Introduction
Interleukin 21 (IL-21), a cytokine identified in
2000 (1), is mainly secreted by
activated CD4+ T cells and NK T cells (2,3). It
effectively enhances T cell proliferation and the killing function
of NK cells, resulting in a strong antitumor immune response
(4,5).
Its receptor (IL-21R) is widely expressed in various cell types
within the immune system, including NK cells, B cells, T cells,
macrophages and dendritic cells (6–8). The
widespread lymphoid distribution of the IL-21R leads to pleiotropic
action of IL-21 in the innate and adaptive immune responses
(6–8).
For this reason, researchers are becoming increasingly interested
in IL-21. Studies have suggested that IL-21 is capable of
suppressing growth in certain tumors, and it has already been used
in phase I or II trials of patients with melanoma and renal cell
carcinoma, with promising antitumor results obtained (9,10).
However, IL-21 may also promote growth in certain other tumors,
including colitis-associated colorectal cancer by impairing tumor
immunosurveillance (11). Therefore,
the function of IL-21 is extremely complex, as its use in different
cancers has been shown to lead to different results (9–11). Further
research is required to investigate this notable cytokine.
Primary hepatic carcinoma (PHC) is currently the
fourth most common cause of cancer-related mortality worldwide
(12). Its incidence is rapidly
increasing (13). To date, there have
been no in-depth studies into the use of IL-21 in PHC. In the
present study, we used adenovirus-mediated IL-21 gene expression in
the mouse hepatic cancer cell line Hepa1–6 to investigate the
influence of IL-21 on antitumor immunity and tumorigenicity in
mice, with the aim of identifying a novel biological treatment for
PHC.
Materials and methods
Materials
Murine hepatocellular carcinoma Hepa1–6 cells and
the murine T-lymphoma YAC-1 cell line sensitive to NK cells were
obtained from the Immunology Laboratory of Weifang Medical
University (Weifang, China). Mouse hepatoma carcinoma cell line
Hepa1–6 cells were maintained in Dulbecco's modified Eagle medium
(DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin solution.
YAC-1 cells were maintained in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS and
1% penicillin/streptomycin solution. The cell counting kit-8
(CCK-8) was purchased from Beyotime Institute of Biotechnology
(Haimen, China).
A replication-defective recombinant Ad5 vector
encoding mouse IL-21 (named Ad5-IL-21-EGFP) and a
replication-defective recombinant Ad5 vector (named Ad5-EGFP) were
purchased from Vector Gene Technology Company Ltd. (Beijing,
China). The physical titer of the recombinant virus (vp/ml) was
5.3×1011, and the infectious titer (TCID50/ml) was
4×1010.
SPF male C57BL/6 mice (6–8 weeks old) were purchased
from the Vital River Laboratory Animal Technology Co. Ltd.
(Beijing, China). The present study was approved by the ethics
committee of Weifang Medical University.
Preparation of spleen mononuclear cells. The mouse
spleen was removed and weighed in a sterile manner. Single spleen
cell suspension was prepared by passing through nylon mesh (200-mm
pore size; BD Biosciences, Baltimore, MD, USA) with a sterile
rubber spatula. Mononuclear cells were obtained by Ficoll-Hypaque
density methods. Briefly, 3–4 ml spleen cell suspension was added
slowly on the surface of the Ficoll along the tube's wall (so as
not to break the interface), then balanced and centrifuged at 2000
× g for 20 min. Mononuclear cells were carefully transferred into
another tube. Following further washes with phosphate-buffered
saline (PBS), the cells were re-suspended in complete RPMI-1640
supplemented with 10% (v/v) heat-inactivated FBS (Gibco; Thermo
Fisher Scientific, Inc.) and antibiotics (100 IU/ml penicillin, 100
mg/ml streptomycin).
Establishment of cell lines
Hepa1–6 cells seeded 24 h earlier were infected with
Ad5-EGFP or Ad5-IL-21-EGFP in serum-free DMEM for 2 h, then the
infection medium was replaced with normal medium. The infection
efficiency was assessed by fluorescence microscopy 24 h later. The
Hepa1–6 cell line infected with Ad5-EGFP was named Ad5-Hepa1–6, and
the Hepa1–6 cell line infected with Ad5-IL-21-EGFP was named
Ad5-IL-21-Hepa1–6.
