells were resuspended in RPMI-1640
medium supplemented with 1,000 U/ml interferon (IFN)-γ (R&D
Systems, Inc., Minneapolis, MN, USA) and incubated at 37°C for 24
h. Then, recombinant human interleukin (IL)-2 protein (cat. no.
202-IL-050; R&D Systems, Inc.) and mouse anti-cluster of
differentiation (CD)3 monoclonal antibody (cat. no. MAB100;
dilution, 1:1,000; R&D Systems, Inc.) were added at 500 U/ml
and 50 ng/ml, respectively. Subsequently, the cells were refreshed
with RPMI-1640 medium supplemented with IL-2 (500 U/ml) every other
day for 10 days prior to being subjected to flow cytometry
analysis.
Flow cytometry
A set of conjugated monoclonal antibodies (BD
Biosciences, Franklin Lakes, NJ, USA), including
anti-CD3-fluorescein isothiocyanate (FITC; cat. no. 561806;
dilution, 1:20) as a T-cell marker, anti-CD4-phycoerythrin (PE;
cat. no. 565999; dilution, 1:20) as a helper T-cell marker,
anti-CD8-PE (cat. no. 561950; dilution, 1:20) as a cytotoxic T-cell
marker and anti-CD56-PE (cat. no. 561903; dilution, 1:20) as a
natural killer (NK) cell-marker, were used to define the phenotypes
of CIK cells. In total, 1×106 CIK cells were harvested
and washed once with phosphate-buffered saline (PBS) containing 1%
bovine serum albumin (BSA; Beyotime Institute of Biotechnology,
Haimen, China), and resuspended in 100 µl PBS/BSA. The cells were
then incubated with the above conjugated monoclonal antibodies
separately for 20 min at 4°C, washed twice with PBS and resuspended
in 400 µl PBS. Flow cytometric analysis was performed with a BD
FACSCalibur™ flow cytometer (BD Biosciences), and the data were
analyzed using the WinMDI software, version 2.9 (The Scripps
Research Institute, La Jolla, CA, USA). The dead cells and debris
were gated out.
HepG2 cell apoptosis was analyzed
using the annexin V/propidium iodide (PI) double staining
method
HepG2 cells were plated at a density of
3×105 cells/well in a BD Falcon® 12-well
plate (BD Biosciences) and cultured in CIK cell-conditioned medium
(CMCIK), HepG2 cell-conditioned medium
(CMcontrol) or a 1:1 mixture of the two conditioned
media (thus, the percentages of CMCIK in the above media
were 100, 0 and 50%, respectively). The pH of the media was
adjusted to 6.5 or 7.4, correspondingly. After 48 h of incubation,
the cells were collected, washed and resuspended in PBS. Then,
annexin V-FITC and PI (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) were added to the cells, which were incubated at 4°C for 20
min prior to being subjected to flow cytometer analysis. All the
experiments were performed in triplicate.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT colorimetric assay was used to determine the
cytotoxic activity of CIK cells against HepG2 cells in vitro
(15). The CIK cells [effector (E)
cells] and the HepG2 cells [target (T) cells] were co-cultured in a
96-well plate with medium at pH 6.5 or 7.4. The ratios of CIK and
HepG2 cells (E/T ratio) were set as 10:1, 20:1, 40:1 and 80:1. In
addition, E cells or T cells alone were used as controls. The MTT
assay was performed 20 h later to evaluate the cell viability, and
the optical density (OD) values were measured at 570 nm. The assays
were conducted in triplicate. The cytotoxic activity was calculated
as follows: Cytotoxic activity (%) = [1-(ODE+T-ODE)/ODT]
× 100%.
Fluorescence assay of cytotoxic
effects of CIK cells on HepG2-luc cells
HepG2-luc cells were co-cultured with CIK cells or
in CMCIK at pH 6.5 or 7.4 respectively. The viability of
HepG2-luc cells was assessed fluorescently using the
IVIS® Spectrum in vivo imaging system (Caliper
Life Sciences; PerkinElmer, Inc., Waltham, MA, USA). In co-culture,
HepG2-luc cells were plated at a density of 8,000 cells/well into a
BD Falcon® 96-well plate (BD Biosciences), and CIK cells
were then added to the 96-well plate at E/T ratios of 0:1, 10:1,
20:1, 40:1 and 80:1. In culture with CMCIK, HepG2-luc
cells were plated at a density of 1,500 cells/well in a BD
Falcon® 96-well plate (BD Biosciences) with medium
containing 0, 25, 50, 75 and 100% CMCIK. The assays were
performed in triplicate. After 20 h of co-culture or 48 h of
culture in CMCIK, 0.15 mg/ml D-luciferin was added into
each well, and the luminescence activity (LA) was measured after
additional 5-min incubation. Based on the LA values, the
cytotoxicities of CIK cells were calculated according to the
following formula: CIK cytotoxicity (%) =
[(LA0:1-LAx:1)/LA0:1] × 100%, where LA0:1 is
the LA of HepG2-luc cells cultured in the absence of CIK cells, and
LAx:1 is a variable that refers to the LA of HepG2-luc cells
co-cultured with 10, 20, 40 or 80-fold CIK cells. The
cytotoxicities present in the CMCIK were calculated
according to the following formula: CMCIK cytotoxicity
(%) = [(LA0-LAy)/LA0] × 100%, where LA is the LA of the HepG2-luc
cells, LA0 is the LA of HepG2-luc cells cultured in the absence of
CMCIK, and LAy is a variable that represents
the LA of HepG2-luc cells cultured with 25, 50, 75 and 100%
CMCIK.
