Introduction
Hepatocarcinoma is one of the major malignant tumors
in humans, with ~600,000 newly diagnosed cases, and leading to
>250,000 mortalities annually (1,2). Surgical
resection, in the form of partial hepatectomy or total hepatectomy,
followed by liver transplantation, may provide an occasional
incidence of cure (3). However, this
can be performed only in selected patients whose tumors are small
and located away from major vessels and have not metastasized to
extrahepatic organs (4). No
chemotherapy agent has better benefit than surgery in controlled
clinical trials due to different reasons, including drug resistance
and toxicity to normal cells (5).
The development of innovative novel therapeutics for
the management of hepatocarcinoma is particularly urgent, and this
development is the long-term objective of the current study.
Curcumin (CUR) has been reported to interrupt the cell cycle, exert
cytotoxic effects, and participate in antiproliferation and
induction of apoptosis in numerous hepatocarcinoma cell lines in
vitro (6). A considerable number
of reports have also described the anticancer effects of CUR on
hepatocellular carcinoma (HCC) in vivo (7). Although CUR is remarkably non toxic and
has promising anticancer activities, preclinical and clinical
studies indicate that its poor bioavailability and pharmacokinetic
profile due to its instability under physiological conditions have
limited its application in anticancer therapies (8–10). Thus,
the development of synthetic structural analogues of CUR is one
approach for overcoming the poor bioavailability of CUR while
retaining or further enhancing its drug-like effects (11,12). The
present authors have designed and synthesized a series of
mono-carbonyl analogues of CUR by deleting the reactive β-diketone
moiety (13–15). Although previous studies suggest that
the presence of the β-diketone moiety may be necessary for the
biological activities of CUR, a number of studies from several
independent groups demonstrated that certain CUR analogues
containing a 5-carbon enone spacer without β-diketone either
retained or increased the growth-suppressive activities of CUR
against several cancer cells (16,17). Those
studies indicate that certain mono-carbonyl analogues not only have
enhanced stability and antitumor activities in vitro, but
also have better pharmacokinetic profiles in vivo than CUR
(18). One such compound,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one (GL63) was
synthesized as part of a series of novel CUR analogues (19).
Upon oral administration at a dose of 500 mg/kg GL63
and CUR to rats, the concentration of each compound in plasma was
measured by high performance liquid chromatography (20). GL63 was observed to possess a plasma
concentration ~44-fold higher than that of CUR (area under the
curve) and a peak blood concentration ~45-fold higher than that of
CUR (20). Furthermore, there was an
apparent decrease in clearance with GL63 (38.98 l/kg/h) compared
with CUR (835.20 l/kg/h) (20). In
summary, these pharmacokinetics data indicate that the deletion of
the β-diketone moiety significantly decreases the degree and speed
of metabolism of curcuminoids, and that GL63 possesses a much
better pharmacokinetic profile than CUR (20). Previous studies demonstrated that the
cytotoxic effect of GL63 against HepG2 human hepatocellular
carcinoma and CNE2 nasopharyngeal carcinoma cells was due to the
induction of cell cycle arrest and subsequent apoptosis by the
endoplasmic reticulum stress pathway (21,22). Xiao
et al noticed that treatment of H460 human lung epithelial
cancer cells with GL63 facilitated the degradation of
cyclooxygenase-2 messenger (m) RNA, as evidenced by mRNA
degradation assay (19). Xiao et
al also observed that neither GL63 nor CUR induced apoptosis in
normal liver cells, which indicated that GL63 had no significant
toxicity, similar to CUR (21). These
results suggested that the novel CUR-related compound GL63 is a
potent antitumor agent. To date, although GL63 has been used for
various different medical purposes in vitro (21,22), the
underlying cellular and molecular mechanisms by which GL63
suppresses hepatocarcinoma cell growth are unknown. Whether GL63
has potential as a novel therapeutic agent for hepatocarcinoma to
the same extent than CUR remains to be investigated. In the present
study, the inhibitory efficacy of GL63 was evaluated on
hepatocarcinoma in vitro and in vivo, and it was
assessed whether GL63 was more potent than CUR in inhibiting
the growth of liver cancer cell lines (SK-HEP-1) and of
hepatocarcinoma induced by N-nitrosodiethylamin (DEN) in a Wistar
rat model.
Materials and methods
Cell lines and reagents
GL63 was synthesized as reported by Liang et
al (20). The SK-HEP-1 cell line
was purchased from the Cell Bank of the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck Millipore) and antibiotics (100 U/ml
penicillin and 100 µg/ml streptomycin) in cell culture incubators
set at 37°C and aired with 5% CO2.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell viability assay
The MTT assay was used to evaluate cell viability.
Briefly, cells were seeded onto 96-well plates, and allowed to
adhere and grow in 10% FBS-containing RPMI-1640 medium for 24 h.
GL63 (Hebei University of Science and Technology, Shijiazhuang,
China) and CUR (Hebei University of Science and Technology) were
dissolved in dimethyl sulfoxide (DMSO). The cells were then treated
with various concentrations of GL63 and CUR (0, 10, 20 and 40 µM)
for 24 h. MTT (20 µl at 5 mg/ml) was added to each well, and
incubation was conducted for 3.5 h. MTT was aspirated, and 100 µl
DMSO was added to each well. Next, the absorbance at 570 nm was
read in a plate reader. The half-maximal inhibitory concentration
(IC50) was used as the concentration of drug required to obtain 50%
of maximal inhibition in cell viability. Each treatment was
performed in triplicate. The mean of three values was determined,
and the results were expressed as a percentage of the control. Cell
viability was expressed as the percentage of the absorbance in the
treated wells relative to that of the untreated (control) wells.
Three independent experiments were performed.
Cell cycle analysis
SK-HEP-1 cells were seeded in 6-well plates at a
concentration of 5×105 cells/well. Following treatment with various
concentrations of GL63 and CUR (0, 10, 20 and 40 µM) for 24 h, the
cells were harvested and washed with phosphate-buffered saline
(PBS), and then resuspended in 70% ethanol at −20°C overnight.
Next, the cells were washed twice with PBS and 10 mg/ml RNase A was
added. Propidium iodide (PI) was added to the tubes at a final
concentration of 0.05 mg/ml and incubated at 4°C for 30 min in the
dark. Cell cycle analysis was conducted immediately upon staining
using an EPICS Elite ESP flow cytometer (Beckman Coulter, Inc.,
Brea, CA, USA) and WinMDI 2.9 software (Beckman Coulter, Inc.).
Flow cytometric analysis
SK-HEP-1 cells were seeded in 6-well plates at a
density of 5×105 cells/well for 24 h at 37°C, incubated with 20 µM
Z-DEVD-FMK (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or
vehicle for 1 h, followed by treatment with various concentrations
of GL63 and CUR (0, 10, 20 and 40 µM) for 24 h. All cells were
collected and washed twice with cold PBS. Next, the cells were
collected and resuspended in 100 µl binding buffer containing
annexin V and PI. The mixed solution was gently vortexed and
incubated in the dark at room temperature (25°C) for 15 min.
Quantification of annexin V and PI binding was performed with a
FACScan™ (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
Following treatment with 0, 10, 20 and 40 µM CUR and
GL63 for 24 h, the culture medium was collected, and the cells were
washed with ice-cold PBS. Cells were lysed with lysis buffer
(Beijing Biosynthesis Biotechnology Co. Ltd., Beijing, China) for
30 min at 40°C, followed by centrifugation at 20,000 × g for 10 min
at 4°C. The supernatants were collected and the protein
concentrations were determined using a BCA-100 Protein Quantitative
Analysis kit (Beijing Biosynthesis Biotechnology Co. Ltd.).
