Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
fifth most common cancer worldwide (1). Despite diagnostic advances and
improvements in treatment modalities, the 5-year survival rate of
such patients remains poor (2). HNSCC
represents a wide spectrum of neoplasms with different patterns of
biological behaviour (3) therefore,
the current tumor classification is limited in its prediction of
prognosis. Consumption of alcohol or tobacco is the primary risk
factor for cancer of the oral cavity, larynx, oropharynx and
hypopharynx, and accounts for 75% of HNSCCs (4). HNSCC usually presents with symptoms from
the primary site; sore throat, hoarseness, difficulty in breathing
and swallowing or ear pain (5).
Enlargement of a cervical lymph node as the first presenting
feature is not uncommon, particularly within certain ‘silent’
sites, including the tongue base, supraglottis and nasopharynx
(5). Systemic metastases are uncommon
at first presentation of HNSCC (6).
The type of treatment required depends on the location of the
primary tumor, the stage of the disease and the expected
oncological/functional outcomes (4).
Early stage HNSCC (stage I/II) is usually treated
with single-modality therapy (surgery or radiotherapy) while the
management of locally advanced disease (stage III/IV) generally
requires various combinations of treatment modalities (surgery,
radiotherapy, chemotherapy or cetuximab). The 5-year survival rate
for all patients with HNSCC is 40–60% (7).
Nuclear receptors are transcription factors that
modulate cell proliferation, apoptosis and migration (8). Recent data suggests that there is an
association between the intranuclear overexpression of estrogen
receptor (ER) β and higher survival rates in patients with lung
(9,10), prostate (11), breast (12) and colon cancer (13). A number of studies have demonstrated
that nuclear receptors are expressed in head and neck cancer
(14–20). However, the clinical significance of
sex hormone receptor expression in HNSCC remains unclear. The aim
of the present study was to assess ERβ expression in HNSCC and
investigate its association with clinical and histopathological
factors, disease recurrence and patient survival. To the best of
our knowledge this is the largest study examining the role of ERβ
in HNSCC.
Patients and methods
Patients
A total of 174 patients with squamous cell carcinoma
of oral cavity, larynx and hypopharynx were included in the present
study. The patients all underwent primary surgical treatment
between January 2000 and December 2006 at the Division of Head and
Neck Surgery, Department of Surgical Oncology, University Hospital
for Tumors (Zagreb, Croatia). The inclusion criteria for the
current retrospective study were as follows: No prior treatment for
head and neck cancer and a patient follow-up period of ≥5 years.
Cases were classified based on the international
tumor-node-metastasis (TNM) classification from 2002 (21). Patients with adverse histopathological
features (extracapsular spread, multiple positive lymph nodes,
stage pT3 or pT4) underwent postoperative (adjuvant) irradiation.
Informed consent was obtained from each patient and the study was
carried out with the approval of the Ethical Committee of the
Clinical Hospital Center Sisters of Charity.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue samples
from 174 primary head and neck squamous cell carcinomas were
obtained from the Department of Pathology. Representative 3 µm
sections of tumour tissue were cut, dewaxed in xylene and
rehydrated in graded ethanol and destilled water. Antigen retrieval
was performed in Tris/Ethylenediaminetetraacetic acid (EDTA), pH
9.0 buffer (Dako Target retrieval solution; catalogue number S2367;
Dako, Glostrup, Denmark) for 1 h at 98°C in a water bath.
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide for 5 min. Sections were washed with the buffer and
subsequently incubated with mouse monoclonal anti-ERβ antibody
(Product code: NCL-ER-β Clone EMR02, dilution, 1:40, Novocastra,
Laboratories, Ltd., Newcastle upon Tyne, UK) for 45 min at room
temperature. Bound primary antibody was detected using biotinylated
anti-mouse secondary antibody (EnVision FLEX, High pH Kit,
catalogue number 8010; Dako, ready for use) for 45 min and
visualized with diaminobenzidine as chromogen on Autostainer Link
48 (Dako). Slides were counterstained with hematoxilyn, dehydrated,
cleared and cover-slipped. A breast cancer tissue sample was used
as a positive control.