After cells had been infected with Ad5-IL-21-EGFP or
Ad5-EGFP for 24 h, cell culture supernatant was collected to
measure the IL-21 expression level using the mouse IL-21
enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go kit
(eBioscience, San Diego, CA, USA). Next, total cellular RNA of
certain cells was extracted using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and reverse transcription was
carried out by converting 0.5 µg RNA into complementary DNA (cDNA)
using the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The target gene was amplified by polymerase
chain reaction (PCR) using the GreenTaq™ Ready Mix PCR reaction mix
(Sigma-Aldrich). The following primers were used: forward,
5′-CCGCTAGCCTGGAGACTCAGTTCTG-3′; and reverse,
5′-CCCAAGCTTCTAGGAGAGATGCTGATG-3′. Certain cells were washed with
pre-cooled PBS twice, and lysed in RIPA buffer containing
proteinase inhibitor cocktail on ice. The protein sample was
quantified using a Bio-Rad DC protein assay kit (Bio-Rad
Laboratories, Inc.; catalog no. 500–0114). Total protein samples
were separated by 12% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis prior to electrophoretic transfer to a
nitrocellulose membrane. Western blot analysis was carried out in
pre-cooled transferring buffer at 100 mA for 1 h. The
nitrocellulose membrane was then blocked in 5% milk in
Tris-buffered saline with 0.05% Tween-20 solution. The membrane was
then incubated with rat anti-mouse IL-21 mAbs (Peprotech 500-p278)
at 4°C overnight. After washing three times with PBS with 0.05%
Tween-20 (PBST), the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rat antibody for 2 h at room
temperature. The membrane was then washed five times with PBST and
developed using a chemiluminescence detection kit (Sangon Biotech,
Co., Ltd., Shanghai, China). The target protein bands were detected
following exposure of the membranes to X-ray film and β-actin was
used as an internal control.
Establishment of tumor model
For the subcutaneous Hepa1–6 tumor model,
Ad5-Hepa1–6, Ad5-IL-21-Hepa1–6 and Hepa1–6 wild type (WT) were
washed with sterilized PBS twice, then the cell density was
adjusted to 1×107/ml. Cell suspension (100 µl) was
inoculated intradermally into the right flank of each mouse. Each
group contained four mice, and the studies were repeated three
times. Four weeks later, tumors were removed and weighed, and the
tumor length and width were measured with calipers after the mice
had been sacrificed in a humane manner. The tumor volume (in
mm3) was estimated using the standard formula: tumor
volume (mm3) = length × width2x 0.5. Mouse
serums were separated and stored at −70°C for cytokine detection.
Mouse tumor tissues were weighed following sacrifice and fixed in
formaldehyde for immunohistochemistry. The mouse spleen and body
were weighed in order to estimate the spleen index using the
standard formula: spleen index = spleen weight (mg) / mouse weight
(g).
Assessment of IL-21, IL-4 and
interferon (IFN)-γlevels by immunohistochemistry
IL-21, IL-4 and IFN-γ levels in mouse serum were
detected using the ELISA Ready-SET-Go kit as described previously.
Proteins expressed in the mouse tumor tissues were detected by
immunohistochemistry, which was performed on serial 4-µm-thick
paraffin sections. The slides were deparaffinized in xylene and
re-hydrated through four decreasing grades of ethanol (100, 95, 80
and 70%) for 2 min each. Endogenous peroxidase activity was blocked
by immersing the slides in 3% hydrogen peroxide in methanol for 15
min at room temperature. Heat-induced antigen retrieval was
performed for 5 min with 1X citrate buffer (pH 6.3) in a microwave
and then cooled for 5 min. This process was performed three times.
To reduce the nonspecific binding of antibodies to the tissues, the
slides were pre-incubated with blocking serum in 1% bovine serum
albumin for 30 min at room temperature. They were left to incubate
with rabbit polyclonal anti–IL21, IL-4 and IFN-γ (antibody
dilution, 1:200), respectively, overnight at 4°C. Following
incubation with the primary antibodies, the slides were rinsed with
PBS for 10 min. Chromogen was then for the detection of the
antibody reactions. The color was developed using diaminobenzidine.
Finally, the sections were counterstained with Mayer's hematoxylin
solution for 1 min, and dehydrated with graded alcohols, dipped in
two changes of xylene, and mounted.