Proteins were then subjected to electrophoresis on 10% SDS-PAGE
gels and transferred to a polyvinylidene difluoride blotting
membrane for 1 h at room temperature. Membranes were blocked with
5% skim milk at room temperature for 1 h, followed by incubation
with polyclonal rabbit phosphorylated (p)-Janus kinase 2 (JAK2;
cat. no. sc-16566; 1:1,000), JAK2 (cat. no. sc-278; 1:1,000),
p-signal transducer and activator of transcription 3 (STAT3; cat.
no. sc-135649; 1:1,000), STAT3 (cat. no. sc-7179; 1:1,000),
cytochrome c (Cyt-c; cat. no. sc-33174; 1:1,000), caspase-9 (cat.
no. sc-7885; 1:1,000), caspase-3 (cat. no. sc-98785; 1:1,000),
poly(ADP-ribose) polymerase (PARP; cat. no. sc-133888; 1:1,000),
B-cell lymphoma (Bcl)-2 (cat. no. sc-783; 1:1,000), Bcl– extra
large (xL) (cat. no. sc-7195; 1:1,000) and Bcl-2 associated X
protein (Bax; cat. no. sc-493; 1:1,000) rabbit polyclonal antibody
(Santa Cruz Biotechnology Inc.) primary antibodies at 4°C
overnight. Following three washes with TBST, the membranes were
incubated with horseradish peroxidase-conjugated polyclonal goat
anti-rabbit IgG secondary antibody (cat. no. sc-2004; 1:10,000;
Santa Cruz Biotechnology Inc.) for 1 h at room temperature.
Chemiluminescence detection was performed using an ECL Western
Blotting Detection kit (GE Healthcare Life Sciences, Chalfont, UK).
The quantities of the proteins were analyzed using ImageJ 1.48
analysis software and normalized to their respective control.
Tumorigenicity assays in Wistar
rats
A total of 105 healthy 4-week-old male Wistar rats
(100–110 g) were provided by the Animal Center of Beijing Medical
University (Beijing, China). All animals received humane care, and
protocols were approved by the Hebei Medical University Animal
Ethics Committee (Shijiazhuang, China). The animals were housed in
a polypropylene cage at temperature of 22±2°C and 50–60% relative
humidity, with a 12 h light/dark cycle for 1 week prior to and
during the experiment. Rats were allowed to access standard
pelleted rat chow and water. Group 1 (control non-treated group;
n=10) was considered as the normal control group. GL63 in group 2
(control GL63-treated group; n=10) was administered
intraperitoneally (80 mg/kg) twice a week for 12 weeks. CUR in
group 3 (control CUR-treated group; n=10) was administered
intraperitoneally (80 mg/kg) twice a week for 12 weeks. The rats in
group 4 (DEN-bearing non-treated group; n=25) were administered an
intraperitoneal injection of DEN (50 mg/kg) twice a week for 12
weeks. The rats in group 5 (DEN-bearing GL63-treated group; n=25)
were administered both DEN as in group 4 and GL63 as in group 2.
The rats in group 6 (DEN-bearing, CUR-treated group; n=25) were
administered both DEN as in group 4 and CUR as in group 3. Body
weight was recorded at the end of every week for 24 weeks. The
experiment was terminated at the end of 24 weeks, and all the
surviving rats were anaesthetized and sacrificed at the end of the
experiment, following animal ethics guidelines (23).
Cytomorphological evaluation and
immunohistochemistry
Autopsy specimens were obtained from the liver of
rats in all groups. All the liver tissues were fixed routinely and
embedded in paraffin to prepare 4-µm sections for hematoxylin and
eosin staining. The diagnosis and grade of hepatocarcinoma was
established based on the morphological findings identified on the
cell block sections using the World Health Organization criteria
(24). The expression strength of
proliferating cell nuclear antigen (PCNA; polyclonal rabbit
antibody; cat. no. sc-7909; 1:100; Santa Cruz Biotechnology, Inc.)
was based on the staining intensity (0, negative; 1, weak; 2,
intermediate; and 3, strong) and the percentage of positive cells
(0, 0% positive cells; 1, ≤25% positive cells; 2, 26–50% positive
cells; and 3, >50% positive cells) identified following
incubation with the PCNA antibody for 1 h at room temperature. The
two scores were added, with a maximum score of 6. A score of >2
represented a positive immunohistochemical identification of the
marker.
Statistical analysis
All statistical analysis was performed using SPSS
13.0 statistical software (SPSS, Inc., Chicago, IL, USA). All
qualitative variables are expressed as frequencies and percentages.
χ2 test and Fisher's exact test were used to determine associations
between categorical variables. All quantitative data are expressed
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
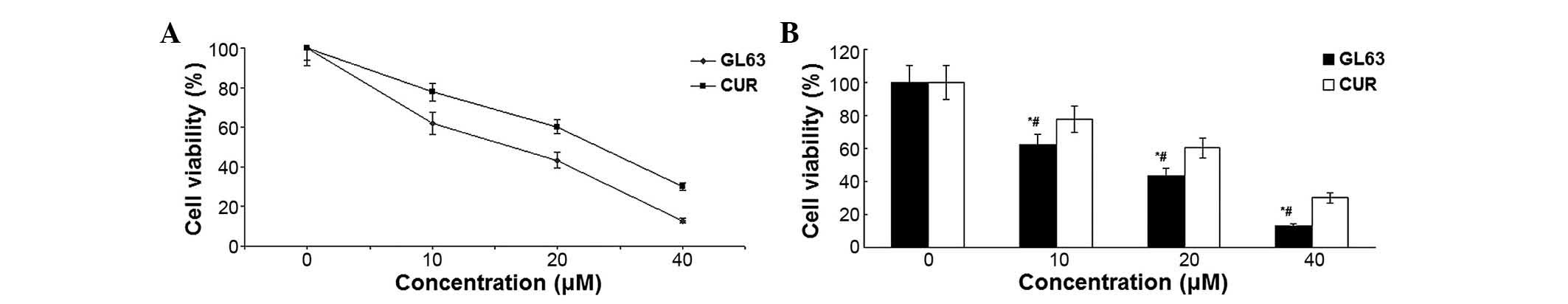
GL63 inhibits cell viability and
proliferation more potently than CUR
The cytotoxicity that GL63 and CUR effected on
SK-HEP-1 cells was assessed by MTT assay. When cultured with
various concentrations of GL63 (0, 10, 20 and 40 µM), the
proliferation of SK-HEP-1 cells was significantly suppressed in a
dose-dependent manner, with an IC50 value of 14.8±1.4 µM, which
indicated that GL63 was substantially more potent than CUR
(Fig. 1). Therefore, the results
indicated that GL63 could efficiently inhibit the growth of
SK-HEP-1 cells to a better extent than CUR.