Stained tissue sections were evaluated by an
experienced pathologist. Nuclear staining was considered positive
and >100 cells were counted. Separate scores were assigned to
each sample according to the percentage of positive nuclei observed
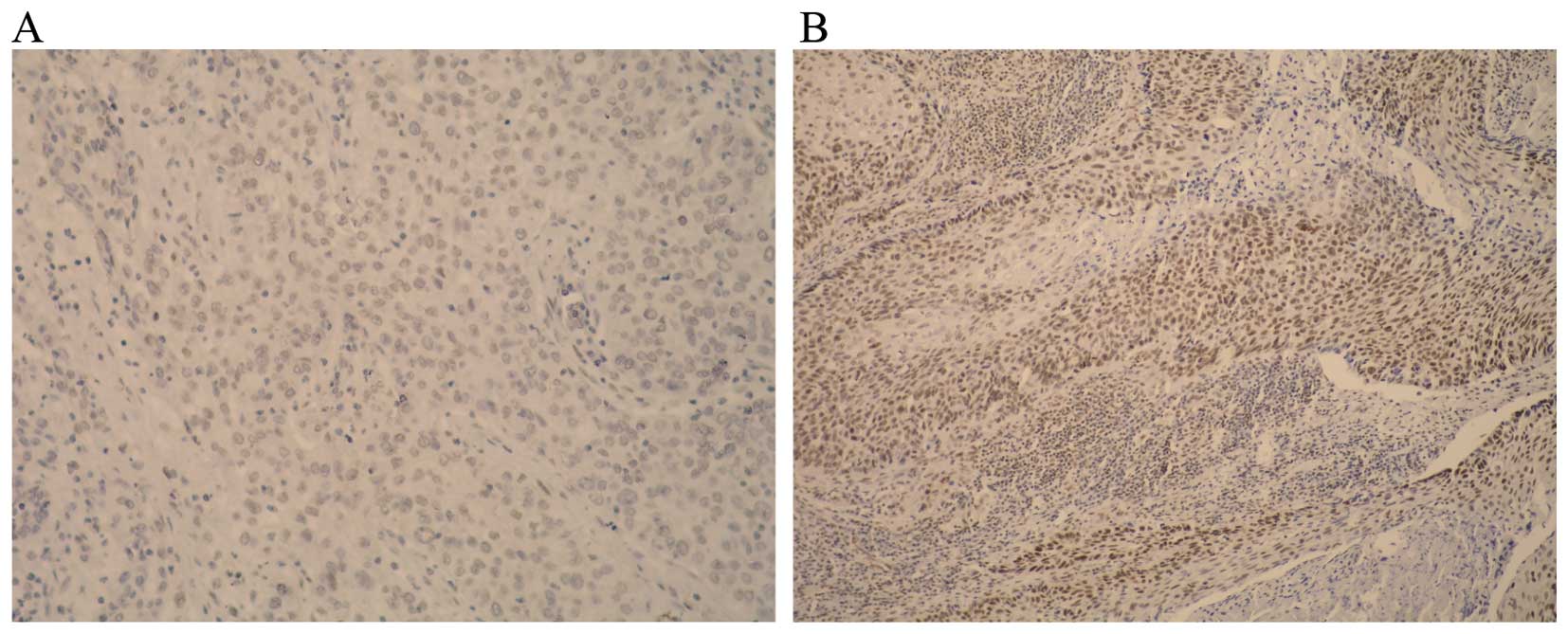
(0–100%). The following scoring system was used: 0, no staining of
tumour cells (Fig. 1A); 1, positive
staining <10% of tumour cells; 2, positive staining in 11–50% of
tumour cells; 3, positive staining in >51% tumour cells
(Fig. 1B). For the purpose of
statistical analysis, cases with scores of 0 and 1 were considered
negative and cases with scores of 2 or 3 were considered positive
(14).
Statistical analysis
Normality of data distribution was assessed with
Smirnov-Kolmogorov test and due to the results obtained,
appropriate non-parametric tests were used in analyses. Differences
between ER groups were analyzed with the χ2 test
(categorical values) and Mann-Whitney U test (quantitative values).
Spearman correlation coefficients were used to analyze associations
between total survival time and other clinical variables for each
tumor location. Overall survival (OS) was calculated using the
Kaplan-Meier method, while the log-rank test tested the differences
between the actuarial curves. OS was calculated from the time of
surgery to death resulting from all causes. IBM SPSS Statistics
ver. 21 (Armonk, NY, USA) was used in all statistical tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The present study included 165 male and 9 female
patients with a median age of 60.8 years, (range, 39–79), of which
80 (46%) exhibited no evidence of cervical lymph node metastasis,
while 94 (54%) had neck metastases. The clinicopathological
characteristics of the study group are listed in Table I.
 | Table I.Clinical and histopathological
characteristics of the cohort. |
Table I.