T cell proliferation assay
The proliferation of T lymphocytes was observed
using CCK-8 assay. Briefly, the spleen mononuclear cells were
adjusted to a final concentration of 1×107 cells/ml in
complete RPMI-1640 medium. The cell suspensions were added to
96-well plates (100 µl/well) in triplicate, and another medium (100
µl/well) containing 40 mg/ml concanavalin A (Sigma-Aldrich) was
added. Medium was also added to another triplicated well as a
control. Following incubation for 72 h, 20 µl CCK-8 was added to
each well and the plate was incubated for an additional 2 h at
37°C. The absorbance at 450 nm of each aliquot was determined using
a microplate reader. The stimulation index was calculated as a
percentage of the absorbance in treated wells relative to the
absorbance in untreated (control) wells.
T lymphocyte and NK cell killing
ability assay
Effector cells generated from spleen mononuclear
cells from Ad5-IL-21-Hepa1–6, Ad5-Hepa1–6 and Hepa1–6 mice were
mixed with the target cell Hepa1–6 (for T lymphocyte killing assay)
or YAC-1 (for NK cell killing assay) at a ratio of 20:1 in
triplicated wells and incubated for 4 h in 96-well plates. The
target cell control and effector cell control were plated in
triplicate. Cells were incubated for 3 h after CCK-8 reagent was
added to the mixture (20 µl/well). Finally, the optical density
(OD) value of each well was measured by the microplate reader at a
wavelength of 450 nm. The cytotoxic activity of the effector cells
was determined using the standard formula: killing rate (%) = 1 −
(OD value of experimental well − OD value of effector cell control)
/ OD value of target cell control. The killing rates among the
three groups were compared.
Statistical analysis
All the data were analyzed with SPSS 11.5 (SPSS,
Inc., Chicago, IL, USA). Statistical comparisons were performed by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference between data.
Results
Establishment of cell lines
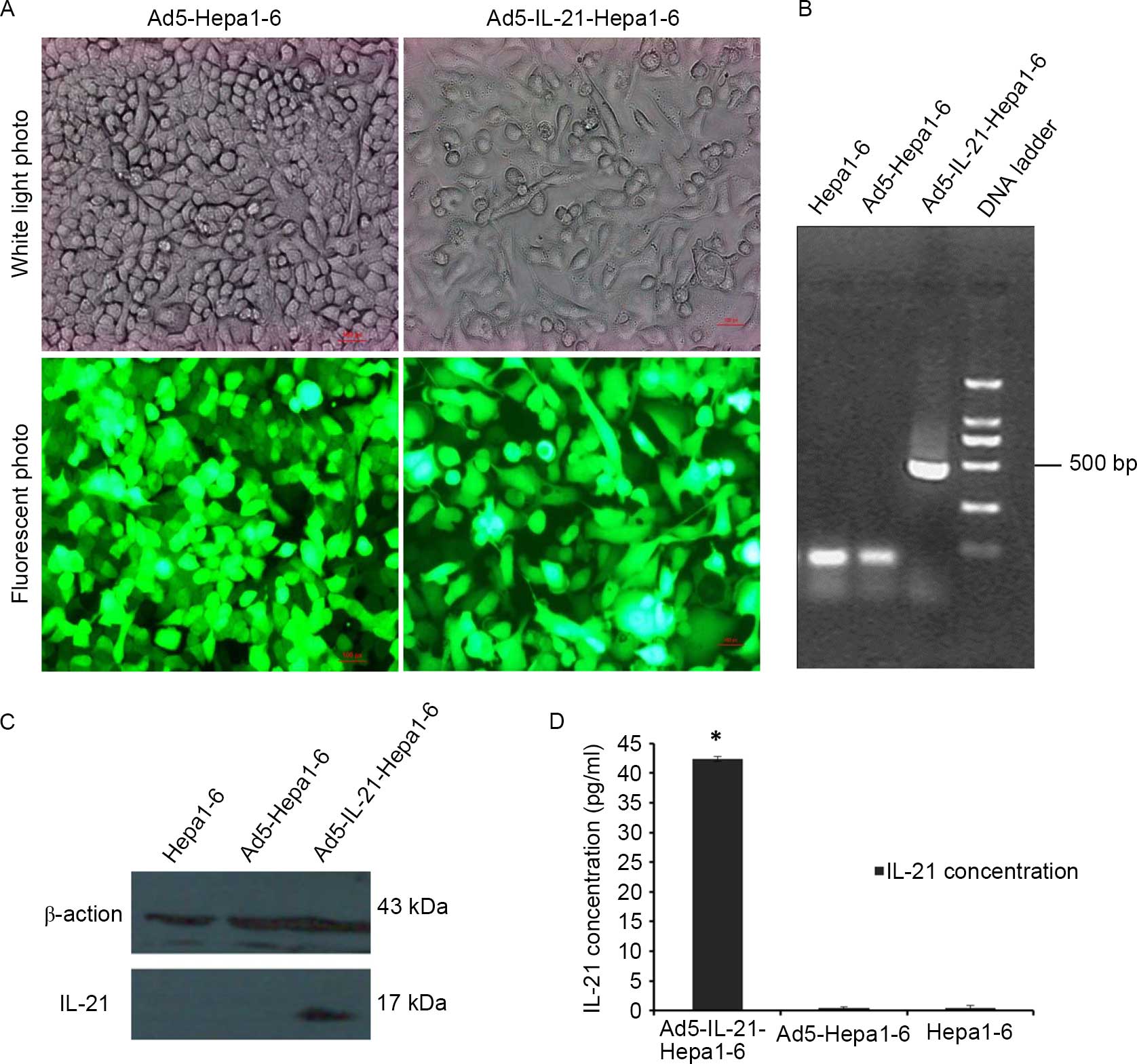
The expression of EGFP reporter genes was analyzed
24 h after infection. Fluorescence microscopy analysis confirmed
EGFP expression in cells infected with Ad5-EGFP and Ad5-IL-21-EGFP
(Fig. 1A). Reverse
transcription-polymerase chain reaction confirmed the expression of
IL-21 mediated by Ad5-IL-21-EGFP at the RNA level (Fig. 1B). Significant amounts of IL-21
expression were observed in the Ad5-IL-21-Hepa1–6 cells.
Ad5-Hepa1–6 and Hepa1–6 cells were not amplified. Western blot