Xenograft assay
A total of 20 female nude mice (6–8 weeks-old) were
purchased from the Animal Experiment Center of Wuhan University
(Wuhan, China). All mice were maintained in the Animal Experiment
Center of the Hubei University of Medicine (Shiyan, China) at a
controlled temperature and humidity, under a 12-h light/dark cycle,
with ad libitum access to sterile food and water. The
present study was performed with the approval of the Hubei
University of Medicine ethical committee, and all the experiments
were performed according to the National Institutes of Health Guide
for the Care and Use of Laboratory Animals. In order to eradicate
residual NK cells, the nude mice were administered 200 cGy of whole
body irradiation and subcutaneously injected with 1×107
HepG2-luc cells. Subsequently, the mice were randomly divided into
four groups according to the different treatments received: i) CIK
cells injection plus oral administration of NaHCO3; ii)
CIK cells injection plus drinking water feeding; iii) normal saline
injection plus oral administration of NaHCO3; and iv)
normal saline injection plus drinking water feeding (which served
as control). CIK cells (1.5×107 cells/injection) or an
equivalent volume of normal saline were intravenously injected in
the mice through their tail veins on days 0, 7, 14, 21 and 28,
while NaHCO3 (200 mmol/l) or drinking water feeding were
conducted on a daily basis. The LA of the tumors was monitored and
measured with the IVIS® Spectrum in vivo imaging
system on days 0, 10, 20 and 30. The mice were sacrificed on day
30, and the tumor weights were measured.
Immunohistochemical analysis
The grafted tumor masses were fixed in 10% formalin,
embedded in paraffin and sectioned with a thickness of 3 µm for
immunohistochemical studies. Rabbit anti-human CD3 polyclonal
antibody (cat. no. ab828; dilution, 1:50; Abcam, Cambridge, MA,
USA) and mouse anti-human perforin monoclonal antibody (cat. no.
ab47225; dilution, 1:50; Abcam) were used for immunostaining.
Horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
ab6721; dilution, 1:1,000; Abcam) and goat anti-mouse (cat. no.
ab6789; dilution, 1:1,000; Abcam) secondary antibodies were used
for detection of the primary antibodies. All procedures were
conducted according to the manufacturer's protocol. Images of the
sections were acquired using an Olympus BX-60 microscope (Olympus
Corporation, Tokyo, Japan) to determine the CD3+ and
perforin+ cell densities. The number of positive cells
was blindly counted in 10 randomly selected independent fields
(0.16 mm2 at ×400 magnification) for each section by two
independent observers.
Statistical analysis
Differences between groups were compared using
analysis of variance, and the least significant difference test was
used for multiple mean comparisons. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was conducted using SPSS software v13.0 (SPSS Inc.,
Chicago, IL, USA).
Results
Characteristics of CIK cells
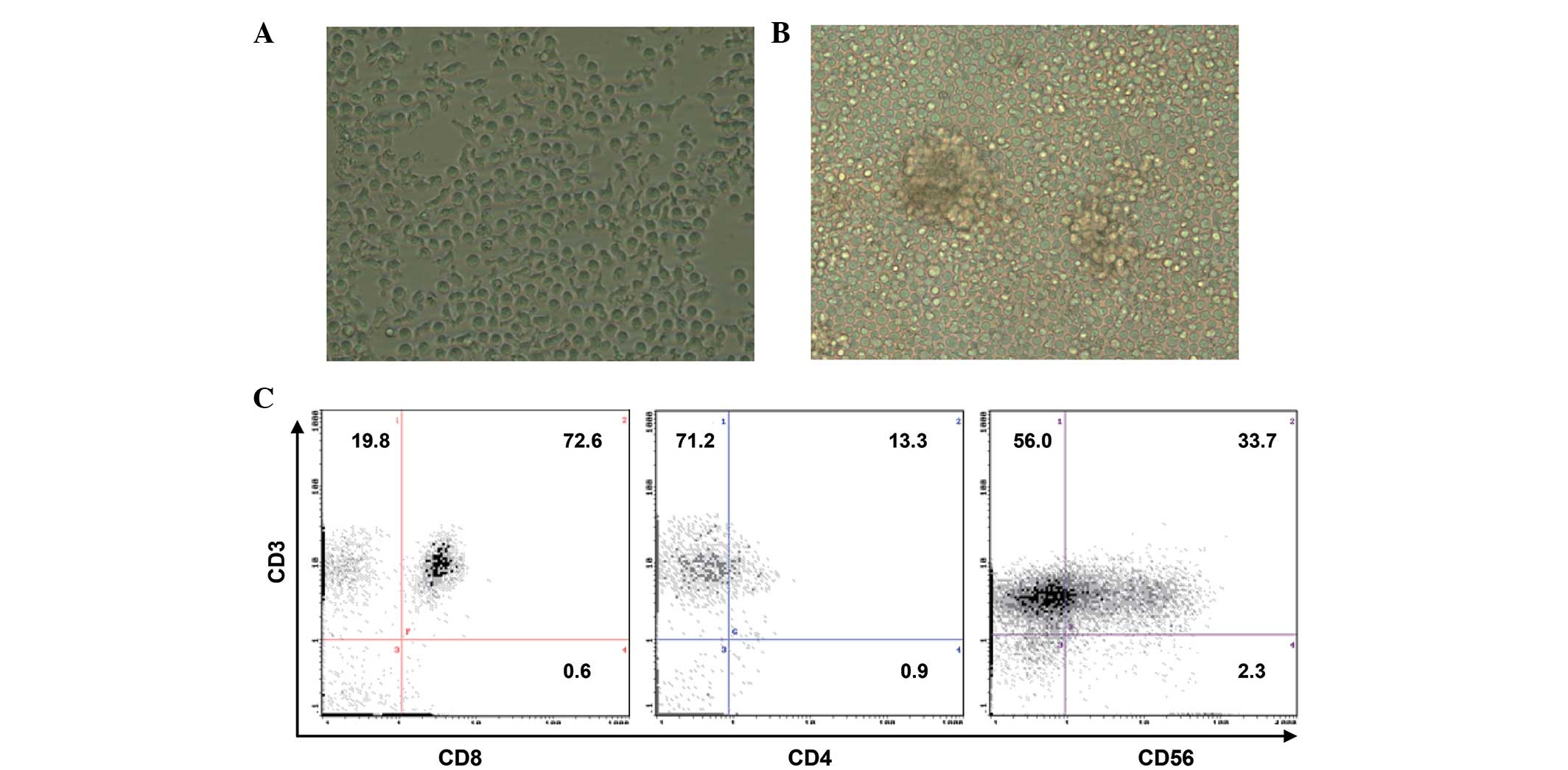
The CIK cells induced with the method described
above appeared regular, round, transparent and variable in size
when observed under a microscope. They exhibited the ability of
growth in suspension, and multiple characteristic cell clusters
were formed (Fig. 1A and B). After 14
days, the absolute number of human PBMCs cultured in the presence
of IL-2 and anti-CD3 antibody increased by >250-fold, from 5
million PBMCs to 1,300 million CIK cells. When the phenotypes of
the cultured cell population were examined by
fluorescence-activated cell sorting analysis, the cell population
was observed to be composed of 92% CD3+, 34%
CD3+ CD56+, 14% CD4+ and 73%
CD8+ cells (Fig. 1C).
