Introduction
Hepatocellular carcinoma (HCC) is a significant
health problem in the world and has a very poor prognosis (1). To date, a treatment strategy does not
exist to effectively treat HCC patients (1,2). Although
the overall 5-year survival rate of HCC patients receiving
conventional chemotherapy after surgical resection or liver
transplantation at a sufficiently early stage is increasing
(3), the long-term prognosis remains
unsatisfactory due to high recurrence rates. Therefore, novel
approaches are needed to prevent and treat HCC.
Stem cell research may provide a novel insight into
identifying a better strategy to treat HCC. Both embryonic and
adult stem cells have self-renewal capacities and have the ability
to produce differentiated progenitors. During tumorigenesis, stem
cells from normal tissues are able to migrate into tumor lesions
and suppress tumor cell growth (4,5). For
example, a previous study demonstrated that human bone
marrow-derived mesenchymal stem cells could be used to treat
gliomas (4). These findings led to
other studies that investigated whether modified stem cells can
express anticancer molecules as targeted anticancer agents
(6–9)
and whether normal stem cells have antitumor activity.
In liver tissue, the ability of hepatocytic stem
cells to migrate into HCC tissue has been reported previously
(10). Thus, stem cells may be used
as a vector to deliver therapeutic genes for targeted cancer
therapy (11–13). Indeed, the microenvironment of stem
cells is considered to serve a role in regulating HCC cell growth
(14,15) by downregulating multiple signal
transduction pathways, such as the Wnt/β-catenin (14) and TGF-β/Smad (16) pathways. However, the molecular
mechanisms of the inhibitory effect of stem cells on tumor cells
remain to be defined.
Constitutive activation of Akt and nuclear
factor-kappaB (NF-κB) are major cellular abnormalities associated
with HCC pathogenesis and progression (17). Inactivation of NF-κB signaling
inhibits growth and induces apoptosis of HCC cells (18–21).
Similarly, a previous study demonstrated that inhibition of Akt
signaling resulted in HCC cell growth, arrest and apoptosis
(22,23). Another previous study demonstrated
that Akt activates NF-κB transcription by phosphorylating IκBκ
(24,25). Thus, inhibiting Akt/NF-κB signaling
may be a novel therapeutic approach for the control of HCC
(17).
The present authors previous study demonstrated that
hepatocytic precursor (stem-like) cells reduced the tumorigenicity
and induced apoptosis of hepatoma cells in a co-culture system
(16). In the present study, the
effect of co-culturing hepatoma cells with hepatocytic precursor
(stem-like) cells on the regulation of HCC phenotypic and gene
expression was assessed in vitro and in nude mice. The
present study aimed to provide useful insight into the development
of stem cell-initiated antitumor therapy for HCC patients.
Materials and methods
Cell lines and culture
The diploid rat liver epithelial cell line WB-F344
was obtained from the Shanghai Cell Bank (Shanghai, China). These
cells are oval in shape and have the capacity to differentiate into
hepatocyte and biliary lineages under suitable conditions (26); thus, these cells are considered to be
hepatocytic precursor (stem-like) cells. The rat hepatoma cell line
CBRH-7919 was also obtained from the Shanghai Cell Bank.
WB-F344 and CBRH-7919 cells were co-cultured using a
Transwell chamber culture system containing 4.0 µm pore
polycarbonate membrane inserts (Corning, NY, USA). The WB-F344 to
CBRH-7919 cell ratio was 5:1 (2×105:4×104
cells/well). CBRH-7919 cells cultured alone in the Transwell
chamber culture system were used as a control. All the cells were
cultured in serum-free conditioned medium from established cultures
at 37°C with 95% air, 5% CO2, and 100% humidity for 7
days before the cells were used for subsequent assays. The
serum-free conditioned medium was composed of Dulbecco's modified
Eagle's medium/Ham's F12 medium (DMEM/F12, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20 ng/ml of
basic fibroblast growth factor (Sigma-Aldrich, St. Louis, MO, USA),
20 ng/ml of epidermal growth factor (Sigma-Aldrich), and 20 µl/ml
of B27 supplement (Invitrogen; Thermo Fisher Scientific, Inc.).
Nude mouse experiments
The present study was approved by the Institutional
Animal Care and Use Committee (IACUC) of the Second Military
Medical University (Shanghai, China). The mice used in the
experiment were maintained under specific pathogen-free conditions
and handled in accordance with the procedures and guidelines set by
the Institutional Animal Care and Use Committee of The Second
Military Medical University (Shanghai, China). Co-cultured WB-F344
and CBRH-7919 cells and single culture CBRH-7919 cells
(1×106 cell/mouse) were subcutaneously inoculated into
the axillary fossae of female nude mice (age, 6–8 weeks old). The
tumor size was monitored every 3 days by measuring the length and
width with calipers. The tumor volume was calculated using the
formula: [(L × W2) × 0.5 mm3], in which L was
the length and W was the width of each tumor. At day 35
post-injection, mice were sacrificed for pathological analysis.