analysis revealed IL-21 expression in Ad5-IL-21-Hepa1–6 cells
(Fig. 1C), but no band was detected
in Ad5-Hepa1–6 and Hepa1–6 cells. IL-21 expression in the cell
culture supernatant of Hepa1–6 infected with Ad5-IL21-EGFP was
detected by the ELISA method (Fig.
1D).
IL-21 significantly reduces Hepa1–6
tumorigenicity
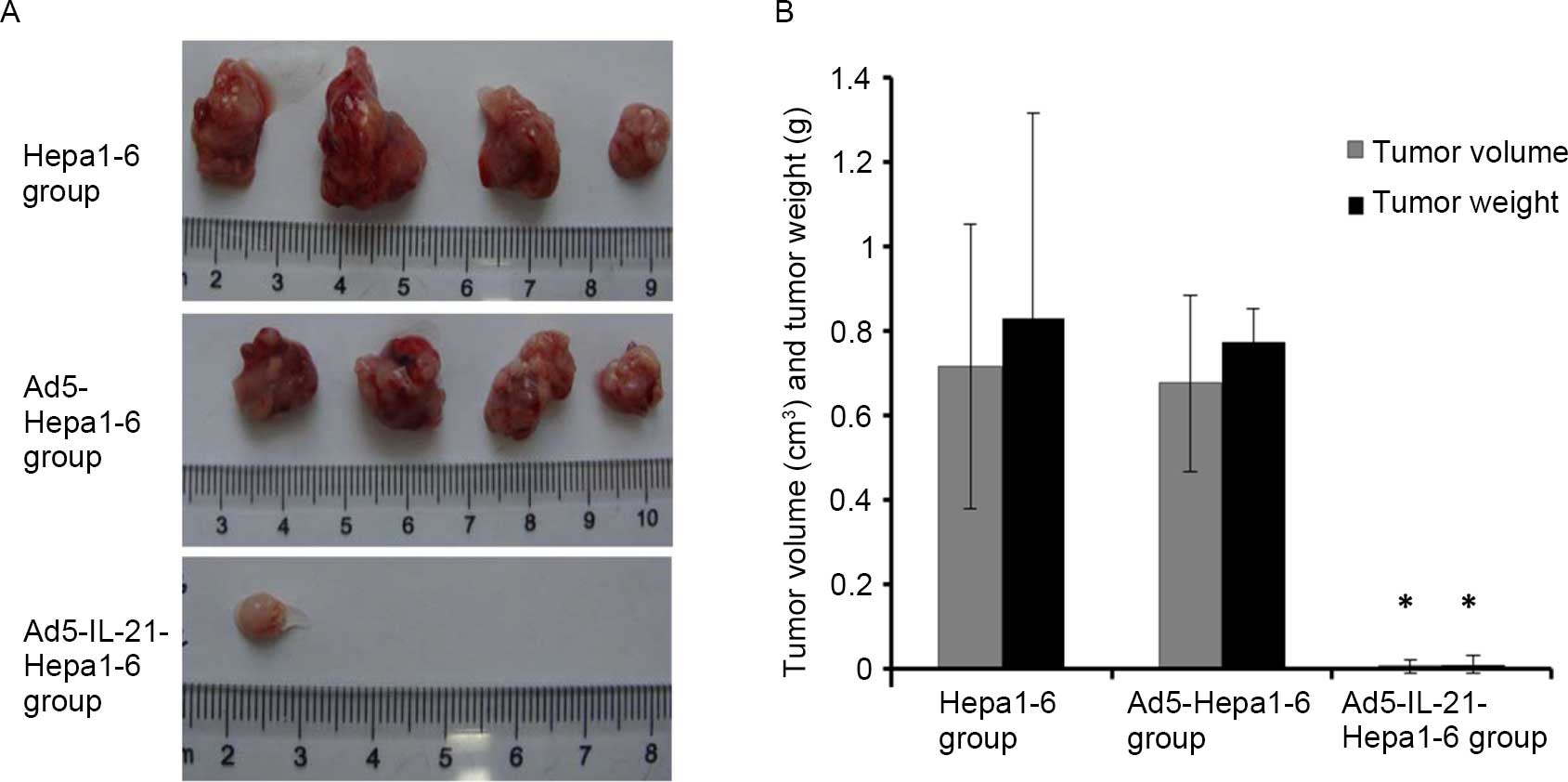
The subcutaneous tumor mouse model revealed that
IL-21 significantly reduced the tumorigenicity of Hepa1–6. In the
Ad5-IL-21-Hepa1–6 group, only one of the four mice grew an
extremely small tumor, while in the other two groups all four mice
grew larger tumors. The tumor volumes of the Ad5-IL-21-Hepa1–6,
Ad5-Hepa1–6 and Hepa1–6 WT group mice were 0.008±0.015
cm3, 0.677±0.208 cm3 and 0.716±0.335
cm3, respectively, and the tumor weights were
0.011±0.022 g, 0.772±0.080 g and 0.828±0.486 g, respectively
(Fig. 2A and B). The tumor volume and
weight in the IL-21-Hepa1–6 group were much smaller than those in
the other two groups, and the difference was significant
(P<0.01).
IL-21 enhances antitumor immunity in
tumor-bearing mice
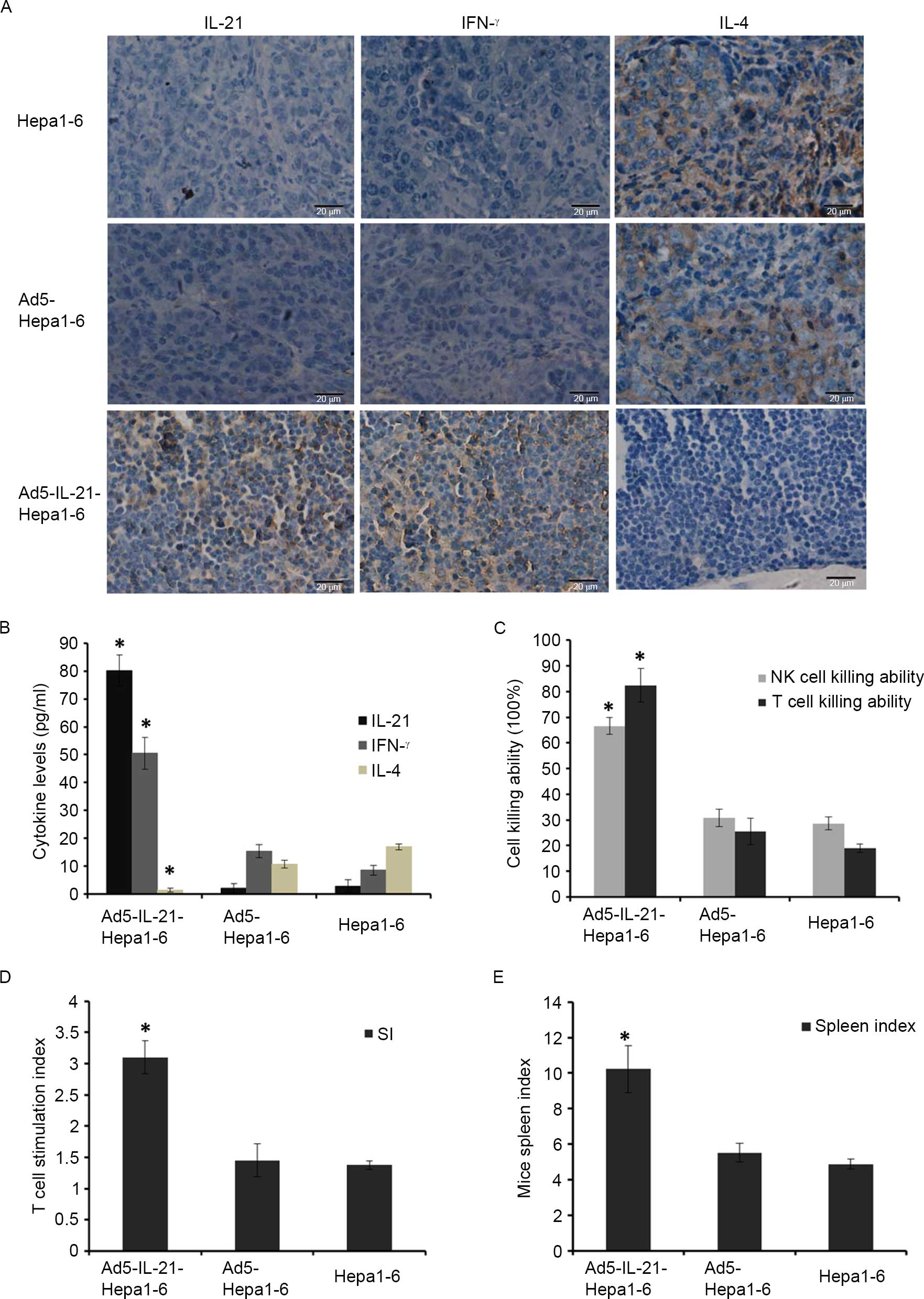
Immunohistochemistry confirmed that IL-21 and IFN-γ
levels were much higher in Ad5-IL-21-Hepa1–6 mouse tumor tissues