The cytotoxic activity of CIK cells is
significantly compromised under an acidic environment
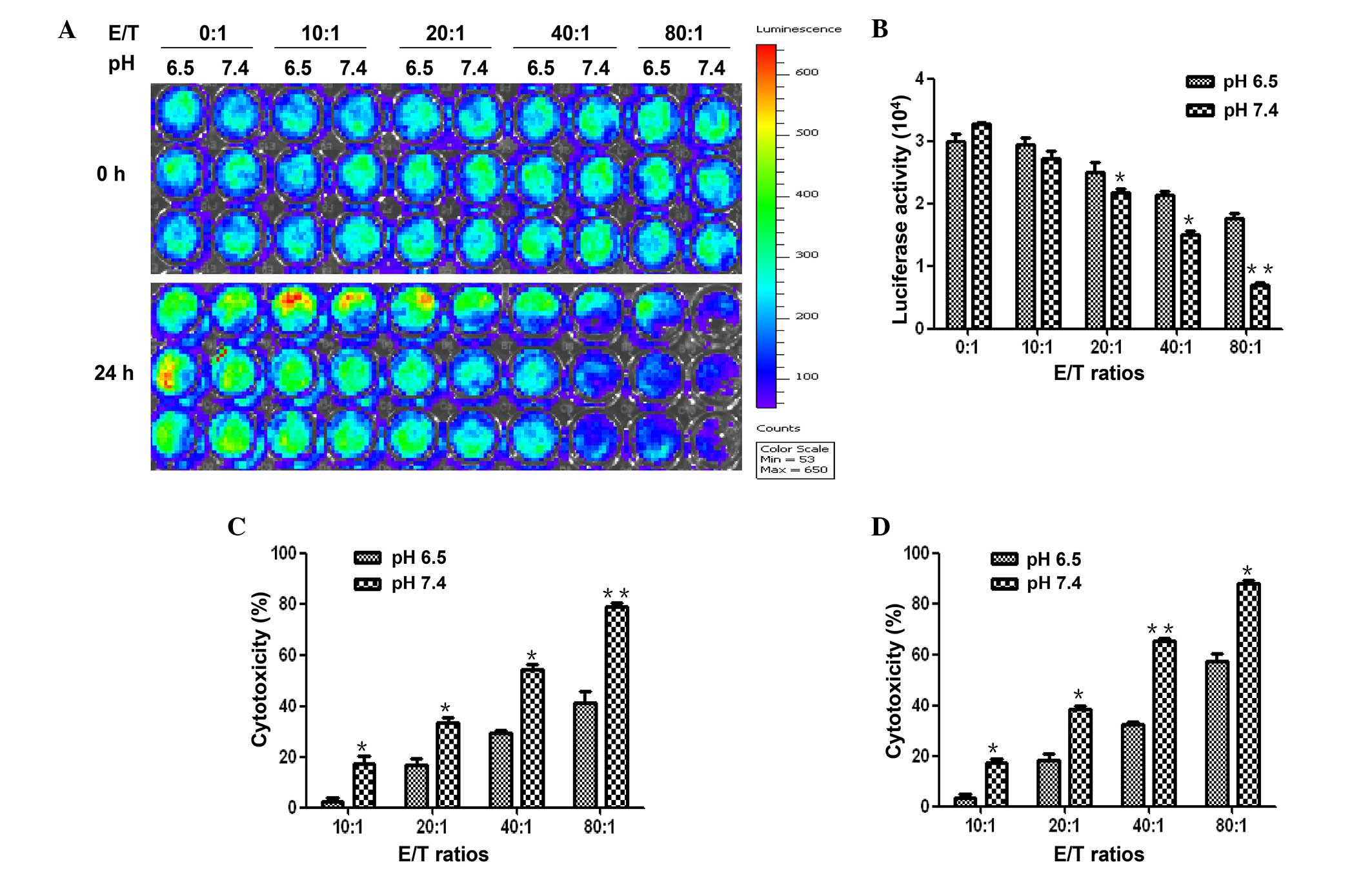
Both the MTT assay and the fluorescence assay
demonstrated that CIK cells had a significantly higher cytotoxic
activity against HepG2-luc cells in the medium with pH 7.4 than in
the medium with pH 6.5. In the fluorescence assay, HepG2-luc cells
were co-cultured with CIK cells at an E/T ratio of 0:1 10:1, 20:1,
40:1 or 80:1 at pH 6.5 or pH 7.4. The fluorescence intensities were
significantly lower with pH 7.4 than with pH 6.5 for each paired
cultures (P<0.05; Fig. 2A and B).
The cytotoxic activity calculated based on the fluorescence
intensity showed that the cytotoxicity of CIK cells were
significantly higher with pH 7.4 than with pH 6.5 (P<0.05;
Fig. 2C).
In the MTT assay, 19, 38, 60 and 80% of HepG2-luc
cells died when co-cultured with CIK cells at an E/T ratio of 10:1,
20:1, 40:1 and 80:1, respectively, in the medium with pH 7.4.
