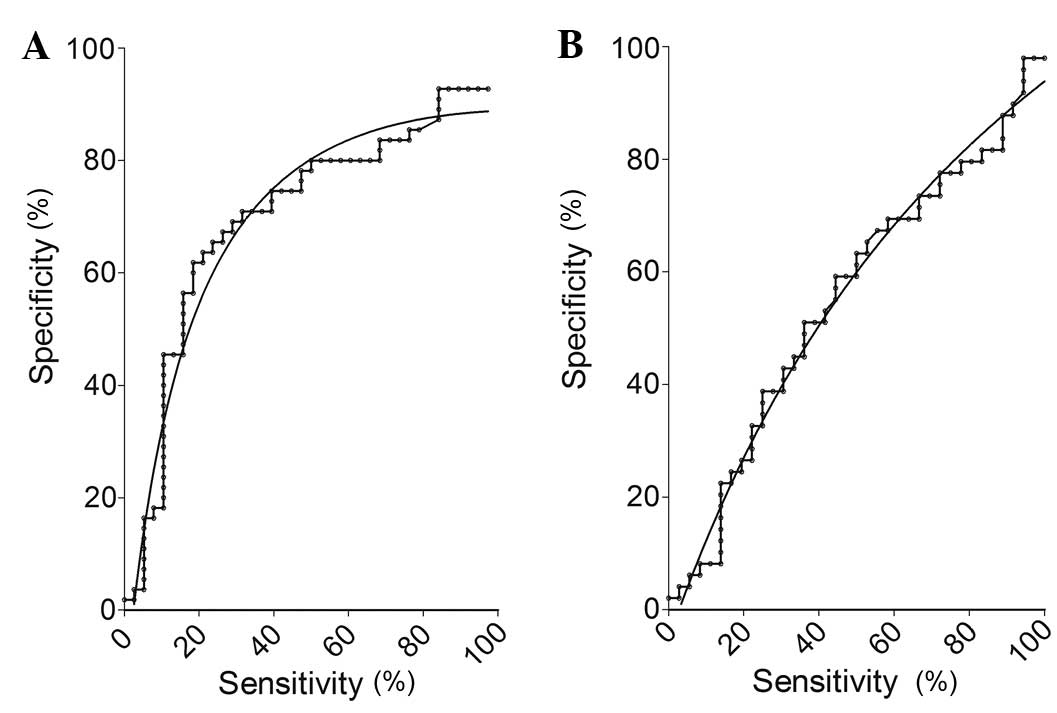
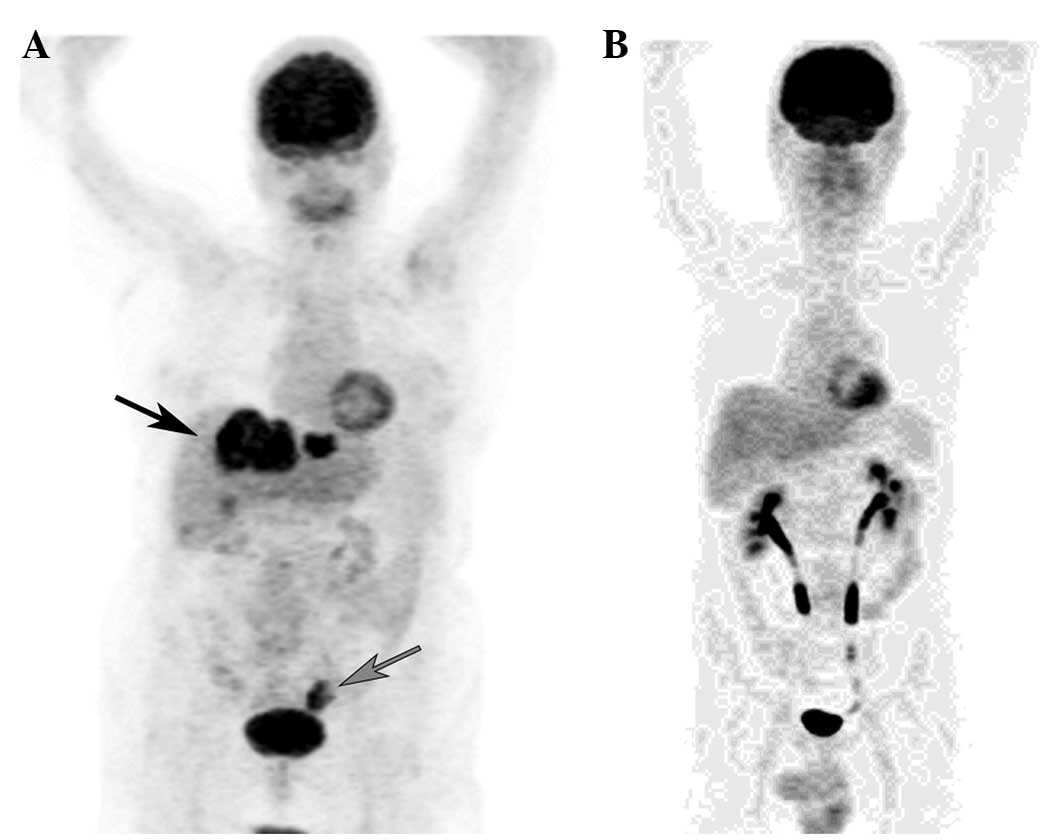
|
1
|
van de Velde CJ, Boelens PG, Borras JM,
Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek
CB, Brown G, Van Cutsem E, et al: EURECCA colorectal:
Multidisciplinary management: European consensus conference colon
& rectum. Eur J Cancer. 50:1.e1–1.e34. 2014. View Article : Google Scholar
|
|
2
|
Bowne WB, Lee B, Wong WD, Ben-Porat L,
Shia J, Cohen AM, Enker WE, Guillem JG, Paty PB and Weiser MR:
Operative salvage for locoregional recurrent colon cancer after
curative resection: An analysis of 100 cases. Dis Colon Rectum.
48:897–909. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CH, Hsieh MC, Lai CC, Yeh CY, Chen
JS, Hsieh PS, Chiang JM, Tsai WS, Tang R, Changchien CR and Wang
JY: Lead time of carcinoembryonic antigen elevation in the
postoperative follow-up of colorectal cancer did not affect the
survival rate after recurrence. Int J Colorectal Dis. 25:567–571.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan E, Gouvas N, Nicholls RJ, Ziprin P,
Xynos E and Tekkis PP: Diagnostic precision of carcinoembryonic
antigen in the detection of recurrence of colorectal cancer. Surg
Oncol. 18:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magnani JL, Steplewski Z, Koprowski H and
Ginsburg V: Identification of the gastrointestinal and pancreatic
cancer-associated antigen detected by monoclonal antibody 19-9 in
the sera of patients as a mucin. Cancer Res. 43:5489–5492.
1983.PubMed/NCBI
|
|
6
|
Filella X, Molina R, Piqué JM,
Garcia-Valdecasas JC, Grau JJ, Novell F, Astudillo E, de Lacy A,
Daniels M and Ballesta AM: Use of CA 19-9 in the early detection of
recurrences in colorectal cancer: Comparison with CEA. Tumour Biol.
15:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bast RC Jr, Ravdin P, Hayes DF, Bates S,
Fritsche H Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG and
Somerfield MR: American Society of Clinical Oncology Tumor Markers