than in the other two groups, but IL-4 levels were much lower in
Ad5-IL-21-Hepa1–6 mouse tumor tissues than the other two groups
(Fig. 3A). Similar results were
observed in the ELISA assay (Fig.
3B), which confirmed the cytokine IL-21 levels in
Ad5-IL-21-Hepa1–6 mice serum to be 80.092±5.560 ng/ml; much higher
than those of the Ad5-Hepa1–6 group and Hepa1–6 WT groups. IFN-γ
levels were 50.464±5.679 ng/ml, much higher than those of the
Ad5-Hepa1–6 and Hepa1–6 WT groups, which were 15.49±2.290 ng/ml and
8.58±1.678 ng/ml, respectively. IL-4 levels in Ad5-IL-21-Hepa1–6
mouse serum were 1.41 ng/ml±0.692, much lower than those in the
Ad5-Hepa1–6 and Hepa1–6 WT groups, which were 10.843±1.398 ng/ml
and 16.998±1.046 ng/ml, respectively.
The spleen cell killing assay revealed the killing
ability of NK and T cells in the Ad5-IL-21-Hepa1–6 mice to be
66.61±3.230 and 82.46±6.531%, respectively, which was much higher
than in the Ad5-Hepa1–6 and Hepa1–6 WT groups (Fig. 3C). T cell proliferation assay revealed
that the stimulation index of spleen T cells in Ad5-IL-21-Hepa1–6
mice was 3.1±0.261, which was much higher than that of the
Ad5-Hepa1–6 and Hepa1–6 WT groups, which were 1.45±0.266 and
1.37±0.072, respectively (Fig. 3D).