However, only 4, 18, 30 and 45% of HepG2 cells died, respectively,
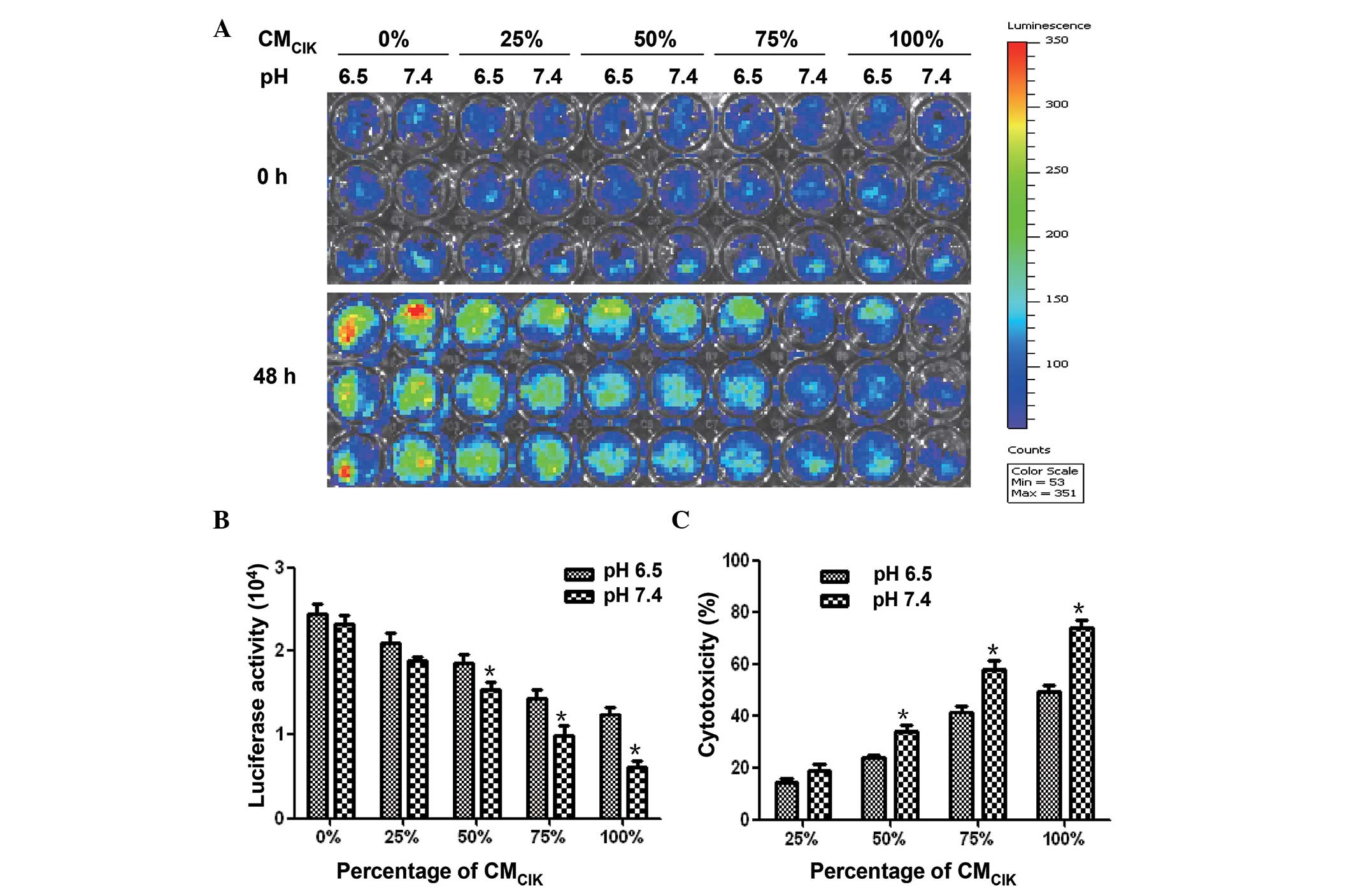
at the same E/T ratios with pH 6.5 (P<0.05; Fig. 2D). Highly consistent with the results
of CIK cells in fluorescence assay, the CMCIK displayed
stronger cytotoxicity activities in pH 7.4 condition than in pH 6.5
condition. The fluorescence intensities of HepG2-luc cells cultured
in medium containing 50, 75 and 100% CMCIK were
significantly lower with pH 7.4 than with pH 6.5 (P<0.05;
Fig. 3A and B). The cytotoxicity of
CMCIK in pH 7.4 condition was stronger than that in the
pH 6.5 condition (Fig. 3C).