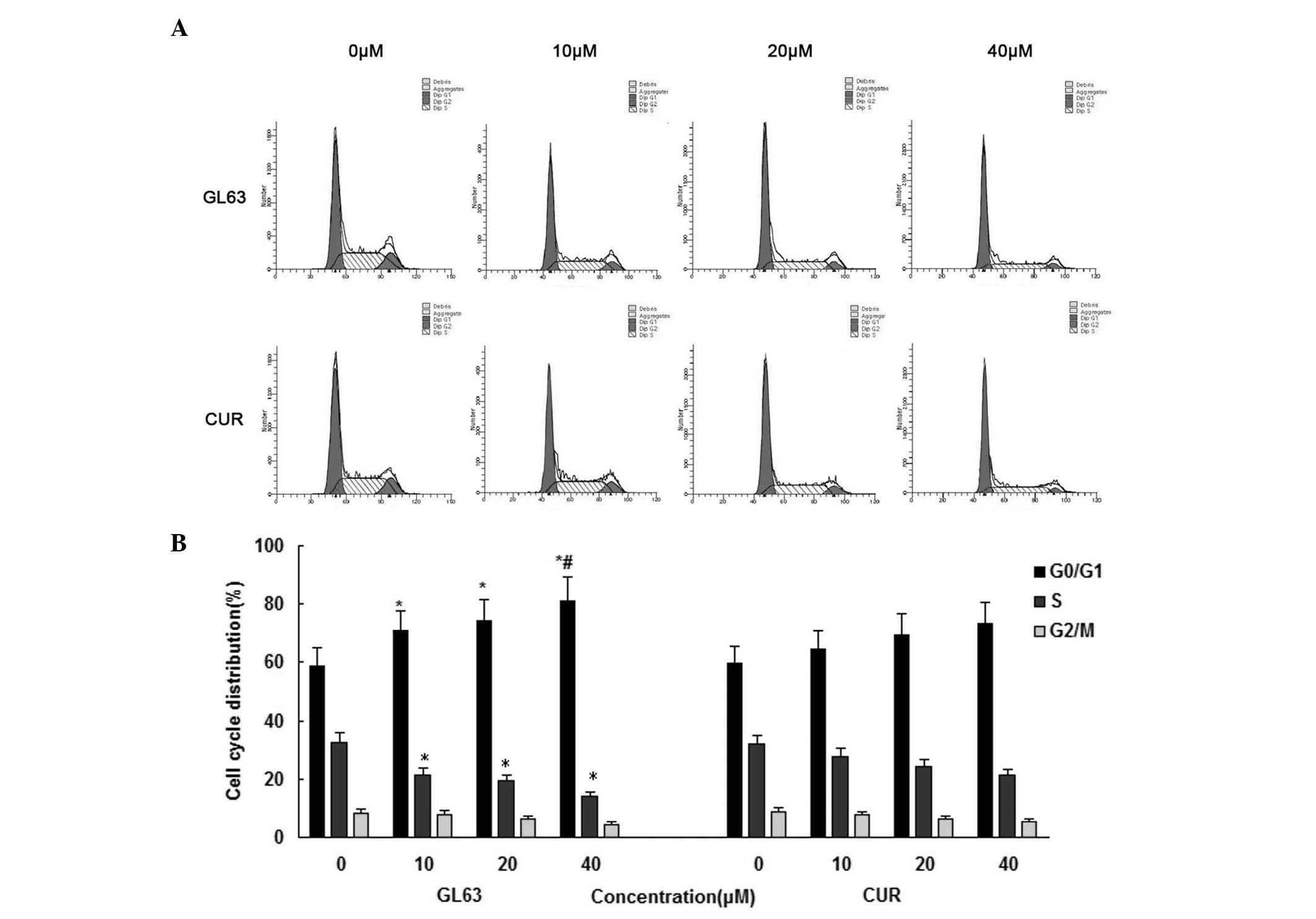
GL63 is more potent than CUR in
inducing cell cycle arrest
In order to gain further information about the
mechanism of the cell-growth inhibitory effect of GL63, the cell
cycle distribution of SK-HEP-1 cells was assessed by flow cytometry
following 24-h treatment with various concentrations of GL63 and
CUR (0, 10, 20 and 40 µM). As indicated in Fig. 2, GL63 increased the number of SK-HEP-1
cells in the G0/G1 phase of the cell cycle, whereas CUR caused a
weaker change in the cell cycle distribution. The average histogram
plot of cell-cycle analysis also indicated that GL63 treatment
induced a dose-dependent accumulation of SK-HEP-1 cells in G0/G1
phase. These results suggested that GL63 caused a cell-cycle arrest
in G0/G1 phase.
GL63 promotes cell apoptosis more
potently than CUR
To further investigate the underlying mechanism of
decreased cell proliferation, the apoptosis of SK-HEP-1 cells
induced by GL63 was examined using annexin V/PI assay (Fig. 3A and B). The apoptosis of SK-HEP-1
cells (including early and late apoptotic cell death) increased in
a dose-dependent manner following treatment with GL63 for 24 h.
Furthermore, GL63 significantly induced apoptosis compared with CUR
at the same concentrations in SK-HEP-1 cells. Bcl and caspase
family proteins are widely accepted to mediate the apoptotic
pathway (25). Mitochondrial
apoptotic pathway-related proteins (caspase-3, caspase-9, Cyt-c and
PARP) were upregulated by GL63 treatment, suggesting that this
apoptotic pathway was activated (Fig. 3C
and D). It was observed an increased activation of caspase-3 in
SK-HEP-1 cells treated with increasing concentrations of GL63,
indicating that GL63-induced apoptosis was dependent upon caspase-3
activity. A similar tendency was observed for caspase-9, Cyt-c and
PARP. Since caspase-9 activation involves mitochondria permeability
changes and the release of Cyt-c (21), a significant increase in released
Cyt-c was detected by GL63 treatment at various doses. Cells were
pretreated with the caspase-3 inhibitor Z-DEVD-FMK prior to and
throughout treatment with GL63, and it was observed that Z-DEVD-FMK
almost completely inhibited GL63-induced apoptosis in SK-HEP-1
cells, indicating that GL63-induced apoptosis was dependent upon
caspase-3 activity (Fig. 3E).
Notably, CUR did not induce caspase activation markedly, compared
with GL63. These in vitro results support a
mitochondria-dependent caspase activation to mediate GL63-induced
apoptosis. The details of the mechanisms by which GL63 affects the
mitochondria to initiate apoptosis signaling pathways will require
further investigation.
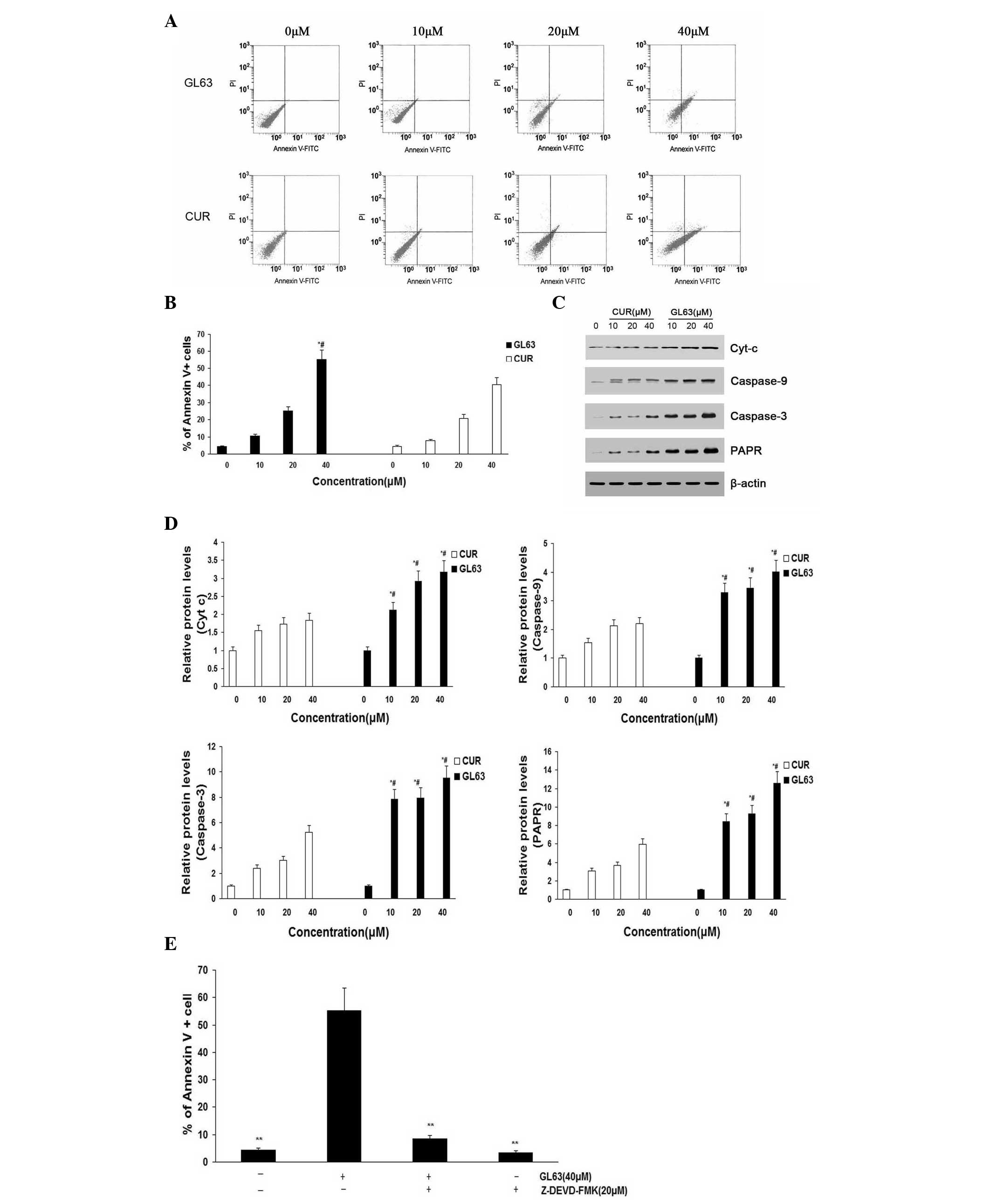 | Figure 3.Apoptosis rates of SK-HEP-1 cells
subjected to GL63 and CUR treatment, as detected by flow cytometry
and protein expression analysis. SK-HEP-1 cells were treated with
GL63 and CUR (0, 10, 20 and 40 µM) for 24 h. (A) Representative
diagrams of annexin V-fluorescein isothiocyanate/propidium iodide
staining upon treatment with different concentrations of GL63 and
CUR. (B) Bar diagram of apoptotic cell rate from three FACS
analyses from three separate treatments. (C) Caspase-9, caspase-3,
Cyt-c and PARP levels were determined in SK-HEP-1 cells treated
with GL63 and CUR at various concentrations for 24 h. β-actin was
used as a protein loading control. (D) Bar diagram represents the
expression levels of caspase-9, caspase-3, Cyt-c and PARP, which
displayed a concentration-dependent increase upon treatment with
GL63 or CUR. (E) Z-DEVD-FMK inhibits GL63-induced apoptosis.