The spleen index of Ad5-IL-21-Hepa1–6 mice was 10.22±1.329, which
was much higher than that of the Ad5-Hepa1–6 and Hepa1–6 WT groups,
which were 5.5±0.519 and 4.8±0.271, respectively (Fig. 3E).
Discussion
As a promising cytokine for cancer immunotherapy,
whilst IL-21 suppresses growth in certain tumors, including renal
cell carcinoma and melanoma (9,14), it may
promote growth in others, including colitis-associated colorectal
cancer and follicular lymphoma (11,15). The
function of IL-21 is complex, as it appears to have different
functions in different tumors.
PHC is currently the fourth-leading cause of
cancer-related mortality (12), and
its incidence is rapidly increasing (13). In a previous study, Pan et al
(16) used the naked plasmids pmIL-21
and/or psPD-1 for local gene transfer by injection into
experimental H22 murine hepatocarcinoma. The immunotherapy with
IL-21 in combination with sPD-1 was observed to induce antitumor
immune response. Cheng et al (17) injected recombinant plasmid capable of
co-expressing GM-SCF, IL-21 and Rae-1 into a H22 cell-bearing
mouse, and the recombinant expression plasmid inhibited liver
cancer by a mechanism that involved activation of cell-mediated
immunity in liver cancer. However, the efficiency of gene
expression by naked plasmid injection was limited, and the
therapeutic effect requires improvement. At present, no other study
has investigated whether IL-21 is able to prevent liver cancer, and
there is no available vaccine, so further study is required in this
field. In the present study, we used adenovirus-mediated
transfection of the IL-21 gene in the hepatic cancer cell line
Hepa1–6 and investigated the effect of IL-21 on the tumorigenicity
of Hepa1–6 and the influence of IL-21 on antitumor immunity in a
mouse model.
Using the subcutaneous liver tumor model, we first
revealed that IL-21 gene expression in the Hepa1–6 cell line
significantly decreases the tumorigenicity of Hepa1–6. In the
Ad5-IL-21-Hepa1–6 group, only one of the four mice grew a small
tumor, and the other three mice did not grow any tumors, while in
the Ad5-Hepa1–6 and Hepa1–6 groups, all the mice grew tumors, and
the tumor weights and volumes were much larger than in the
Ad5-IL-21-Hepa1–6 group. We speculated that this was due to the
infection and expression efficiency of IL-21 mediated by Ad5
compared with naked plasmid injection, as well as the effective
strategy we used in our study. This is positive news for vaccine
development.
We also observed that the antitumor immune response
was significantly enhanced in Ad5-IL-21-Hepa1–6 mice, which is
consistent with the results of Pan et al (16) and Cheng et al (17), but the antitumor effect is much
better, due to the infection and expression efficiency of IL-21
mediated by Ad5 compared with naked plasmid injection. The killing
ability of NK and T cells in mouse spleen was significantly
enhanced. We also detected high IFN-γ and low IL-4 levels in the
serum and tumor tissue in the Ad5-IL-21-Hepa1–6 group. Since IFN-γ
is a typical Th1 cytokine and IL-4 is a typical Th2 cytokine, the
results of the present study indicated that IL-21 promoted the
immune response shift to a Th1 response in mice, which is superior
to the Th2 response for antitumor reactions. These data explain why
IL-21 reduced the tumorigenicity of Hepa1–6. Our study is likely to
lay a strong foundation for future biological treatments of liver
cancer and vaccine development.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81373185 and 30901779), the
Natural Science Foundation of Shandong, China (ZR2009CM019),
Shandong Province Department of Education Foundation of China
(J10LF62), Shandong Province Health Department (2013WS0287 and
2014WS0462), and funding from the International Cooperation Program
for Excellent Lecturers of 2012 by Shandong Provincial Education
Department, China.