Cell proliferation and clonogenic
assays
Cell counting kit-8 (CCK-8) is a sensitive,
nonradioactive colorimetric assay that assesses cell proliferation
and detects the number of living cells. In the present study, a
CCK-8 (Dojindo Molecular Technologies, Inc., Tokyo, Japan) assay
was performed to assess the effect of rat WB-F344 stem cells on
CBRH-7919 cell proliferation. In brief, after co-culturing these
cell lines for 7 days in serum-free conditioned medium, CBRH-7919
cells were trypsinized, counted, and 5×104 cells were
seeded in 24-well plates in triplicate and cultured for up to 8
days. At the end of each experiment, the cells were further
incubated with an additional equal amount of fresh medium
containing 10% CCK-8 at 37°C for 4 h, and the cell number was then
counted. The data are presented as the mean cell number of each
count in the curve diagrams.
For the clonogenic assay, CBRH-7919-only cultured
cells and CBRH-7919 cells co-cultured with WB-F344 stem cells were
seeded in 12-well plates in triplicate at a density of 100
cells/well and grown for 14 days. Subsequently, cell colonies were
stained with 0.5% crystal violet and images were captured (EOS 600D
Digital SLR; Canon, Inc., Tokyo, Japan) using an Olympus 1×71
inverted microscope (Olympus Corp., Tokyo). The number of colonies
was counted 14 days after seeding. A colony was counted only if it
contained ≥50 cells. The rate of colony formation was calculated
with the following equation: colony formation rate = (number of
colonies/number of seeded cells) ×100%.
Tumor cell migration and invasion
assay
The ability of CBRH-7919-only cultured cells and
CBRH-7919 cells co-cultured with WB-F344 stem cells to invade
through Matrigel-coated filters was investigated using the 8-µm BD
Falcon™ cell culture insert (BD Biosciences, San Jose, CA, USA).
Briefly, 1×105 cell were suspended in 500 µl serum-free
DMEM/F12 and then seeded into the upper compartments of each
chamber. The lower compartments were filled with 1 ml DMEM/F12
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). After 24 h of incubation at 37°C in 5%
CO2, non-invading cells were removed by scrubbing the
upper surface of the membrane. Cells that invaded into the bottom
surface of the membrane were fixed with methanol and stained with
0.5% crystal violet. The cells were then reviewed and counted under
a microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan) in 5
microscopic fields (x× magnification).
Wound healing assay
Scratch assays were performed to assess the effect
of WB-F344 stem cells on the migration of hepatoma cells. Briefly,
cells were seeded in 6-well plates at a density of 2×105
cells/well. When the cells were 90–100% confluent, the monolayer
was scratched manually with a plastic pipette tip across each
plate. The cells were then washed twice with phosphate buffered
saline (PBS) and further incubated for up to 96 h. At the end of
each experiment, images of each plate were captured to demonstrate
‘wound healing’ using a PowerShot G10 camera (Canon, Inc.). The
distance of each wound was measured and the mean was calculated for
the capacity of tumor cell migration.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from the cultured
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and then reverse transcribed into cDNA using a Two-Step
RT-PCR kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The PCR cycling conditions were as
follows: One cycle at 95°C for 30 sec, followed by 30 cycles at
95°C for 5 sec, one cycle at 60°C for 30 sec, with a final
extension step at 72°C for 1 min, using the SYBR Green Realtime PCR
Master Mix (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. The samples were held at 4°C until
required. To determine the levels of E-cadherin, β-catenin, α-SMA
and vimentin, qPCR was performed with an ABI 7300 instrument
(Thermo Fisher Scientific, Inc.) using a SYBR PrimeScript RT-PCR
kit (Takara Suzo, Kyoto, Japan) according to the manufacturer's
instructions. The mRNA β-actin level was used as an internal
control. The primer sequences used for amplification were as
follows: E-cadherin, 5′-TGAGGTCGGTGCCCGTATTG-3′ and
5′-GAATGCCCTCGTTGGTCTTGG-3′; β-catenin, 5′-ACTCTAGTGCAGCTTGGTTC-3′
and 5′-ATGGCAGGCTCGGTAATGTC-3′; α-SMA,
5′-ATGACCCAGATTATGTTTGAGACC-3′ and 5′-CCAGAGTCCAGCACAATACCAG-3′;
vimentin, 5′-CGCAGCCTCTATTCCTCGTC-3′ and
5′-TCTTGAACTCGGTGTTGATGG-3′; and β-actin,
5′-ACGTTGACATCCGTAAAGAC-3′ and 5′-GAAGGTGGACAGTGAGGC-3′. The
relative mRNA levels were calculated based on the Ct values and
normalized to the level of β-actin mRNA according to the equation:
2−ΔΔCt [ΔCt=Ct (Gene)-Ct (β-actin)]. All of the
experiments were performed in triplicate and repeated once.