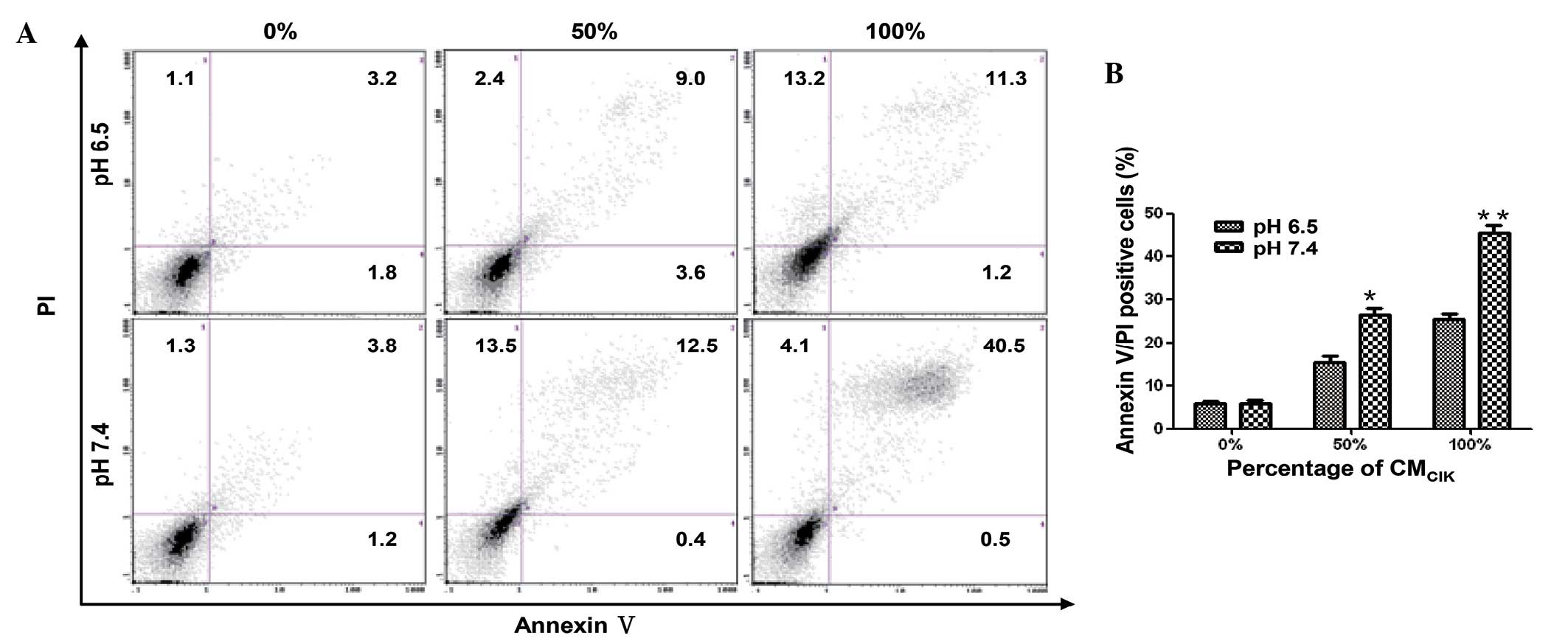
In addition, the annexin V/PI double staining method
was used to determine the percentage of cells that exhibited
apoptosis and necrosis (Fig. 4). At a
CM ratio of 50%, the CMCIK-induced apoptosis and
necrosis in pH 6.5 and pH 7.4 medium were 15 and 26%, respectively.
Furthermore, at a CMCIK ratio of 100%, the percentage of
apoptosis and necrosis in pH-6.5 and pH-7.4 medium were 25 and 45%,
respectively. These results indicated that the acidic environment
inhibits the antitumor activity of CIK cells.
The activity of CIK cells against HCC
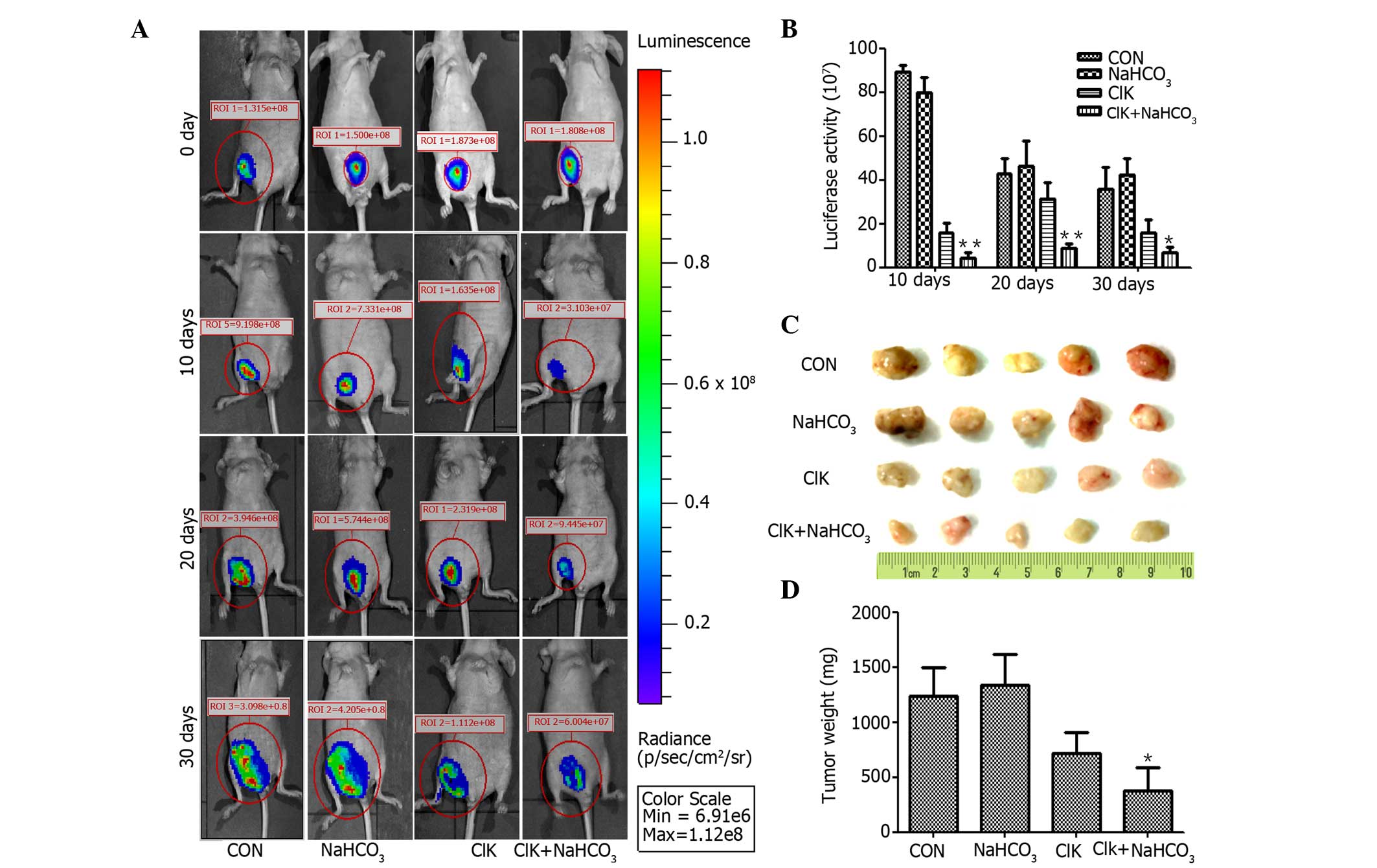
is enhanced by NaHCO3 feeding in nude mice
In order to further investigate whether
NaHCO3 treatment would enhance the antitumor activity of
CIK therapy in vivo, HepG2-luc carcinoma-bearing nude mice
were grouped and treated as above described. Nude mice were
selected due to their lack of cellular immunity (16). The results revealed that there was no
difference between the NaHCO3 feeding group and the
control group in terms of luminescence intensities from the tumors.