Clinical and histopathological
characteristics of the cohort.
| Characteristic | No. patients | % |
|---|
| Age, years |
|
|
|
Median | 60.78 |
|
|
Range | 39–79 |
|
| Gender |
|
|
|
Female | 9 | 5.2 |
| Male | 165 | 94.8 |
| Tumor site |
|
|
| Oral
cavity | 37 | 21.3 |
|
Oropharynx | 33 | 18.9 |
|
Larynx | 63 | 36.2 |
|
Hypopharynx | 41 | 23.6 |
| pT stage |
|
|
| T1 | 33 | 19 |
| T2 | 80 | 46 |
| T3 | 41 | 23.5 |
| T4 | 20 | 11.5 |
| pN stage |
|
|
| N0 | 80 | 46 |
| N1 | 22 | 12.3 |
| N2 | 64 | 36.8 |
| N3 | 8 | 4.6 |
| Differentiation |
|
|
| Well | 19 | 10.9 |
|
Moderate | 112 | 64.4 |
| Poor | 43 | 24.7 |
| Adjuvant
radiotherapy |
|
|
| No | 43 | 24.7 |
| Yes | 131 | 75.3 |
| Survival |
|
|
| AwD | 70 | 40.2 |
| StD | 54 | 31.1 |
| StC | 50 | 28.7 |
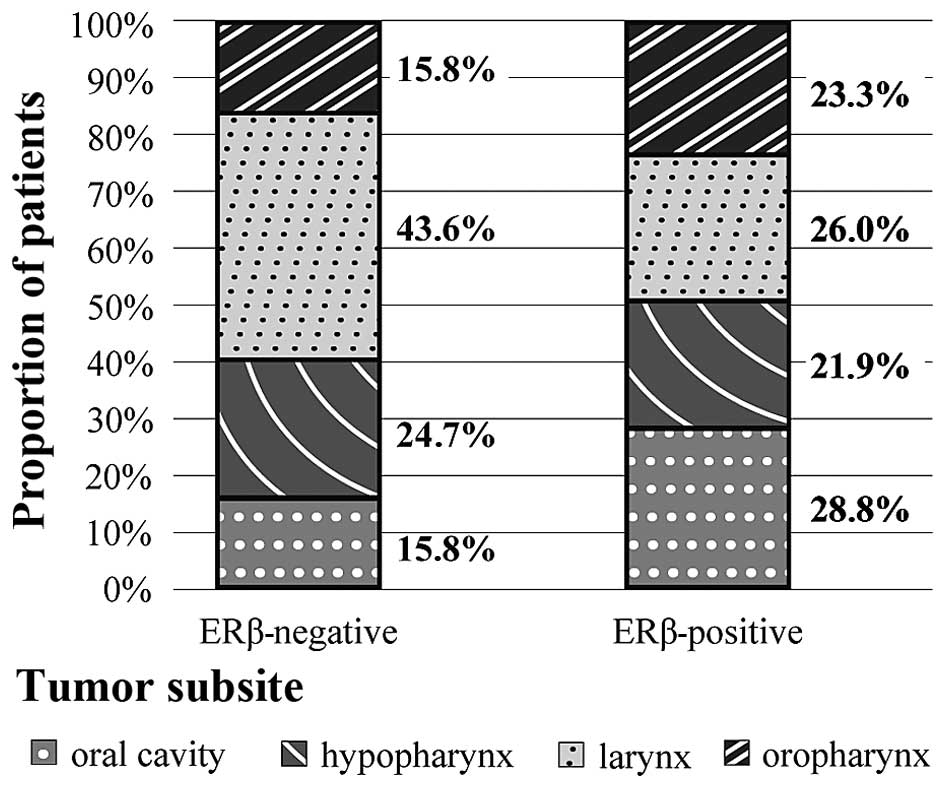
Immunohistochemistry for ERβ indicated positive
staining in 73 patients (42%). Distribution of ERβ status among
different head and neck subsites is listed in Fig. 2. A higher proportion of the ERβ
negative cases (>40%) were located in laryngeal primaries
compared with other areas, whereas incidence of other
sublocalization within ERβ positive cases were similar (Fig. 2). This association was statistically
significant (P=0.04; Table II).
 | Table II.Comparison of the clinicopathological
features with respect to ERβ status. |
Table II.