Protein extraction and western blot
analysis
The levels of NF-κB (p65), phospho-Akt,
phospho-IKKα/β and phospho-IκBα proteins were assayed in CBRH-7919
cells co-cultured with WB-F344 cells and CBRH-7919-only cultured
cells using western blot analysis. Briefly, total cell extracts
were prepared using RIPA lysis buffer (50 mM Tris-HCl, 1% NP-40, 2
mM EDTA, 10 mM NaCl, 2 mg/ml aprotinin, 5 mg/ml leupeptin, 2 mg/ml
pepstatin, 1 mM DTT, 0.1% SDS and 1 mM phenylmethylsulfonyl
fluoride). Protein concentrations were determined using the BCA
assay (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal
amounts of protein (20 µg) were then separated by SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore, Billerica, MA, USA)
by electroblotting. The membranes were then blocked with 5% non-fat
milk in PBS-T (PBS with 0.05% Tween-20) for 1 h and then incubated
with specific primary antibodies against rat NF-κB (p65; rabbit
monoclonal; dilution, 1:500; catalog no., CST-8242), phospho-Akt
(rabbit monoclonal; dilution, 1:1,000; catalog no., CST-12178),
phospho-IKKα/β (rabbit monoclonal; dilution, 1:1,000; catalog. no.,
CST-2697), phospho-IκBα (rabbit monoclonal; dilution, 1:500;
catalog no., CST-2859; all purchased from Cell Signaling
Technology, Inc., Danvers, MA, USA) and GAPDH (rabbit polyclonal;
dilution, 1:500; catalog no., SC-25778; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) overnight at 4°C. The membranes were then
washed with PBS-T and incubated with a horseradish peroxidase
conjugated goat anti-rabbit immunoglobulin G (dilution, 1:2,000;
catalog no., SC-2004; Santa Cruz Biotechnology, Inc.) for 1 h. The
protein bands were visualized using an enhanced chemiluminescence
kit (Pierce Biotechnology, Inc.) and exposed on X-ray films. The
level of protein expression was normalized to GAPDH protein.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS version 19 (IBM SPSS, Armonk, NY, USA) was used to analyze the
data. Statistical analysis was performed using the two-tailed
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Hepatocytic precursor WB-F344 cells
suppressed the growth of hepatoma cell xenografts in nude mice
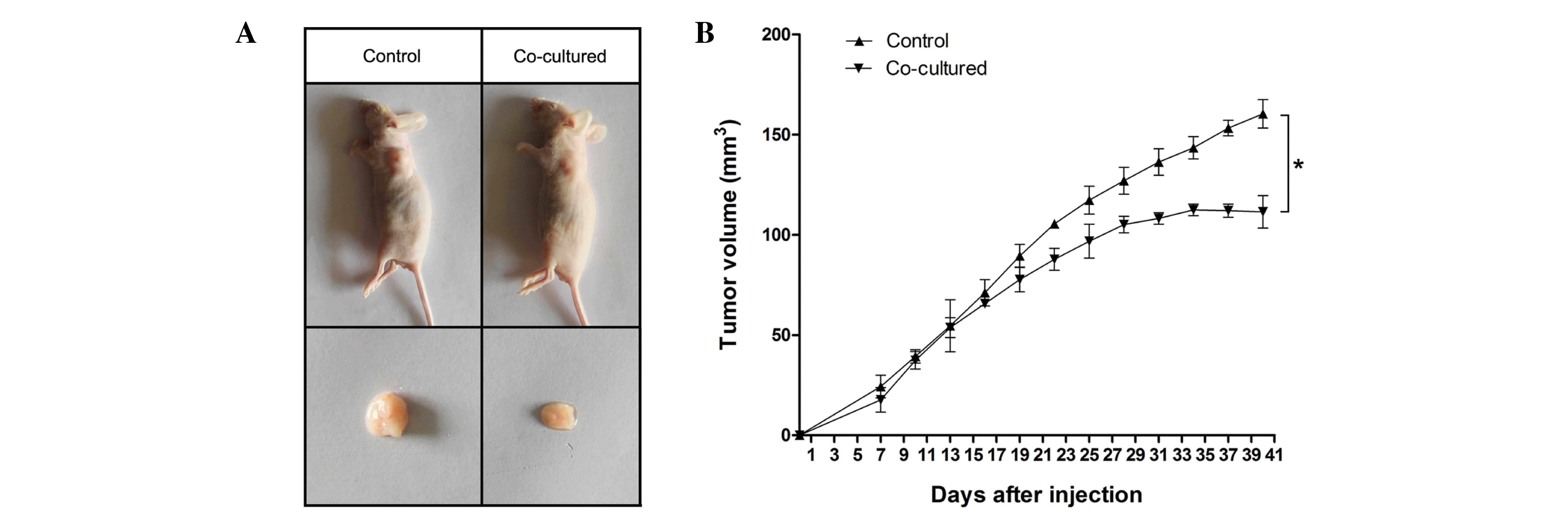
In the present study, CBRH-7919 cells were
co-cultured with WB-F344 cells or were cultured alone for 7 days
and then injected into nude mice. On day 22 post-injection, nude
mice bearing co-cultured or control CBRH-7919 cells had similar
tumor xenograft volumes. From ~4 weeks, mice bearing co-cultured
CBRH-7919 cells had a significantly reduced tumor volume compared
with mice bearing control xenografts (Fig. 1, P<0.05). The data indicated that
WB-F344 cells inhibited the growth of hepatoma CBRH-7919 cell
xenografts in nude mice.