SK-HEP-1 cells were incubated with Z-DEVD-FMK (20 µM) or vehicle
for 1 h, followed by treatment with or without GL63 (40 µM) for
another 24 h, and then apoptosis was evaluated by FACS (**P<0.01
vs. GL63 alone treatment). All data were expressed as the mean ±
standard deviation of ≥3 separate experiments. *P<0.05 vs.
dimethyl sulfoxide control. #P<0.05 vs. CUR group at the same
concentration. CUR, curcumin; FITC, fluorescein isothiocyanate; PI,
propidium iodide; Cyt-c, cytochrome c; PARP, poly(ADP-ribose)
polymerase; FACS, fluorescence-activated cell sorting; GL63,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one. |
GL63 potently suppresses the
JAK2/STAT3 signaling pathway and regulates the expression of its
downstream targets in vitro
To characterize the molecular mechanism by which the
JAK2/STAT3 signaling pathway alters GL63-induced apoptosis in
SK-HEP-1 cells, the expression of proteins in response to GL63 was
examined by immunoblotting. The results indicated that the levels
of JAK2 and STAT3 were not altered, while the levels of p-JAK2 and
p-STAT3 were markedly and concentration-dependently reduced
following treatment with GL63 and CUR in SK-HEP-1 cells. GL63
significantly reduced the level of p-JAK2 and p-STAT3 at various
concentrations, while CUR only slightly reduced p-JAK2 and p-STAT3
expression (Fig. 4A and C). To
further investigate whether GL63 could affect STAT3 downstream
genes, western blot analysis was used to examine the expression of
Bcl-2, Bcl-xL and Bax. The results indicated that cells treated
with GL63 exhibited a strong reduction in the protein expression
levels of Bcl-2 and Bcl-xL and a concomitant increase in Bax
expression in a concentration-dependent manner, compared with CUR
(Fig. 4B and D).
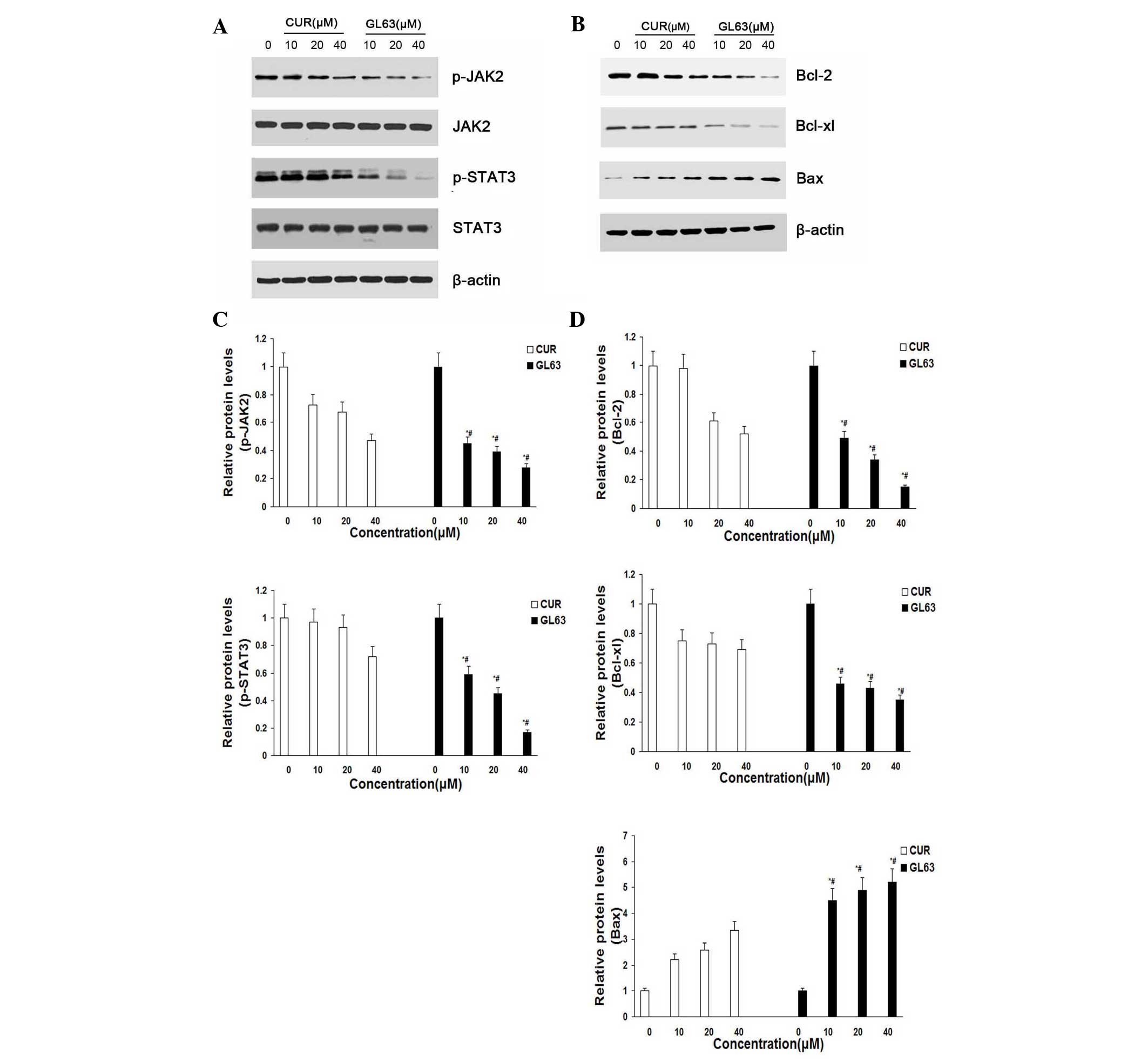 | Figure 4.GL63 and CUR both reduce cell
viability in human SK-HEP-1 cells by inhibiting the JAK2/STAT3
signaling pathway and its downstream gene expression. (A) SK-HEP-1
cells were treated with the indicated concentrations of GL63 and
CUR for 24 h prior to be subjected to western blot analysis with
anti-p-JAK2, anti-JAK2, anti-p-STAT3 and anti-STAT3 antibodies. (B)
Bar diagram of the effects of the different concentrations of GL63
and CUR on p-JAK2 and p-STAT3 from three separate treatments for 24
h. (C) Representative immunoblots of Bcl-2, Bcl-xl and Bax from the
nuclear extracts of SK-HEP-1 cells treated with GL63 and CUR (0,
10, 20 and 40 µM) for 24 h. β-actin served as a loading control.