The luminescence intensities of the tumors in the CIK group were
significantly reduced compared with those in the control group,
while those in the CIK plus NaHCO3 feeding group were
also significantly reduced compared with those in the CIK group
(Fig. 5A and B). The tumor masses
were isolated on day 30 (Fig. 5C)
after grafted from each group, and the average weights of the
tumors were consistent with the luminescence intensity analysis
(Fig. 5D).
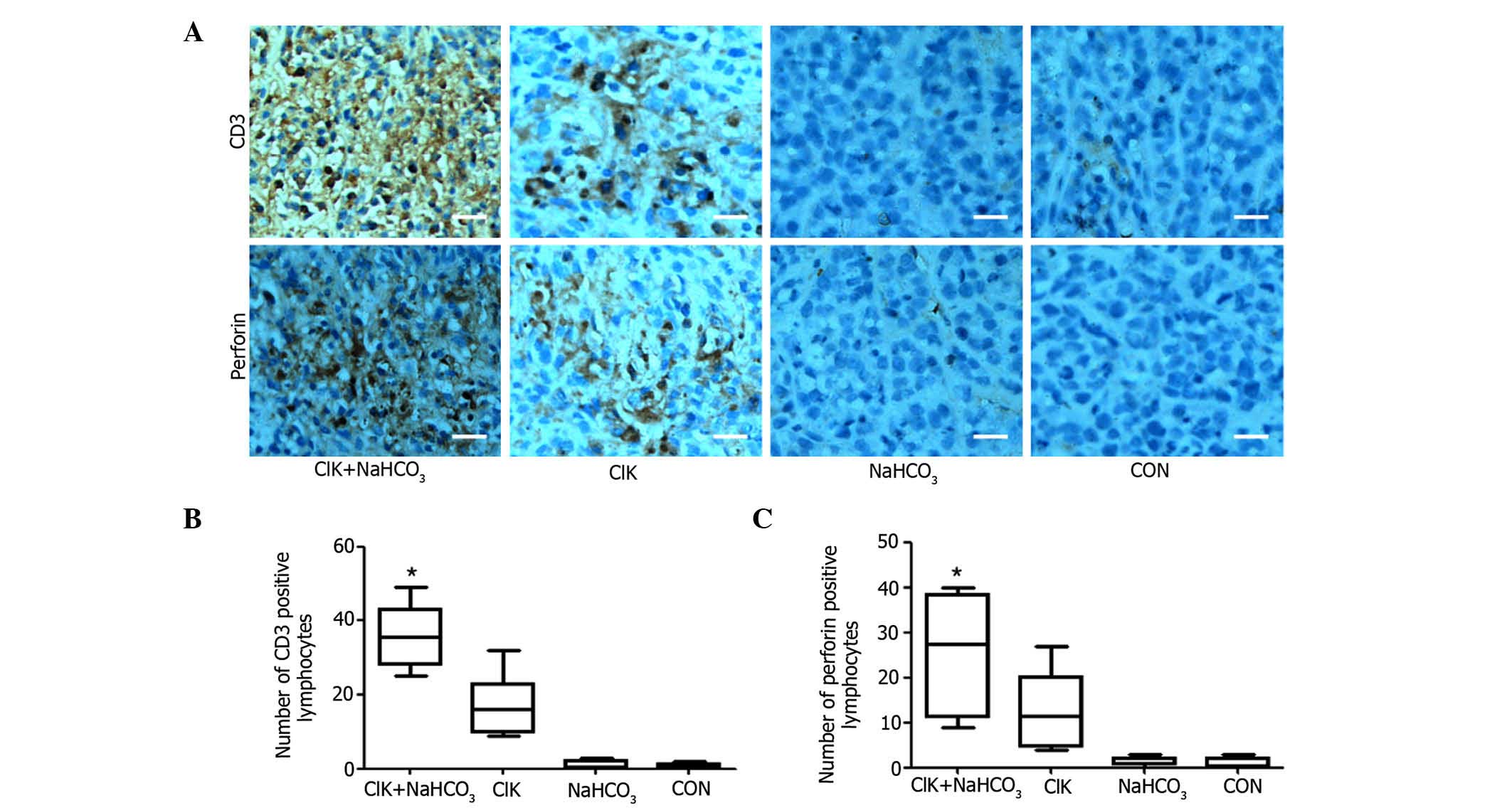
NaHCO3 enhances the
infiltration of CIK cells within the tumor tissue
Since CD3+ T lymphocytes are the main
components of CIK cells and perforin is important in the antitumor
effects of CIK cells (17), an
immunohistochemical study was further performed on the excised
tumor tissue with anti-CD3 and anti-perforin antibodies. The
results revealed that there was significantly more accumulation of
CD3+ T lymphocytes and perforin in tumor tissue treated
with CIK cells plus NaHCO3 compared with tumor tissue
treated with CIK cells alone (Fig.
6). There were almost no accumulation of CD3+ T
lymphocytes or perforin in the tumor tissue from the other two
groups. This result could explain why tumor growth was remarkably
suppressed in the CIK+NaHCO3 group.
Discussion
The acidic pH of the extracellular environment has a
direct influence on a broad range of immunological functions
(18). Previous studies on
polymorphonuclear leukocytes demonstrate that this acidic pH mainly
causes inhibition of chemotaxis, respiratory activity and
bactericidal capacity (19,20). Evidence of impaired lymphocyte
cytotoxicity and proliferation at acidic pH is also beginning to
emerge (21). In addition, clinical
acidosis is accompanied by immunodeficiency (22). Furthermore, other evidence has
suggested that acidic microenvironments may play a role during
neoplastic growth and invasion (23).