Hepatocytic precursor (stem-like)
WB-F344 cells suppressed the clonogenic growth of CBRH-7919
cells
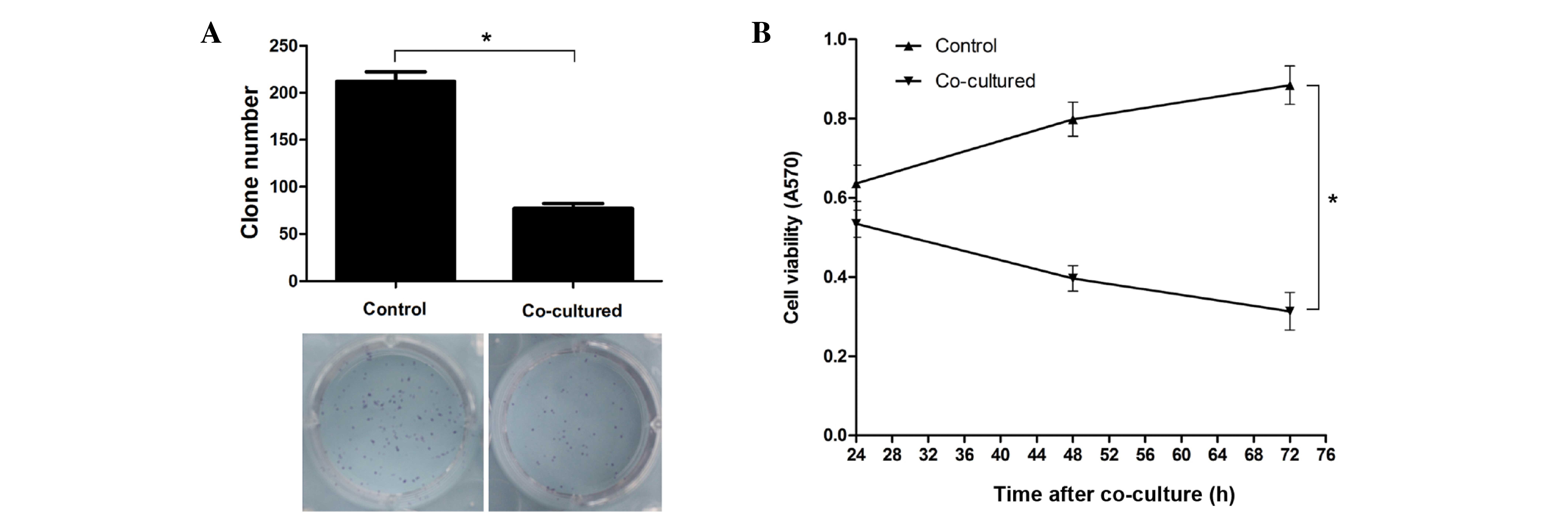
After co-culture with hepatocytic precursor
(stem-like) WB-F344 cells, hepatoma CBRH-7919 cells exhibited a
reduced cell growth and proliferation rate; there was a significant
reduction in the number of CBRH-7919 cells in both monolayer and
colony formation (P<0.05, Fig. 2A and
B).