(D) Bar diagram of the effects of the different concentrations of
GL63 and CUR on STAT3 downstream gene expression from three
separate treatments. Data are presented as the mean ± standard
deviation. *P<0.05 vs. dimethyl sulfoxide control. #P<0.05
vs. CUR group at the same concentration. CUR, curcumin; JAK2, Janus
kinase 2; STAT3, signal transducer and activator of transcription
3; p-, phosphorylated; Bcl-2, B-cell lymphoma-2; Bcl-XL, B-cell
lymphoma-extra large; Bax, Bcl-2 associated X protein; GL63,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one. |
Liver tumor formation
Table I presents the
results of survival, tumor incidence and number of nodules per
nodule-bearing liver in rats. The hepatocarcinogenic rats were
infiltrated with a large number of nodules, thus making the precise
assessment of tumor mass impossible. The literature reports that,
in previous studies, nodules measuring >6 mm in diameter at week
24 were always hepatocarcinoma (26);
thus, in the present study, those nodules were quantified according
to this standard when detected at the surface of the liver. As
indicated in Table I, no tumors were
observed in the untreated control group (group 1) or in the GL63 or
CUR control groups (groups 2 and 3). A significant reduction in
tumor incidence was observed in the GL63 and CUR-treated groups
(group 5 and 6) compared with the DEN-bearing non-treated group
(group 4). Groups 5 and 6 exhibited a significant decrease in the
number of nodules as compared with group 4. Furthermore, GL63
appeared to be a more potent compound than CUR, with regard to
decreasing tumor incidence and nodules numbers in rats. Table II presents data regarding body
weight, absolute liver weight and relative liver weight of the rats
from each control and experimental group. The body weights were
significantly decreased in the DEN-bearing non-treated group
compared with the control groups (P<0.001). GL63 and CUR
treatment of rats with DEN significantly improved their body weight
(P<0.001), compared with rats in group 4. Group 4 exhibited an
increased liver/body weight ratio compared with group 1. There was
an appreciable decrease in the absolute and relative liver weight
in groups 5 and 6 compared with rats administered DEN alone,
particularly in group 5.
 | Table I.Effect of GL63 and CUR on the
survival and development of nodules on DEN-induced
hepatocarcinogenesis in rats. |
Table I.
Effect of GL63 and CUR on the
survival and development of nodules on DEN-induced
hepatocarcinogenesis in rats.
| Group | Treatment | Survival, % | Tumor incidence,
% | Average count of
nodules/nodules bearing liver |
|---|
| 1 | Control | 100.0 (10/10) | – | – |
| 2 | GL63 alone | 100.0 (10/10) | – | – |
| 3 | CUR alone | 100.0 (10/10) | – | – |
| 4 | DEN alone | 52.0
(13/25)b | 100.0
(25/25)b |
17.53±1.29b |
| 5 | DEN + GL63 | 92.0
(23/25)d | 20.0
(5/25)d |
10.75±1.72d |
| 6 | DEN + CUR | 80.0
(20/25)c | 36.0
(9/25)d |
13.14±1.64d |
 | Table II.Effect of GL63 and CUR on body, liver
and relative liver weights on DEN-induced hepatocarcinogenesis in
rats. |
Table II.
Effect of GL63 and CUR on body, liver
and relative liver weights on DEN-induced hepatocarcinogenesis in
rats.
| Group | Treatment | Gain in body
weight, g | Final body weight,
g | Liver weight,
g | Relative liver
weight, g |
|---|
| 1 | Control | 267.1±4.7 | 371.9±5.5 | 11.38±0.12 | 3.06±0.04 |
| 2 | GL63 alone | 265.0±8.7 | 371.2±9.5 | 11.47±0.12 | 3.10±0.08 |
| 3 | CUR alone | 263.9±4.6 | 368.9±4.8 | 11.48±0.11 | 3.11±0.06 |
| 4 | DEN alone |
196.2±10.8b |
302.0±11.1b |
14.23±0.27b |
4.73±0.16b |
| 5 | DEN + GL63 |
245.5±9.7d |
350.2±9.7d |
12.01±0.20c |
3.44±0.10d |
| 6 | DEN + CUR |
243.4±8.1d |
347.5±8.2d |
11.95±0.30c |
3.44±0.09d |
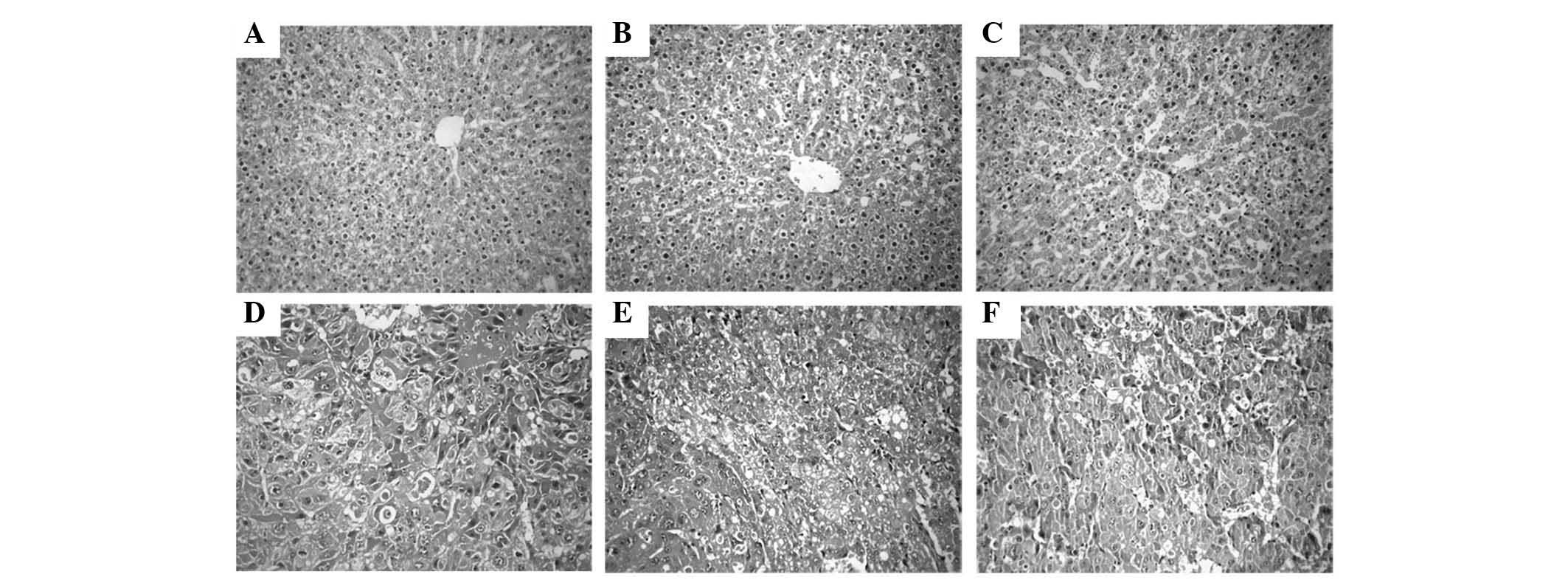
Histopathological observations
The histology of the liver tissue was examined under
a light microscope and represented in (Fig. 5). The livers from the control
non-treated group (group 1) exhibited a normal architecture and
cytoplasm of hepatic cells, displaying granulated cytoplasm, a
central vein, small uniform nuclei and nucleoli. Although liver
tissues from rats treated with GL63 only (group 2) displayed no
significant differences compared with those of the rats in the
control non-treated group (P=0.267), they exhibited minor
sinusoidal congestions. Compared with group 2, the liver tissues
from rats treated with CUR only (group 3) also had a normal lobular
organization, represented by a central vein, hepatic cords and
sinusoids, but displayed moderate-to-severe sinusoidal and venous
congestions. DEN treatment alone (group 4) resulted in the loss of
the normal architecture, with the tissues displaying instead a
granular cytoplasm with large hyperchromatic nuclei. Although the
DEN-bearing GL63-treated group (group 5) lost the normal
organization of hepatic lobules, it exhibited cancerous foci with
patchy necrosis compared with group 4. The tissues of rats in the
DEN-bearing CUR-treated group (group 6) exhibited disorganized
cells bordering wide sinusoids, but displayed moderately malignant
features such as focal necrosis and a low mitotic count.