References
|
1
|
Parrish-Novak J, Dillon SR, Nelson A,
Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West
J, et al: Interleukin 21 and its receptor are involved in NK cell
expansion and regulation of lymphocyte function. Nature. 408:57–63.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bryant VL, Ma CS, Avery DT, Li Y, Good KL,
Corcoran LM, de Waal Malefyt R and Tangye SG: Cytokine-mediated
regulation of human B cell differentiation into Ig-secreting cells:
predominant role of IL-21 produced by CXCR5+T follicular helper
cells. J Immunol. 179:8180–8190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coquet JM, Kyparissoudis K, Pellicci DG,
Besra G, Berzins SP, Smyth MJ and Godfrey DI: IL-21 is produced by
NKT cells and modulates NKT cell activation and cytokine
production. J Immunol. 178:2827–2834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ertelt JM, Johanns TM, Rowe JH and Way SS:
Interleukin (IL)-21-independent pathogen-specific CD8+
T-cell expansion, and IL-21-dependent suppression of
CD4+ T-cell IL-17 production. Immunology. 131:183–191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Yang L, Cui Y, Wang X, Guo C, Huang
Z, Kan Q, Liu Z and Liu Y: Il-21 enhances NK cell activation and
cytolytic activity and induces Th17 cell differentiation in
inflammatory bowel disease. Inflamm Bowel Dis. 15:1133–1144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruffin N, Lantto R, Pensieroso S,
Sammicheli S, Hejdeman B, Rethi B and Chiodi F: Immune activation
and increased IL-21R expression are associated with the loss of
memory B cells during HIV-1 infection. J Intern Med. 272:492–503.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Dienz O, Roberts B, Moussawi M,
Rincon M and Huber SA: IL-21R expression on CD8+ T cells
promotes CD8+ T cell activation in coxsackievirus B3
induced myocarditis. Exp Mol Pathol. 92:327–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamming OJ, Kang L, Svensson A, Karlsen
JL, Rahbek-Nielsen H, Paludan SR, Hjorth SA, Bondensgaard K and
Hartmann R: Crystal structure of interleukin-21 receptor (IL-21R)
bound to IL-21 reveals that sugar chain interacting with WSXWS
motif is integral part of IL-21R. J Biol Chem. 287:9454–9460. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashmi MH and Van Veldhuizen PJ:
Interleukin-21: updated review of Phase I and II clinical trials in
metastatic renal cell carcinoma, metastatic melanoma and
relapsed/refractory indolent non-Hodgkin's lymphoma. Expert Opin
Biol Ther. 10:807–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson JA, Curti BD, Redman BG, Bhatia
S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA and
Hausman DF: Phase I study of recombinant interleukin-21 in patients
with metastatic melanoma and renal cell carcinoma. J Clin Oncol.
26:2034–2039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kesselring R, Jauch D and Fichtner-Feigl
S: Interleukin 21 impairs tumor immunosurveillance of
colitis-associated colorectal cancer. Oncoimmunology. 1:537–538.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao FF, Yu S, Jiang ZY and Bao YX:
Diagnostic accuracy of Golgi protein 73 in primary hepatic
carcinoma using ELISA: a systematic review and meta-analysis. Clin
Lab. 60:587–597. 2014.PubMed/NCBI
|
|
13
|
Dongiovanni P, Romeo S and Valenti L:
Hepatocellular carcinoma in nonalcoholic fatty liver: role of
environmental and genetic factors. World J Gastroenterol.
20:12945–12955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grünwald V, Desar IM, Haanen J, Fiedler W,
Mouritzen U, Olsen MW and van Herpen CM: A phase I study of
recombinant human interleukin-21 (rIL-21) in combination with
sunitinib in patients with metastatic renal cell carcinoma (RCC).
Acta Oncol. 50:121–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wood B, Sikdar S, Choi SJ, Virk S,
Alhejaily A, Baetz T and LeBrun DP: Abundant expression of
interleukin-21 receptor in follicular lymphoma cells is associated
with more aggressive disease. Leuk Lymphoma. 54:1212–1220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan XC, Li L, Mao JJ, Yao W, Zheng JN, Liu
M and Fu JJ: Synergistic effects of soluble PD-1 and IL-21 on
antitumor immunity against H22 murine hepatocellular carcinoma.
Oncol Lett. 5:90–96. 2013.PubMed/NCBI
|
|
17
|
Cheng M, Zhi K, Gao X, He B, Li Y, Han J,
Zhang Z and Wu Y: Activation of cellular immunity and marked
inhibition of liver cancer in a mouse model following gene therapy
and tumor expression of GM-SCF, IL-21, and Rae-1. Mol Cancer.
12:1662013. View Article : Google Scholar : PubMed/NCBI
|