Hepatocytic precursor (stem-like)
WB-F344 cells suppressed the migration and invasion of CBRH-7919
cells
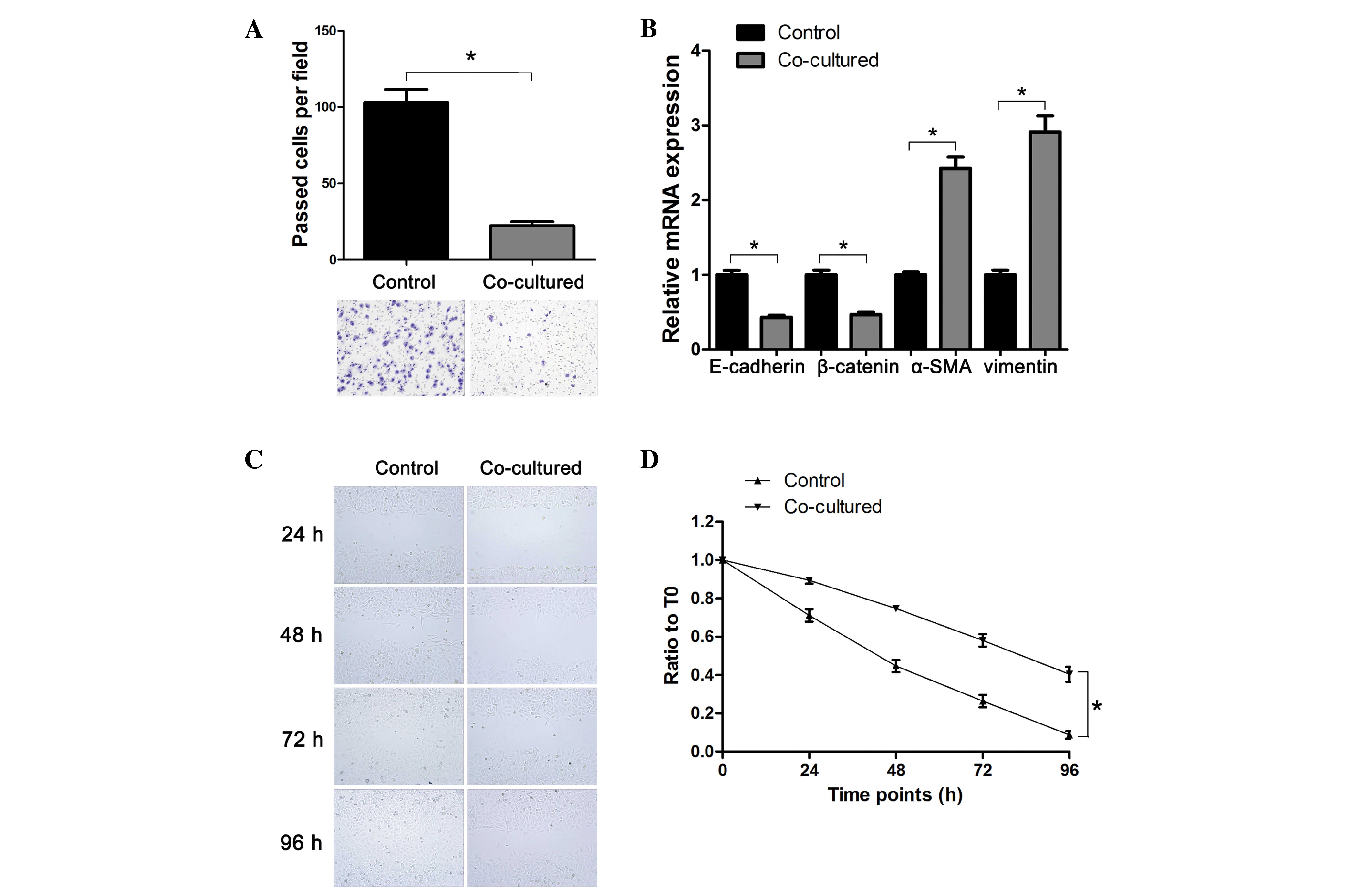
As presented in Fig.
3A, the Transwell assay demonstrated that co-cultured CBRH-7919
cells demonstrated a reduced invasion capacity compared with that
of the control cells (P<0.05).
In addition, epithelial-mesenchymal transition
(EMT), a key event in tumor invasion and metastasis (27,28), was
also characterized in the present study. The RT-qPCR analysis
results demonstrated that the mRNA expression levels of the
epithelial markers E-cadherin and β-catenin were downregulated in
co-cultured CBRH-7919 cells, while the mesenchymal markers α-SMA
and vimentin were upregulated when compared with control cells
(P<0.05; Fig. 3B).
The tumor cell wound healing assay demonstrated that
CBRH-7919 cells co-cultured with WB-F344 cell demonstrated a
reduced invasion capacity compared with that of the control cells
(P<0.05; Fig. 3C and D).
These results indicated that co-culture with WB-F344
cells suppressed the migration and invasion of CBRH-7919 cells via
regulation of tumor cell EMT activity.