CIK cells are ex vivo-expanded T lymphocytes
that share phenotypic and functional properties with both NK and T
cells (24). In view of the
conclusions of previous studies, it is reasonable for us to infer
that acidic microenvironments of solid tumors will affect the
antitumor activity of CIK cells. Based on the mechanism underlying
the antitumor effects of CIK cells, including the fact that CIK
cells bind to T cells, form cellular conjugates with tumor cells
and secrete a large number of cytotoxic cytoplasmic granules that
are cytolytic to T cells (25), and
the fact that CIK cells secrete a number of cytokines (including
IFN-γ, IL-2 and tumor necrosis factor) that attack tumor cells
(26), the present study compared the
antitumor activities of CIK cells or CMCIK against HCC
at pH 6.5 and 7.4. The results revealed that the antitumor
activities of both CIK cells and CMCIK against HepG2
cells were significantly compromised under an acidic environment,
with 35 and 25% reduction in cytotoxicity and tumor inhibition,
respectively. These results strongly suggested that neutralization
of the acidic environment in the tumor tissue should be equally
important as the CIK cell therapy itself. CIK cells would not be
fully active unless the acidic environment in tumor tissue is
neutralized.
Previous experimental and mathematical models have
successfully demonstrated that it is possible to upregulate the pHe
of tumors with little effect on systemic pH by chronic
administration of sodium bicarbonate (27,28). In
addition, recent studies documented that oral administration of
HCO3− could actually inhibit tumor metastasis in
prostate, breast and colon cancer cell line-derived tumors
(29–31). This apparently occurred by reducing
the extracellular acidity of the acid-extruding tumors, in the
absence of changes in the pH of the blood or normal tissues
(29–31). Previous findings suggested that
certain enzymes such as cathepsins and matrix metalloproteases,
which are involved in tumor invasion, were inhibited by
alkalinization (32). In the present
study, oral administration of NaHCO3 was combined with
CIK cell therapy in HCC-bearing nude mice to investigate whether
NaHCO3 could enhance the antitumor activity of CIK cells
against HCC. The present data revealed a remarkable synergy between
oral administration of NaHCO3 and CIK cell therapy in
treating grafted HepG2-luc carcinomas in nude mice, suggesting that
oral NaHCO3 administration may be sufficient to reduce
the acidity of the tumor microenvironment, eventually enhancing the
efficacy of CIK cell therapy. These findings and the in
vitro results could explain one of the reasons why tumor cells
thrive in the acidic environment, that is, due to the fact that the
immune system is compromised in the acidic environment, thus
facilitating the survival of tumor cells. Notably, oral
administration of NaHCO3 alone failed to inhibit the
growth of the tumor in nude mice. This result was consistent with a
previous study (28), in which
NaHCO3 administration was observed to have no effect on
the growth of primary tumors in severe combined immunodeficiency
mice. Thus, although NaHCO3 inhibits spontaneous
metastases by attenuating the function of certain enzymes, without
the immune system, NaHCO3 is unable to inhibit the
primary tumor growth. However, this is not the true situation of
the patients with tumors in the clinic, since the majority of these
patients are immunologically intact (33). Therefore, even without receiving any
immunotherapy such as CIK cell therapy, it would be beneficial for
these patients to ingest alkaline food or drinks, since it may aid
the T lymphocytes to fight against the tumor cells in their
bodies.
The present immunohistochemical studies on the tumor
tissue from each group revealed a significantly augmented number of
CD3+ T lymphocytes infiltrating into the tumor mass when
NaHCO3 feeding was combined with CIK cell therapy,
compared with CIK cell therapy alone. In addition, the perforin
secreted by T lymphocytes, which is important in killing T cells,
was also abundantly detected by immunohistochemistry. These results
suggest that the acidic environment appeared to prevent CIK cells
from migrating into the tumor tissue, which may partially explain
why immune cells such as CIK cells could not function well in an
acidic environment. However, there may be other mechanisms
involved, such as the acidic environment inhibiting the cytokine
secretion of CIK cells, thus compromising their binding ability to
the T cells. Further studies to explore the exact molecular
mechanisms are warranted.
In summary, both the in vitro and in
vivo experiments of the present study demonstrated that the
antitumor activities of CIK cells against HepG2 cells were
significantly enhanced under alkaline or relatively less acidic
environments, although the molecular mechanism involved require to
be further investigated. The present results have provided novel
insights for oncologists to treat their cancer patients,
particularly when immunotherapy is considered.
Acknowledgements
The present study was supported by grants from the
Ministry of Education of China (Beijing, China; grant no.
20151092900), the Science and Technology Department of Hubei
Province (Wuhan, China; grant no. 2014CFA068) and the Science and
Technology Department of Shiyan City (Shiyan, China; grant no.
16Y01).
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264.e1–1273.e1. 2012. View Article : Google Scholar
|
|
2
|
Su Y, Yang Y, Ma Y, Zhang Y, Rao W, Yang G
and Kou C: The Efficacy and Safety of Dendritic Cells Co-Cultured
with Cytokine-Induced Killer Cell Therapy in Combination with
TACE-Predominant Minimally-Invasive Treatment for Hepatocellular
Carcinoma: A Meta-Analysis. Clin Lab. 62:599–608. 2016.PubMed/NCBI
|
|
3
|
Zhang L, Zhu W, Li J, Yang X, Ren Y, Niu J
and Pang Y: Clinical outcome of immunotherapy with dendritic cell
vaccine and cytokine-induced killer cell therapy in hepatobiliary
and pancreatic cancer. Mol Clin Oncol. 4:129–133. 2016.PubMed/NCBI
|
|
4
|
Schmidt-Wolf IG, Finke S, Trojaneck B,
Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M,
Takeya M, et al: Phase I clinical study applying autologous
immunological effector cells transfected with the interleukin-2
gene in patients with metastatic renal cancer, colorectal cancer
and lymphoma. Br J Cancer. 81:1009–1016. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rettinger E, Meyer V, Kreyenberg H, Volk
A, Kuçi S, Willasch A, Koscielniak E, Fulda S, Wels WS, Boenig H,
et al: Cytotoxic capacity of IL-15-stimulated cytokine-induced
killer cells against human acute myeloid leukemia and
rhabdomyosarcoma in humanized preclinical mouse models. Front
Oncol. 2:322012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Chen YT, Song HZ, Huang GC and
Chen LB: Cisplatin pretreatment enhances antitumor activity of
cytokine-induced killer cells. World J Gastroenterol. 17:3002–3011.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang D, Zhang B, Gao H, Ding G, Wu Q,
Zhang J, Liao L and Chen H: Clinical research of genetically
modified dendritic cells in combination with cytokine-induced
killer cell treatment in advanced renal cancer. BMC Cancer.