Comparison of the clinicopathological
features with respect to ERβ status.
| Clinical
parameter | ER β+ No. patients
(%) | ER β- No. patients
(%) | P-value |
|---|
| Age, years |
|
|
|
≤61 | 38 (52.1) | 53 (52.5) | 0.956 |
|
>61 | 35 (47.9) | 48 (47.5) |
|
| Gender |
|
|
|
Male | 69 (94.5) | 96 (95.0) | 0.876 |
|
Female | 4 (5.5) | 5 (5.0) |
|
| Tumor site |
|
|
| Oral
cavity | 21 (28.8) | 16 (15.8) | 0.040a |
|
Oropharynx | 17 (23.3) | 16 (15.8) |
|
|
Larynx | 19 (26.0) | 44 (43.6) |
|
|
Hypopharynx | 16 (21.9) | 25 (24.8) |
|
| pT stage |
|
|
|
T1/T2 | 40 (54.8) | 73 (72.3) | 0.448 |
|
T3/T4 | 33 (45.2) | 28 (27.7) |
|
| pN stage |
|
|
| N0 | 31 (42.5) | 49 (48.5) | 0.429 |
| N+ | 42 (57.5) | 52 (51.5) |
|
| pTNM stage |
|
|
| Stage
I/II | 21 (28.8) | 34 (33.7) | 0.493 |
| Stage
III/IV | 52 (71.2) | 67 (66.3) |
|
|
Differentiation |
|
|
|
Well | 7 (9.6) | 12 (11.9) | 0.261 |
|
Moderate | 52 (71.2) | 60 (59.4) |
|
|
Poor | 14 (19.2) | 29 (28.7) |
|
The univariate correlations between
clinicopathological features and ERβ status are presented in
Table II. Positive ERβ status was
not significantly correlated with any clinicopathohistological
parameter other than the survival of patients with respect to the
primary tumor site.
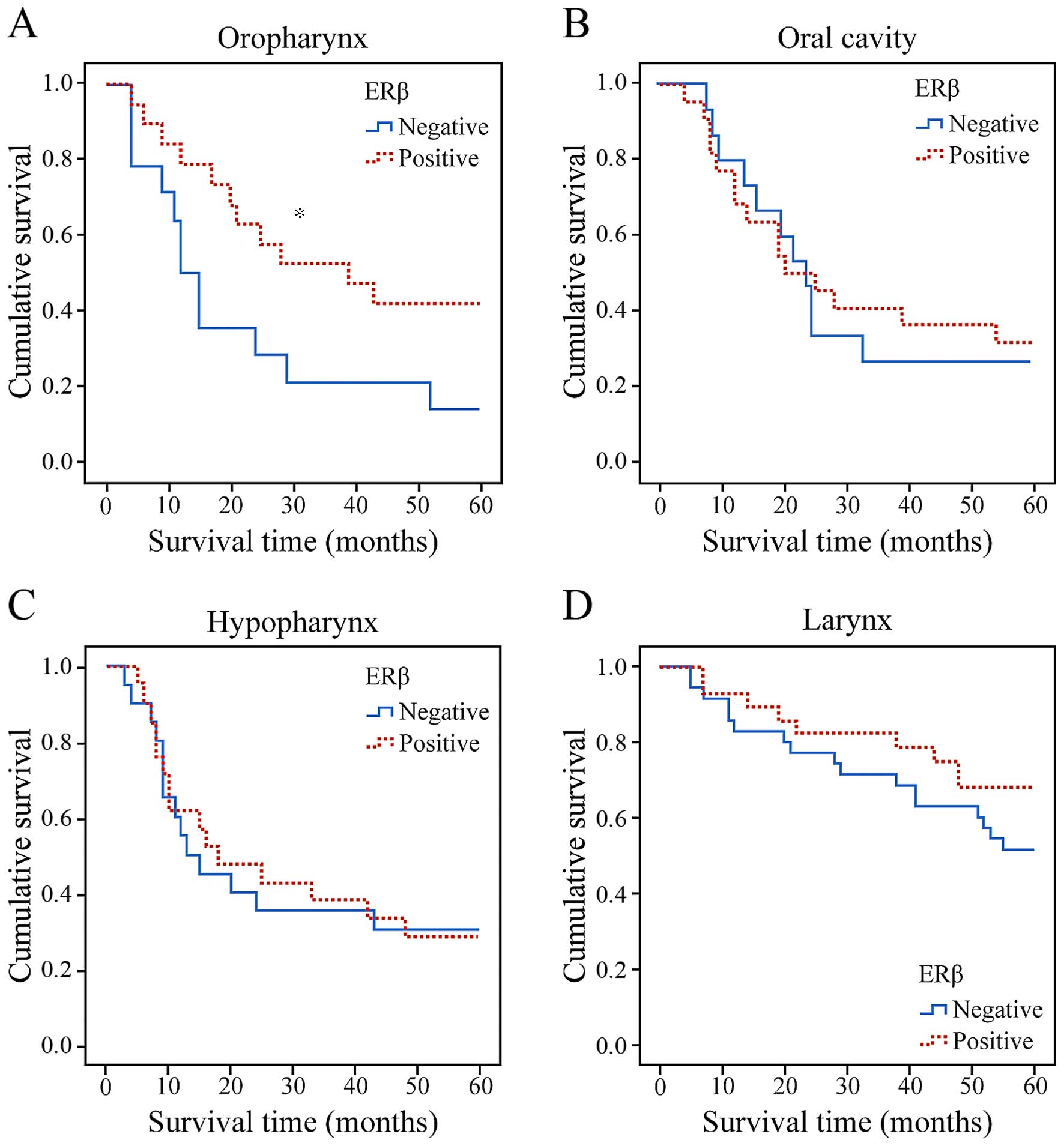
Patients with oropharyngeal cancer with specimens
that demonstrated an ERβ positive intranuclear reaction had an OS
rate of 35.3% at 5 years compared with 25% for patients with
negative reaction, a statistically significant difference (log-rank
test, P=0.045) (Fig. 3A). By
contrast, there was no significant association between ERβ
expression and other primary tumor subsites with respect to
survival (Fig. 3B-D). By the end of
the study, 54 (31.1%) patients had died of disease recurrence while
50 (28.7%) had died of other causes. Follow-up information was
available for all patients and average follow-up time was 38.5
months (range, 3–60 months).
Discussion
A previous report has suggested that ER-mediated
signals and pathways serve a critical role in malignant tumor