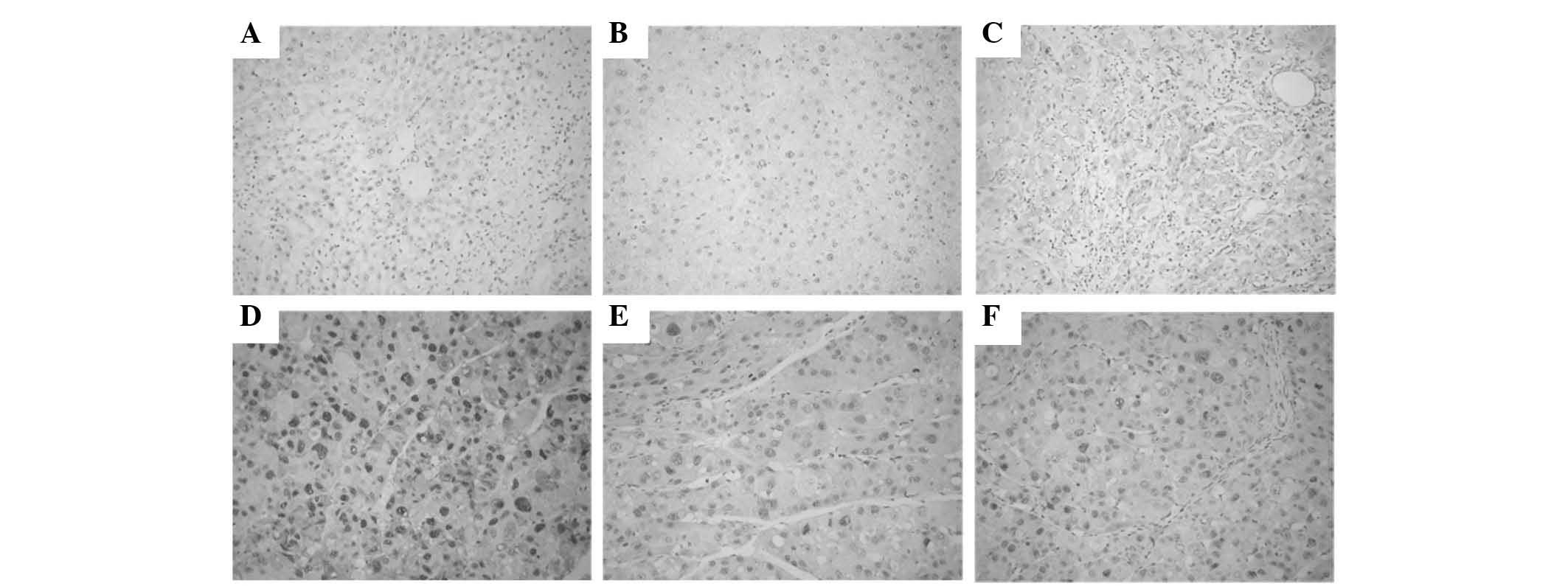
Immunohistochemical examinations
PCNA was expressed in the nuclei of hepatic cells.
The number of PCNA-positive tumor cells was significantly lower in
the DEN-bearing treated with GL63 or CUR group than in the
DEN-bearing non-treated group, particularly in group 5 (all
P<0.05). Photomicrographs of PCNA immunohistochemical sections
of various groups are represented in Fig.
6.
Discussion
The liver is the key organ of the metabolic,
secretory and excretory functions in the body, and its disorders
are numerous and varied (27).
Several environmental carcinogens have been reported to elicit
hepatic tumorigenesis (28).
Animal models such as Wistar rats can be used to
simulate carcinogenesis and development of human hepatocarcinoma,
and to study its molecular mechanism and intervention (29). DEN is a powerful carcinogen known to
induce carcinoma in all animal species including humans, and is
considered as an initiating agent in certain two-stage (initiation
and promotion) protocols for hepatocarcinogenic studies (30). DEN-induced lesions and tumors in
rodents exhibit marked biochemical, histological and molecular
similarity to the progression of HCC in humans (31). DEN is an established powerful
hepatocarcinogen in rats, possibly by altering the DNA structure,
forming alkyl DNA adducts, and inducing chromosomal aberrations and
micronuclei in the liver (32,33).
Granado-Serrano et al reported a decrease in
food intake, feed efficiency ratio and body weight gain, as well as
a significant increase in relative liver weight, in the DEN group
compared with the normal control group (34). The present results also confirmed this
tendency. The reduction in food intake, and consequently, the
reduction in body weight gain observed in DEN-treated animals may
be due to losses of skeletal muscle and adipose tissue, with
relative sparing of visceral proteins, as mentioned by Sreepriya
and Bali (35), and it could be
considered as an indirect indication of the declining hepatic
function following exposure to DEN. Sivaramakrishnan et al
reported a marked loss in body weight and an increase in liver
weight induced by DEN in animals treated with DEN compared with the
control group. In addition, the administration of DEN to animals
also caused a significantly increase in liver weight due to the
formation of nodules and tumors in the liver following carcinogen
exposure (36). In accordance to the
above report, the present study also observed a decrease in body
weight and an increase in liver weight in animals treated with DEN.
PCNA, as an index of the state of cell proliferation and indication
of the malignant degree, was significantly enhanced in the nucleus
of hepatocarcinoma cells, which has been confirmed in numerous
studies (37). Therefore, enhanced
expression of PCNA indicates abnormal proliferation of liver
tissue. The present results demonstrated that GL63 treatment led to
a decreased expression of PCNA in Wistar rats, which supported the
preventive effects of GL63 against DEN-induced hepatocarcinogenesis
in rats suggested by the present histopathological and
immunohistochemical findings.
Due to its poor solubility and stability in water,
the therapeutic benefit of CUR is limited (10). A series of mono-carbonyl analogues of
CUR with higher potencies and improved water solubility,
formulation and delivery system have been developed, since these
properties are considered to be responsible for their stability
in vitro and the pharmacokinetic advantages in vivo
(20). In the present study, annexin
V/PI staining demonstrated that, compared with CUR, GL63 increased
the efficacy of induction of apoptosis in SK-HEP-1 cells. The
results of flow cytometry also revealed that treatment with GL63
resulted in G0/G1 arrest of cancer cells, which may account for the
inhibition of proliferation and cell cycle arrest exhibited by
treated cells. Furthermore, the apoptotic rate induced by GL63 was
higher than that induced by CUR. Based on these findings, a series
of experiments were performed to further determine the potential
apoptotic signaling pathways.