14:2512014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie F, Zhang X, Li H, Zheng T, Xu F, Shen
R, Yan L, Yang J and He J: Adoptive immunotherapy in postoperative
hepatocellular carcinoma: A systemic review. PLoS One.
7:e428792012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantor JR and Sabatini DM: Cancer cell
metabolism: One hallmark, many faces. Cancer Discov. 2:881–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen AP, Moreira JM and Pedersen SF:
Interactions of ion transporters and channels with cancer cell
metabolism and the tumour microenvironment. Philos Trans R Soc Lond
B Biol Sci. 369:201300982014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallagher FA, Kettunen MI, Day SE, Hu DE,
Ardenkjaer-Larsen JH, Zandt RI, Jensen PR, Karlsson M, Golman K,
Lerche MH and Brindle KM: Magnetic resonance imaging of pH in vivo
using hyperpolarized 13C-labelled bicarbonate. Nature. 453:940–943.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rofstad EK, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barar J and Omidi Y: Dysregulated pH in
tumor microenvironment checkmates cancer therapy. Bioimpacts.
3:149–162. 2013.PubMed/NCBI
|
|
14
|
von Schwarzenberg K, Lajtos T, Simon L,
Müller R, Vereb G and Vollmar AM: V-ATPase inhibition overcomes
trastuzumab resistance in breast cancer. Mol Oncol. 8:9–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrari M, Fornasiero MC and Isetta AM:
MTT colorimetric assay for testing macrophage cytotoxic activity in
vitro. J Immunol Methods. 131:165–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pyo KH, Jung BK, Xin CF, Lee YW, Chai JY
and Shin EH: Prominent IL-12 production and tumor reduction in
athymic nude mice after Toxoplasma gondii lysate antigen treatment.
Korean J Parasitol. 52:605–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JH, Xiang JY, Ding GP and Cao LP:
Cholangiocarcinoma-derived exosomes inhibit the antitumor activity
of cytokine-induced killer cells by down-regulating the secretion
of tumor necrosis factor-α and perforin. J Zhejiang Univ Sci B.
17:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lardner A: The effects of extracellular pH
on immune function. J Leukoc Biol. 69:522–530. 2001.PubMed/NCBI
|
|
19
|
Rabinovich M, DeStefano MJ and
Dziezanowski MA: Neutrophil migration under agarose: Stimulation by
lowered medium pH and osmolality. J Reticuloendothel Soc.
27:189–200. 1980.PubMed/NCBI
|
|
20
|
Leblebicioglu B, Lim JS, Cario AC, Beck FM
and Walters JD: pH changes observed in the inflamed gingival
crevice modulate human polymorphonuclear leukocyte activation in
vitro. J Periodontol. 67:472–477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loeffler DA, Juneau PL and Masserant S:
Influence of tumour physico-chemical conditions on
interleukin-2-stimulated lymphocyte proliferation. Br J Cancer.
66:619–622. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kellum JA, Song M and Li J: Science
review: Extracellular acidosis and the immune response: Clinical
and physiologic implications. Crit Care. 8:331–336. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kraus M and Wolf B: Implications of acidic
tumor microenvironment for neoplastic growth and cancer treatment:
A computer analysis. Tumour Biol. 17:133–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimura R, Baker J, Beilhack A, Zeiser
R, Olson JA, Sega EI, Karimi M and Negrin RS: In vivo trafficking
and survival of cytokine-induced killer cells resulting in minimal
GVHD with retention of antitumor activity. Blood. 112:2563–2574.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pievani A, Borleri G, Pende D, Moretta L,
Rambaldi A, Golay J and Introna M: Dual-functional capability of
CD3+CD56+CIK cells, a T-cell subset that acquires NK function and
retains TCR-mediated specific cytotoxicity. Blood. 118:3301–3310.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao L, Run-Ming J and Yi J: T-Bet
mediated anti-neoplastic effects of dendritic cell-cytokine induced
killer cells in vitro. Iran J Pediatr. 22:43–51. 2012.PubMed/NCBI
|
|
27
|
Martin NK, Gaffney EA, Gatenby RA, Gillies
RJ, Robey IF and Maini PK: A mathematical model of tumour and blood
pHe regulation: The HCO3-/CO2 buffering system. Math Biosci.
230:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robey IF, Baggett BK, Kirkpatrick ND, Roe
DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby
RA and Gillies RJ: Bicarbonate increases tumor pH and inhibits
spontaneous metastases. Cancer Res. 69:2260–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wojtkowiak JW, Verduzco D, Schramm KJ and
Gillies RJ: Drug resistance and cellular adaptation to tumor acidic
pH microenvironment. Mol Pharm. 8:2032–2038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg
JM, Sloane BF, et al: Acidity generated by the tumor
microenvironment drives local invasion. Cancer Res. 73:1524–1535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Silva AS, Yunes JA, Gillies RJ and Gatenby
RA: The potential role of systemic buffers in reducing intratumoral
extracellular pH and acid-mediated invasion. Cancer Res.
69:2677–2684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robey IF and Nesbit LA: Investigating
mechanisms of alkalinization for reducing primary breast tumor
invasion. Biomed Res Int. 2013:4851962013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu H, Du F, Jiang L, Wang Z, Gong Z, Lian
P, Li P and Chen J: The Efficacy of CIK-Based Immunotherapies for
Advanced Solid Tumors. Technol Cancer Res Treat. Jul 19–2016.(Epub
ahead of print).
|