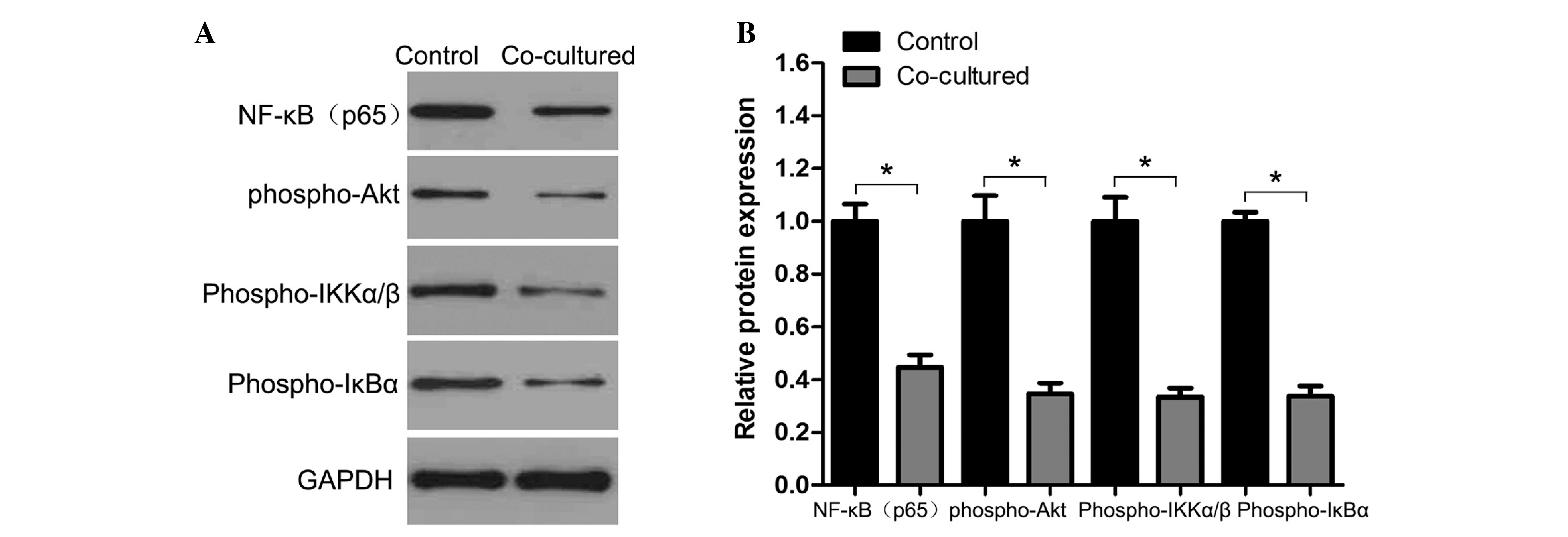
Akt/NF-κB signaling mediated the
migration and invasion capacity of hepatoma CBRH-7919 cells
Since AKT and NF-κB are essential regulators of
tumor cell properties, including metastasis (29,30), the
present authors proposed that the Akt/NF-κB signaling pathway may
be involved in the inhibition of hepatoma CBRH-7919 cell migration
and invasion capacity through its pro-apoptotic role. As
illustrated in Fig. 4, western blot
analyses demonstrated that co-culture of hepatoma CBRH-7919 cells
with WB-F344 cells resulted in the downregulation of NF-κB and
phospho-Akt expression in CBRH-7919 cells.
Discussion
HCC is a leading cause of cancer-associated
mortality in the world and has an extremely poor prognosis
(31,32). The development and identification of
novel therapeutic strategies to effectively control HCC metastasis
may increase patient survival. Previous studies have indicated that
stem cells may be useful to treat different types of human cancer
(4–15). Regardless of their distinct origins,
stem cells and tumor cells share a number of common properties,
including the loss of contact inhibition and immortality, and
therefore, similar signaling pathways may be involved in their
control. It has been reported that the stem cell microenvironment
is essential in preventing carcinogenesis by providing signals to
inhibit cell proliferation and to promote differentiation (33). In the current study, it was
demonstrated that co-culture with WB-F344 cells suppressed the
growth, colony formation and tumor cell migration and invasion
capacity of CBRH-7919 cells. Co-cultured CBRH-7919 cells also
expressed downregulated levels of the epithelial markers E-cadherin
and β-catenin and upregulated expression levels of the mesenchymal
markers α-SMA and vimentin via downregulating NF-κB and phospho-Akt
expression. In addition, the co-culture of CBRH-7919 cells with
WB-F344 cells inhibited xenograft formation and growth in a nude
mouse model. The current study therefore indicates that hepatocytic
precursor (stem-like) WB-F344 cells regulate HCC cell phenotype and
gene expression. However, this study is a proof-of-principle study
and additional studies are needed to understand the underlying
molecular mechanisms by which stem cells act on HCC cells.