It is known that the dysregulation of the JAK/STAT
signaling pathway may cause immunodeficiencies and cancers
(38,39). Activated JAK2/STAT3 signaling pathway
can modulate the expression of target genes that are involved in
various physiological functions, including anti-apoptotic proteins
(Bcl-xl and Bcl-2), pro-apoptotic proteins (Bax and BH3
interacting-domain death agonist) and mitochondrial apoptosis
pathway-related proteins (caspase-3, caspase-9, Cyt-c and PARP)
(40). Importantly, the activated
JAK2/STAT3 signaling pathway has been extensively validated as a
novel molecular target for the treatment of human tumors (41). The present study further explored the
role of the JAK2/STAT3 signaling pathway in the
anti-hepatocarcinoma effect of GL63 for the first time, and
evaluated that GL63 could efficiently inhibit the phosphorylation
of JAK2 and STAT3, thus affecting cell viability and inducing
apoptosis. GL63 can inhibit the JAK2/STAT3 signaling pathway with
lower doses than those required for CUR, thus being a more
efficient form of treatment for hepatocarcinoma than CUR. The
present authors suggest that GL63 may have inhibitory effects on
the proliferation of SK-HEP-1 cells, which may be mediated through
the suppression of the JAK2/STAT3 signaling pathway.
Previous studies have demonstrated that the
regulation of the JAK2/STAT3 signaling pathway by diverse drugs can
induce apoptosis through the intrinsic mitochondrial pathway
(42). The intrinsic pathway is
initiated at the level of the mitochondria under the regulation of
the Bcl-2 family of proteins (43).
Mitochondrial integrity and apoptosis are regulated mainly by the
Bcl-2 family of cell-death regulatory molecules, which include the
pro-apoptotic Bax and the anti-apoptotic Bcl-2 (44). Mitochondria mediate apoptotic
signaling via the activation of the cell-death initiator
pro-caspase-9 (45). Activated
caspase-9 in turn cleaves executioner caspase-3 (46). The activated caspase-3 then cleaves
PARP, a nuclear protein associated with the process of programmed
cell death (47). The present data
support the scenario that GL63 treatment induced apoptosis of
SK-HEP-1 cells possibly through the caspase family proteins,
leading to Cyt-c release and the activation of caspase-9, caspase-3
and PARP. It was observed that the antitumor activity of GL63 was
also associated with the induction of apoptosis in HCC cells, as
indicated by increased caspase-9, caspase-3 and PARP cleavage in
vitro. In the present study, it was also revealed that the
activation of caspase was involved in GL63-induced apoptosis, and a
general caspase inhibitor markedly blocked GL63-induced cell death.
These results suggested that GL63 induced apoptosis of SK-HEP-1
cells via a mitochondria-mediated intrinsic apoptosis pathway.
Activation of the intrinsic pathway with GL63 was demonstrated by
the activation of caspase-9 and caspase-3, along with the altered
expression of Bcl-2 family proteins. In the current study, the
significantly increased levels of Bcl-2 and Bcl-xL, and the
activation of caspases-9 and −3, suggested that the intrinsic
pathway is important in GL63-induced apoptosis in SK-HEP-1 cells.
Compared with CUR, GL63 increased the efficacy of induction of
apoptosis of SK-HEP-1 cells. GL63 treatment resulted in a reduction
in JAK2 and STAT3 phosphorylation, activated caspase-9, caspase-3,
Cyt-c and PARP cleavage, and caused apoptotic death of cells
remarkably. This finding verifies previous studies reporting that
inhibiting the JAK2/STAT3 signaling pathway is associated with the
induction of apoptosis by the intrinsic mitochondrial pathway in
hepatocarcinoma cells (48). It was
also concluded that GL63 treatment inhibits hepatocarcinoma cell
growth via downregulation of the JAK2/STAT3 signaling pathway.
The activity of GL63 was also evaluated in the
present study using male Wistar rats, which is an animal model for
studies of human hepatocarcinoma (49). For these in vivo studies, rats
were divided into six different groups according to the
requirements of the experimental design, and the animals were
injected DEN into the peritoneum to induce hepatocarcinoma. A
series of indexes of hepatocarcinoma were recorded for each group
to judge the protective role of GL63 and CUR against
hepatocarcinoma in rats. Although the results of the present study
provide support for the chemopreventive effects of GL63 and CUR,
GL63 could be a more potent compound than CUR for the prevention of
DEN-induced hepatocarcinogenesis in rats.
In conclusion, the present results demonstrated
that, compared with CUR, GL63 was more effective in inhibiting the
JAK2/STAT3 signaling pathway and regulating the activation of the
intrinsic mitochondrial pathway with a more favorable
pharmacological activity than CUR, which contributed to the
suppression of cell proliferation and the induction of cell
apoptosis. GL63 may have translational potential as an effective
drug or preventive agent for hepatocarcinoma. The doses of GL63
that effectively demonstrate chemopreventive effects without
toxicity for humans must be examined in future clinical
studies.
Acknowledgements
The present study was supported by research grants
from the Health and Family Planning Commission of Hebei Province
(Shijiazhuang, China; grant no. 20150634) and the Tumor Research
Institute, The Fourth Affiliated Hospital, Hebei Medical University
(Shijiazhuang, China). The authors appreciate the generous support
from Dr Yueping Liu, Dr Xiaoling Wang and Dr Huichai Yang for the
pathological images.
References
|
1
|
Hussain SA, Ferry DR, El-Gazzaz G, Mirza
DF, James ND, McMaster P and Kerr DJ: Hepatocellular carcinoma. Ann
Oncol. 12:161–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang TS, Wang CH, Hsieh RK, Chen JS and
Fung MC: Gemcitabine and doxorubicin for the treatment of patients
with advanced hepatocellular carcinoma: A phase I–II trial. Ann
Oncol. 13:1771–1778. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuda H: Hepatocellular carcinoma
development in cirrhosis. Best Pract Res Clin Gastroenterol.
21:161–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeda K: Current therapy for
hepatocellular carcinoma. Nihon Rinsho. 68:1129–1136. 2010.(In
Japanese). PubMed/NCBI
|
|
5
|
Zhao JA, Peng L, Geng CZ, Liu YP, Wang X,
Yang HC and Wang SJ: Preventive effect of hydrazinocurcumin on
carcinogenesis of diethylnitrosamine-induced hepatocarcinoma in
male SD Rats. Asian Pac J Cancer Prev. 15:2115–2121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai XZ, Yin HT, Sun LF, Hu X, Zhou C, Zhou
Y, Zhang W, Huang XE and Li XC: Potential therapeutic efficacy of
curcumin in liver cancer. Asian Pac J Cancer Prev. 14:3855–3859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Appiah-Opong R, de Esch I, Commandeur JN,
Andarini M and Vermeulen NP: Structure-activity relationships for
the inhibition of recombinant human cytochromes P450 by curcumin
analogues. Eur J Med Chem. 43:1621–1631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma RA, Steward WP and Gescher AJ:
Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med
Biol. 595:453–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Padhye S, Chavan D, Pandey S, Deshpande J,
Swamy KV and Sarkar FH: Perspectives on chemopreventive and
therapeutic potential of curcumin analogs in medicinal chemistry.
Mini Rev Med Chem. 10:372–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: Potential anticancer agents. Med Res Rev. 30:818–860.
2010.PubMed/NCBI
|
|
13
|
Liang G, Yang SL, Shao LL, Zhao CG, Xiao
J, Lv YX, Yang J, Zhao Y and Li XK: Synthesis, structure, and
bioevaluation of 2,5-bis(arylmethenyl)cyclopentanones. J Asian Nat
Prod Res. 10:957–965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang G, Yang S, Zhou H, Shao L, Huang K,
Xiao J, Huang Z and Li X: Synthesis, crystal structure and
anti-inflammatory properties of curcumin analogues. Eur J Med Chem.