Expert Panel: 2000 update of recommendations for the use of tumor
markers in breast and colorectal cancer: Clinical practice
guidelines of the American Society of Clinical Oncology. J Clin
Oncol. 19:1865–1878. 2001.PubMed/NCBI
|
|
8
|
Panagiotidis E, Datseris IE, Rondogianni
P, Vlontzou E, Skilakaki M, Exarhos D and Bamias A: Does CEA and CA
19-9 combined increase the likelihood of 18F-FDG in detecting
recurrence in colorectal patients with negative CeCT? Nucl Med
Commun. 35:598–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozkan E, Soydal C, Araz M and Aras G:
Serum carcinoembryonic antigen measurement, abdominal
contrast-enhanced computed tomography, and fluorine-18
fluorodeoxyglucose positron emission tomography/computed tomography
in the detection of colorectal cancer recurrence: A correlative
study. Nucl Med Commun. 33:990–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozkan E, Soydal C, Araz M, Kir KM and Ibis
E: The role of 18F-FDG PET/CT in detecting colorectal cancer
recurrence in patients with elevated CEA levels. Nucl Med Commun.
33:395–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanli Y, Kuyumcu S, Ozkan ZG, Kilic L,
Balik E, Turkmen C, Has D, Isik G, Asoglu O, Kapran Y and Adalet I:
The utility of FDG-PET/CT as an effective tool for detecting
recurrent colorectal cancer regardless of serum CEA levels. Ann
Nucl Med. 26:551–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gade M, Kubik M, Fisker RV,
Thorlacius-Ussing O and Petersen LJ: Diagnostic value of (18)F-FDG
PET/CT as first choice in the detection of recurrent colorectal
cancer due to rising CEA. Cancer Imaging. 15:112015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boellaard R, Delgado-Bolton R, Oyen WG,
Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF,
Pike LC, Weber WA, et al: FDG PET/CT: EANM procedure guidelines for
tumour imaging: Version 2.0. Eur J Nucl Med Mol Imaging.