The results of the present study are consistent with
the effect of human mesenchymal stem cells on HCC cells, as
previously reported (34). In the
current study, suppression of the Akt/NF-κB signaling pathway after
co-culturing CBRH-7919 cells with WB-F344 cells contributed to the
suppression of HCC cell growth and invasion. Indeed, aberrant
activation of the Akt/NF-κB signaling pathway has been widely
investigated and reported in the literature. Akt and NF-κB proteins
regulate a variety of cellular processes, including cell
proliferation, apoptosis, invasion and angiogenesis (35–38).
Furthermore, Akt and NF-κB are being extensively investigated as
molecular targets for cancer therapeutics (38), as studies have demonstrated that
repression of Akt and/or NF-κB activity inhibit cancer cell growth,
migration and invasion, and sensitizes cancer cells to apoptosis
(39–41). In agreement with these previous
studies, the current data demonstrated that Akt-mediated
phosphorylation of IKKα/β and IκBα was necessary for the activation
of NF-κB, further mediating the metastasis potential of hepatoma
CBRH-7919 cells in this co-culture system. In the resting state,
the activity of NF-κB is sequestered in the cytoplasm as an
inactive precursor complex with inhibitory κB (IκB). The biological
activity of NF-κB is tightly regulated by IκBα. It appears that
newly synthesized IκB rapidly re-associates with newly released
NF-κB, thereby markedly reducing the amount of NF-κB translocated
into the nucleus for the activation of cytokine genes. The activity
of the NF-κB transcription factor is regulated by p-IκB through
multiple intracellular signal transduction pathways (42).
In conclusion, hepatocytic precursor (stem-like)
WB-F344 cells inhibited the growth, colony formation and invasion
capacity of hepatoma CBRH-7919 cells in vitro and in
vivo by downregulating Akt/NF-κB signaling. However, further
studies are required to confirm the results of the present study.
Subsequent studies by the present authors will assess whether
overexpressing genes in this signaling pathway overcome the
activity of co-culturing CBRH-7919 cells with WB-F344 cells.
Acknowledgements
The present study was supported in part by grants
from the National Natural Sciences Fund Project of China (nos.
81370553 and 39670699 to Dr. Hong-Yu Yu). The authors would like to
thank Medjaden Bioscience Limited (Hong Kong, China) for
editing and improving English of this manuscript.
References
|
1
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamizo A, Marini F, Amano T, Khan A,
Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, et
al: Human bone marrow-derived mesenchymal stem cells in the
treatment of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
5
|
Aboody KS, Brown A, Rainov NG, Bower KA,
Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: Evidence from intracranial gliomas. Proc Natl Acad Sci U S
A. 97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Lin X, Zhao J, Shi W, Zhang H,
Wang Y, Kan B, Du L, Wang B, Wei Y, et al: A tumor-selective
biotherapy with prolonged impact on established metastases based on
cytokine gene-engineered MSCs. Mol Ther. 16:749–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchibori R, Okada T, Ito T, Urabe M,
Mizukami H, Kume A and Ozawa K: Retroviral vector-producing
mesenchymal stem cells for targeted suicide cancer gene therapy. J
Gene Med. 11:373–381. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura K, Ito Y, Kawano Y, Kurozumi K,
Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y and Hamada H:
Antitumor effect of genetically engineered mesenchymal stem cells
in a rat glioma model. Gene Ther. 11:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong XG, He S, Yin W, Deng JY and Cheng
B: Selective tropism of liver stem cells to hepatocellular
carcinoma in vivo. World J Gastroenterol. 13:3886–3891. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XC, Wang R, Zhao X, Wei YQ, Hu M,
Wang YS, Zhang XW, Zhang R, Zhang L, Yao B, et al: Prophylaxis
against carcinogenesis in three kinds of unestablished tumor models
via IL12-gene-engineered MSCs. Carcinogenesis. 27:2434–2441. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Yao A, Zhang W, et al: Human
mesenchymal stem cells overexpressing pigment epithelium-derived
factor inhibit hepatocellular carcinoma in nude mice. Oncogene.
29:2784–2794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aquino JB, Bolontrade MF, García MG,
Podhajcer OL and Mazzolini G: Mesenchymal stem cells as therapeutic
tools and gene carriers in liver fibrosis and hepatocellular
carcinoma. Gene Ther. 17:692–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao L, Xu Z, Zhao T, et al: Suppression
of tumorigenesis by human mesenchymal stem cells in a hepatoma
model. Cell Res. 18:500–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH
and Zhang XD: NF-kappaB downregulation may be involved the
depression of tumor cell proliferation mediated by human
mesenchymal stem cells. Acta Pharmacol Sin. 29:333–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campos B, Wan F, Farhadi M, Ernst A,
Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G,
et al: Differentiation therapy exerts antitumor effects on
stem-like glioma cells. Clin Cancer Res. 16:2715–2728. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omar HA, Sargeant AM, Weng JR, Wang D,
Kulp SK, Patel T and Chen CS: Targeting of the Akt-nuclear
factor-kappaB signaling network by
[1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol
(OSU-A9), a novel indole-3-carbinol derivative, in a mouse model of
hepatocellular carcinoma. Mol Pharmacol. 76:957–968. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu LF, Li GP, Su JD, Pu ZJ, Feng JL, Ye YQ
and Wei BL: Involvement of NF-kappaB activation in the apoptosis
induced by extracellular adenosine in human hepatocellular
carcinoma HepG2 cells. Biochem Cell Biol. 88:705–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MO, Moon DO, Kang CH, Kwon TK, Choi YH
and Kim GY: beta-Ionone enhances TRAIL-induced apoptosis in
hepatocellular carcinoma cells through Sp1-dependent upregulation
of DR5 and downregulation of NF-kappaB activity. Mol Cancer Ther.