growth, progression and metastasis (8). The most comprehensive analysis was
performed on patients with breast cancer where positive expression
of ERβ was associated with increased response rates to ER
antagonist therapy with tamoxifen (12,22).
However, the role of ER signaling in other malignancies remains
unclear. Similarily, the significance of ER expression in HNSCC and
its biological role in cell invasion, proliferation, metastasis and
survival is controversial.
In the head and neck region, ER expression was
initially evaluated in laryngeal cancer. This type of cancer is 11
times more common in males than females, despite similar rates of
alcohol abuse and smoking in both genders (15,23).
Previous studies have demonstrated that 23–79% of laryngeal cancer
cases express ERs (16–18,24),
concluding that positive ER expression is gender independent with a
positive prognostic influence on survival. However, other authors
have not been able to identify ER expression in laryngeal cancer
(25,26), therefore laryngeal carcinogenesis may
be a hormonally independant process. Non-identification of ER in
epithelial components of the normal larynges and laryngeal
carcinomas has been confirmed (27).
A previous study examined the role of ERβ in
laryngeal cancer and demonstrated that its expression was
positively correlated with the maintenance of E-cadherin and
β-catenin at cell junctions and negatively with increased TNM stage
(19). This suggests that ERβ serves
a protective role in the pathogenesis of this malignancy.
A study by Nozoe et al (28) demonstrated that positive expression of
ERα in combination with negative expression of ERβ proved to be an
unfavorable prognostic indicator with respect to the survival of
patients with esophageal squamous cell carcinoma (SCC). Wang et
al (29) have reported a
correlation between positive ERβ expression and lower malignant
potential in oesophageal SCC. However, Dong et al (30) reported that ERα and ERβ levels were
inversely correlated and that downregulation of ERα and
upregulation of ERβ expression may indicate poor prognosis in
patients with oesophageal SCC.