42:328–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Puri KS, Suresh KR, Gogtay NJ and Thatte
UM: Declaration of Helsinki, 2008: Implications for stakeholders in
research. J Postgrad Med. 55:131–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiaravalloti A, Danieli R, Caracciolo CR,
Travascio L, Cantonetti M, Gallamini A, Guazzaroni M, Orlacchio A,
Simonetti G and Schillaci O: Initial staging of Hodgkin's disease:
Role of contrast-enhanced 18F FDG PET/CT. Medicine (Baltimore).
93:e502014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogunbiyi OA, Flanagan FL, Dehdashti F,
Siegel BA, Trask DD, Birnbaum EH, Fleshman JW, Read TE, Philpott GW
and Kodner IJ: Detection of recurrent and metastatic colorectal
cancer: Comparison of positron emission tomography and computed
tomography. Ann Surg Oncol. 4:613–620. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caobelli F, Alongi P, Evangelista L,
Picchio M, Saladini G, Rensi M, Geatti O, Castello A, Laghai I and
Popescu CE: Predictive value of F-FDG PET/CT in restaging patients
affected by ovarian carcinoma: A multicentre study. Eur J Nucl Med
Mol Imaging. 43:404–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiaravalloti A, Rubello D, Chondrogiannis
S, Giammarile F, Colletti PM and Schillaci O: Low-dose CT and
contrast-medium CT in hybrid PET/CT systems for oncologic patients.
Nucl Med Commun. 36:867–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu YY, Chen JH, Chien CR, Chen WT, Tsai
SC, Lin WY and Kao CH: Use of FDG-PET or PET/CT to detect recurrent
colorectal cancer in patients with elevated CEA: A systematic
review and meta-analysis. Int J Colorectal Dis. 28:1039–1047. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fletcher JW, Djulbegovic B, Soares HP,
Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC,
Avril N, et al: Recommendations on the use of F-18-FDG PET in
oncology. J Nucl Med. 49:480–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delgado-Bolton RC, Fernández-Pérez C,
González-Maté A and Carreras JL: Meta-analysis of the performance
of 18F-FDG PET in primary tumor detection in unknown primary
tumors. J Nucl Med. 44:1301–1314. 2003.PubMed/NCBI
|
|
22
|
Delgado-Bolton RC, Carreras JL and
Pérez-Castejón MJ: A systematic review of the efficacy of F-18-FDG
PET in unknown primary tumors. Curr Med Imaging Rev. 2:215–225.
2006. View Article : Google Scholar
|
|
23
|
Boellaard R, O'Doherty MJ, Weber WA, et
al: FDG PET and PET/CT: EANM procedure guidelines for tumour PET
imaging: Version 1.0. Eur J Nucl Med Mol Imaging. 37:181–200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delbeke D, Coleman RE, Guiberteau MJ,
Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA,
Hubner K, et al: Procedure guideline for tumor imaging with 18F-FDG
PET/CT 1.0. J Nucl Med. 47:885–895. 2006.PubMed/NCBI
|
|
25
|
Boellaard R, Delgado-Bolton R, Oyen WJ,
Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF,
Pike LC, Weber WA, et al: European Association of Nuclear Medicine
(EANM): FDG PET/CT: EANM procedure guidelines for tumour imaging:
Version 2.0. Eur J Nuclear Med Mol Imaging. 42:328–354. 2015.
View Article : Google Scholar
|
|
26
|
de Geus-Oei LF, van Laarhoven HW, Visser
EP, Hermsen R, van Hoorn BA, Kamm YJ, Krabbe PF, Corstens FH, Punt
CJ and Oyen WJ: Chemotherapy response evaluation with FDG-PET
inpatients with colorectal cancer. Ann Oncol. 19:348–352. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan MC, Linehan DC, Hawkins WG, Siegel BA
and Strasberg SM: Chemotherapy-induced normalization of FDG uptake
by colorectal liver metastases does not usually indicate complete
pathologic response. J Gastrointest Surg. 11:1112–1119. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calabria F, Rubello D and Schillaci O: The
optimal timing to perform 18F/11C-choline PET/CT in patients with
suspicion of relapse of prostate cancer: Trigger PSA versus PSA
velocity and PSA doubling time. Int J Biol Markers. 29:e423–e430.
2014. View Article : Google Scholar : PubMed/NCBI
|