9:833–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren K, Zhang W, Shi Y and Gong J: Pim-2
activates API-5 to inhibit the apoptosis of hepatocellular
carcinoma cells through NF-kappaB pathway. Pathol Oncol Res.
16:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho HA, Park IS, Kim TW, Oh YK, Yang KS
and Kim JS: Suppression of hepatitis B virus-derived human
hepatocellular carcinoma by NF-kappaB-inducing kinase-specific
siRNA using liver-targeting liposomes. Arch Pharm Res.
32:1077–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fei HR, Chen G, Wang JM and Wang FZ:
Perifosine induces cell cycle arrest and apoptosis in human
hepatocellular carcinoma cell lines by blockade of Akt
phosphorylation. Cytotechnology. 62:449–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buontempo F, Ersahin T, Missiroli S,
Senturk S, Etro D, Ozturk M, Capitani S, Cetin-Atalay R and Neri
ML: Inhibition of Akt signaling in hepatoma cells induces apoptotic
cell death independent of Akt activation status. Invest New Drugs.
29:1303–1313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nair AS, Shishodia S, Ahn KS, Kunnumakkara
AB, Sethi G and Aggarwal BB: Deguelin, an Akt inhibitor, suppresses
IkappaBalpha kinase activation leading to suppression of
NF-kappaB-regulated gene expression, potentiation of apoptosis and
inhibition of cellular invasion. J Immunol. 177:5612–5622. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsao MS, Smith JD, Nelson KG and Grisham
JW: A diploid epithelial cell line from normal adult rat liver with
phenotypic properties of 'oval' cells. Exp Cell Res. 154:38–52.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin HH, Chen JH, Chou FP and Wang CJ:
Protocatechuic acid inhibits cancer cell metastasis involving the
down-regulation of Ras/Akt/NF-kappaB pathway and MMP-2 production
by targeting RhoB activation. Br J Pharmacol. 162:237–254. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park YP, Choi SC, Kim BY, Kim JT, Song EY,
Kang SH, Yoon DY, Paik SG, Kim KD, Kim JW and Lee HG: Induction of
Mac-2BP by nerve growth factor is regulated by the
PI3K/Akt/NF-kappaB-dependent pathway in the HEK293 cell line. BMB
Rep. 41:784–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuki K, Hirohashi S, Sakamoto M, Kanai T
and Shimosato Y: Growth and spread of hepatocellular carcinoma. A
review of 240 consecutive autopsy cases. Cancer. 66:2174–2179.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genda T, Sakamoto M, Ichida T, Asakura H,
Kojiro M, Narumiya S and Hirohashi S: Cell motility mediated by rho
and Rho-associated protein kinase plays a critical role in
intrahepatocytic metastasis of human hepatocellular carcinoma.
Hepatology. 30:1027–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L and Neaves WB: Normal stem cells and
cancer stem cells: The niche matters. Cancer Res. 66:4553–4557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren
N, Jia HL, Shi J, Wu JC, Dai C, et al: Human mesenchymal stem cells
inhibit metastasis of a hepatocellular carcinoma model using the
MHCC97-H cell line. Cancer Sci. 101:2546–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haefner B: NF-kappaB: Arresting a major
culprit in cancer. Drug Discov Today. 7:653–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci U S A.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: Molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wendel HG, De Stanchina E, Fridman JS,
Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J and Lowe SW:
Survival signalling by Akt and eIF4E in oncogenesis and cancer
therapy. Nature. 428:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|
|
41
|
Li Y, Ahmed F, Ali S, Philip PA, Kucuk O
and Sarkar FH: Inactivation of nuclear factor kappaB by soy
isoflavone genistein contributes to increased apoptosis induced by
chemotherapeutic agents in human cancer cells. Cancer Res.
65:6934–6942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Konrath F, Witt J, Sauter T and Kulms D:
Identification of new IκBα complexes by an iterative experimental
and mathematical modeling approach. PLoS Comput Biol.
10:e10035282014. View Article : Google Scholar : PubMed/NCBI
|