Few studies have investigated the role of ER
expression in HNSCC. Lukits et al (14) performed a study on 67 patients with
carcinoma of the larynx, oral cavity and hypopharynx. ERα and ERβ
expressions were identified in 50% of patients and had no
significant influence on patient prognosis.
Egloff et al (20) demonstrated that treating HNSCC cells
with estrogen and epidermal growth factor (EGF) significantly
increased cell invasion compared to treatment alone, whereas
inhibiting the two pathways reduced cell invasion. Futhermore,
patients with high intranuclear ERα and tumor EGFR levels exhibited
significantly reduced rates of progression-free survival; ERβ
expression, however, did not significantly affect patient survival
(20).
Ishida et al (31) examined 4 cell lines and 15 tumors from
patients with oral SCC. High ERβ expression was observed in the
tumor cells of human primary SCC tissues and various SCC cultured
cell lines. Treatment with the ER antagonists tamoxifen and
raloksifen resulted in SCC apoptotic cell death and Erβ knockdown
by small interfering RNA inhibited SCC proliferation (31).
In the current study, 73 (42%) patients exhibited
intranuclear ERβ expression, which is in accordance with prior
reports (14). Distribution of ERβ
status among different head and neck subsites demonstrated that
almost half of ERβ negative cases were located in the larynx, while
incidence of all tumor sublocalizations within ERβ positive cases
was similar and comparable. Further studies are required to
evaluate the effects of this aberrant distribution.
According to the results of the present study,
positive expression of ERβ indicates improved survival in patients
with oropharyngeal cancer. To the best of our knowledge, this is
the first study suggesting that positive expression of ERβ may be
an indicator of improved survival of patients with oropharyngeal
primaries. However, this is only the case in patients with
oropharyngeal cancer-in other subsites, there was no correlation
between ERβ expression and prolonged survival.
In conclusion, given the positive results of
antihormonal therapy administration in patients with breast cancer
and similar effects observed in HNSCC cell lines, future studies
are necessary in order to implement novel therapeutic
strategies.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takenaka Y, Yasui T, Enomoto K, Miyabe H,
Morizane N, Ashida N, Shimizu K, Hirose M, Yamamoto Y and Uno A:
Health insurance status and survival among patients with head and
neck cancer in Japan. Int J Clin Oncol. 21:517–522. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Razzouk S: Translational genomics and head
and neck cancer: Toward precision medicine. Clin Genet. 86:412–421.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machiels JP, Lambrecht M, Hanin FX, Duprez
T, Gregoire V, Schmitz S and Hamoir M: Advances in the management
of squamous cell carcinoma of the head and neck. F1000Prime Rep.
6:442014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanderson RJ and Ironside JA: Squamous
cell carcinomas of the head and neck. BMJ. 325:822–827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merino OR, Lindberg RD and Fletcher GH: An
analysis of distant metastases from squamous cell carcinoma of the
upper respiratory and digestive tracts. Cancer. 40:145–151. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grégoire V, Lefebvre JL, Licitra L and
Felip E: EHNS- ESMO-ESTRO Guidelines Working Group: Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
21(Suppl 5): v184–v186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schweitzer A, Knauer SK and Stauber RH:
Nuclear receptors in head and neck cancer: Current knowledge and
perspectives. Int J Cancer. 126:801–809. 2010.PubMed/NCBI
|
|
9
|
Wu CT, Chang YL, Shih JY and Lee YC: The
significance of estrogen receptor beta in 301 surgically treated
non-small cell lung cancers. J Thorac Cardiovasc Surg. 130:979–986.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawai H, Ishii A, Washiya K, Konno T, Kon
H, Yamaya C, Ono I, Minamiya Y and Ogawa J: Estrogen receptor alpha
and beta are prognostic factors in non-small cell lung cancer. Clin
Cancer Res. 11:5084–5089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng J, Lee EJ, Madison LD and Lazennec
G: Expression of estrogen receptor beta in prostate carcinoma cells
inhibits invasion and proliferation and triggers apoptosis. FEBS
Lett. 566:169–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reese JM, Suman VJ, Subramaniam M, Wu X,
Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE,
et al: ERβ1: Characterization, prognosis, and evaluation of
treatment strategies in ERα-positive and -negative breast cancer.
BMC Cancer. 14:7492014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jassam N, Bell SM, Speirs V and Quirke P:
Loss of expression of oestrogen receptor beta in colon cancer and
its association with Dukes' staging. Oncol Rep. 14:17–21.