44:915–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang G, Yang S, Jiang L, Zhao Y, Shao L,
Xiao J, Ye F, Li Y and Li X: Synthesis and anti-bacterial
properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull
(Tokyo). 56:162–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson TP, Ehlers T, Hubbard RB IV, Bai
X, Arbiser JL, Goldsmith DJ and Bowen JP: Design, synthesis, and
biological evaluation of angiogenesis inhibitors: Aromatic enone
and dienone analogues of curcumin. Bioorg Med Chem Lett.
13:115–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang
HK, Itokawa H, Su CY, Shih C, Chiang T, Chang E, et al: Antitumor
agents. 217. Curcumin analogues as novel androgen receptor
antagonists with potential as anti-prostate cancer agents. J Med
Chem. 45:5037–5042. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weng Q, Fu L, Chen G, Hui J, Song J, Feng
J, Shi D, Cai Y, Ji J and Liang G: Design, synthesis, and
anticancer evaluation of long-chain alkoxylated mono-carbonyl
analogues of curcumin. Eur J Med Chem. 103:44–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao J, Tan Y, Pan Y, Liang G, Qu C, Zhang
X, Zhang Y, Li X and Yang H: A new cyclooxygenase-2 inhibitor,
(1E,4E)-1,5-bis(2-bromophenyl)penta-1,4-dien-3-one (GL63)
suppresses cyclooxygenase-2 gene expression in human lung
epithelial cancer cells: Coupled mRNA stabilization and
posttranscriptional inhibition. Biol Pharm Bull. 33:1170–1175.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang G, Shao L, Wang Y, Zhao C, Chu Y,
Xiao J, Zhao Y, Li X and Yang S: Exploration and synthesis of
curcumin analogues with improved structural stability both in vitro
and in vivo as cytotoxic agents. Bioorg Med Chem. 17:2623–2631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao J, Chu Y, Hu K, Wan J, Huang Y, Jiang
C, Liang G and Li X: Synthesis and biological analysis of a new
curcumin analogue for enhanced anti-tumor activity in HepG 2 cells.
Oncol Rep. 23:1435–1441. 2010.PubMed/NCBI
|
|
22
|
Pan Y, Xiao J, Liang G, Wang M, Wang D,
Wang S and Yang H: A new curcumin analogue exhibits enhanced
antitumor activity in nasopharyngeal carcinoma. Oncol Rep.
30:239–245. 2013.PubMed/NCBI
|
|
23
|
Reiser D: Animal EthicsEncylopedia of
Corporate Social Responsibility. Springer; Berlin Heidelberg: pp.
106–110. 2013
|
|
24
|
Theise ND, Curado MP and Franceschi S:
Hepatocellular carcinomaWHO Classification of Tumors of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
4th. IARC Press; Lyon: pp. 205–216. 2010
|
|
25
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taras D, Blanc JF, Rullier A, Dugot-Senant
N, Laurendeau I, Vidaud M and Rosenbaum J: Pravastatin reduces lung
metastasis of rat hepatocellular carcinoma via a coordinated
decrease of MMP expression and activity. J Hepatol. 46:69–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Franco-Bourland RE and Méndez-Sánchez N:
The liver is the key organ for the development of metabolic
syndrome. Ann Hepatol. 10:216–217. 2011.PubMed/NCBI
|
|
28
|
Peraino C: Initiation and promotion of
liver tumorigenesis. Natl Cancer Inst Monogr. 58:55–61.
1981.PubMed/NCBI
|
|
29
|
Sivalokanathan S, Ilayaraja M and
Balasubramanian MP: Antioxidant activity of Terminalia arjuna bark
extract on N-nitrosodiethylamine induced hepatocellular carcinoma
in rats. Mol Cell Biochem. 281:87–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pereira MA, Herren-Freund SL, Britt AL and
Khoury MM: Effect of coadministration of phenobarbital sodium on
N-nitrosodiethylamine-induced gamma-glutamyltransferase-positive
foci and hepatocellular carcinoma in rats. J Natl Cancer Inst.
72:741–744. 1984.PubMed/NCBI
|
|
31
|
Fen F, Pascale RM, Simile MM, De Miglio
MR, Muroni MR and Calvisi D: Genetic alterations in liver
carcinogenesis: Implications for new preventive and therapeutic
strategies. Crit Rev Oncog. 11:19–62. 2000.PubMed/NCBI
|
|
32
|
Al-Rejaie SS, Aleisa AM, Al-Yahya AA,
Bakheet SA, Alsheikh A, Fatani AG, Al-Shabanah OA and Sayed-Ahmed
MM: Progression of diethylnitrosamine-induced hepatic
carcinogenesis in carnitine-depleted rats. World J Gastroenterol.
15:1373–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verna L, Whysner J and Williams GM:
N-nitrosodiethylamine mechanistic data and risk assessment:
Bioactivation, DNA-adduct formation, mutagenicity, and tumor
initiation. Pharmacol Ther. 71:57–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: A diet rich in cocoa attenuates
N-nitrosodiethylamine-induced liver injury in rats. Food Chem
Toxicol. 47:2499–2506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sreepriya M and Bali G: Chemopreventive
effects of embelin and curcumin against
N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in
Wistar rats. Fitoterapia. 76:549–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sivaramakrishnan V, Shilpa PN, Kumar VR
Praveen and Devaraj S Niranjali: Attenuation of
N-nitrosodiethylamine-induced hepatocellular carcinoma by a novel
flavonol-Morin. Chem Biol Interact. 171:79–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelloff GJ, Lieberman R, Steele VE, Boone
CW, Lubet RA, Kopelovitch L, Malone WA, Crowell JA and Sigman CC:
Chemoprevention of prostate cancer: Concepts and strategies. Eur
Urol. 35:342–350. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schindler CW: Series introduction.
JAK-STAT signaling in human disease. J Clin Invest. 109:1133–1137.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitchell TJ and John S: Signal transducer
and activator of transcription (STAT) signalling and T-cell
lymphomas. Immunology. 114:301–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Wang L, Wu Y, Lv C, Li X, Cao X,
Yang M, Feng D and Luo Z: Pterostilbene exerts antitumor activity
against human osteosarcoma cells by inhibiting the JAK2/STAT3
signaling pathway. Toxicology. 304:120–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wung BS, Hsu MC, Wu CC and Hsieh CW:
Resveratrol suppresses IL-6-induced ICAM-1 gene expression in
endothelial cells: Effects on the inhibition of STAT3
phosphorylation. Life Sci. 78:389–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Du W, Hong J, Wang YC, Zhang YJ, Wang P,
Su WY, Lin YW, Lu R, Zou WP, Xiong H and Fang JY: Inhibition of
JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via
mitochondrial pathway. J Cell Mol Med. 16:1878–1888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
45
|
Estaquier J, Vallette E, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martin KR: Targeting apoptosis with
dietary bioactive agents. Exp Biol Med (Maywood). 231:117–129.
2006.PubMed/NCBI
|
|
47
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fuke H, Shiraki K, Sugimoto K, Tanaka J,
Beppu T, Yoneda K, Yamamoto N, Ito K, Masuya M and Takei Y: Jak
inhibitor induces S phase cell-cycle arrest and augments
TRAIL-induced apoptosis in human hepatocellular carcinoma cells.
Biochem Biophys Res Commun. 363:738–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park DH, Shin JW, Park SK, Seo JN, Li L,
Jang JJ and Lee MJ: Diethylnitrosamine (DEN) induces irreversible
hepatocellular carcinogenesis through overexpression of G1/S-phase
regulatory proteins in rat. Toxicol Lett. 191:321–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|