2005.PubMed/NCBI
|
|
14
|
Lukits J, Remenár E, Rásó E, Ladányi A,
Kásler M and Tímár J: Molecular identification, expression and
prognostic role of estrogen-and progesterone receptors in head and
neck cancer. Int J Oncol. 30:155–60. 2007.PubMed/NCBI
|
|
15
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budai B, Remenár E, Orosz Z, Számel I,
Kralovánszky J and Kásler M: Steroid hormone receptors in squamous
cell carcinoma of the head and neck. Orv Hetil. 138:723–727.
1997.(In Hungarian). PubMed/NCBI
|
|
17
|
Virolainen E, Tuohimaa P, Aitasalo K,
Kyttä J and Vanharanta- Hiltunen R: Steroid hormone receptors in
laryngeal carcinoma. Otolaryngol Head Neck Surg. 94:512–517. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oğretmenoğlu O and Ayas K: Laryngeal
carcinoma and estrogen receptor analysis in patients after
long-term follow-up. Eur Arch Otorhinolaryngol. 255:457–461. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goulioumis AK, Fuxe J, Varakis J, Repanti
M, Goumas P and Papadaki H: Estrogen receptor-beta expression in
human laryngeal carcinoma: Correlation with the expression of
epithelial-mesenchymal transition specific biomarkers. Oncol Rep.
22:1063–1068. 2009.PubMed/NCBI
|
|
20
|
Egloff AM, Rothstein ME, Seethala R,
Siegfried JM, Grandis JR and Stabile LP: Cross-talk between
estrogen receptor and epidermal growth factor receptor in head and
neck squamous cell carcinoma. Clin Cancer Res. 15:6529–6540. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York: pp. 29–35. 2010
|
|
22
|
Niu XL, Wang Y, Yao Z, Duan H, Li Z, Liu
W, Zhang H and Deng WM: Autocrine interferon-γ may affect malignant
behavior and sensitivity to tamoxifen of MCF-7 via estrogen
receptor β subtype. Oncol Rep. 34:3120–3130. 2015.PubMed/NCBI
|
|
23
|
Licitra L, Bernier J, Grandi C, Locati L,
Merlano M, Gatta G and Lefebvre JL: Cancer of the larynx. Crit Rev
Oncol Hematol. 47:65–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bianchini C, Pastore A, Pelucchi S,
Torreggiani E, Lambertini E, Marchesi E, Magri E, Frasson C,
Querzoli P and Piva R: Sex hormone receptor levels in laryngeal
carcinoma: A comparison between protein and RNA evaluations. Eur
Arch Otorhinolaryngol. 265:1089–1094. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hagedorn HG and Nerlich AG: Analysis of
sex-hormone-receptor expression in laryngeal carcinoma. Eur Arch
Otorhinolaryngol. 259:205–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schuller DE, Abou-Issa H and Parrish R:
Estrogen and progesterone receptors in head and neck cancer. Arch
Otolaryngol. 110:725–727. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferguson BJ, Hudson WR and McCarty KS Jr:
Sex steroid receptor distribution in the human larynx and laryngeal
carcinoma. Arch Otolaryngol Head Neck Surg. 113:1311–1315. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nozoe T, Oyama T, Takenoyama M, Hanagiri
T, Sugio K and Yasumoto K: Significance of immunohistochemical
expression of estrogen receptors alpha and beta in squamous cell
carcinoma of the esophagus. Clin Cancer Res. 13:4046–4050. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang QM, Qi YJ, Jiang Q, Ma YF and Wang
LD: Relevance of serum estradiol and estrogen receptor beta
expression from a high-incidence area for esophageal squamous cell
carcinoma in China. Med Oncol. 28:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong J, Jiang SW, Niu Y, Chen L, Liu S, Ma
T, Chen X, Xu L, Su Z and Chen H: Expression of estrogen receptor α
and β in esophageal squamous cell carcinoma. Oncol Rep.
30:2771–2776. 2013.PubMed/NCBI
|
|
31
|
Ishida H, Wada K, Masuda T, Okura M,
Kohama K, Sano Y, Nakajima A, Kogo M and Kamisaki Y: Critical role
of estrogen receptor on anoikis and invasion of squamous cell
carcinoma. Cancer Sci. 98:636–643. 2007. View Article : Google Scholar : PubMed/NCBI
|