Introduction
The formation of metastasis requires multiple
complex processes, including local invasion by cancer cells into
lymphatic and blood vessels, trafficking and extravasation of
cancer cells to the lymph node and distant organs, and development
of small tumor nodules (1). Various
factors and signaling networks enhance the metastatic potential of
cancer cells by allowing tumor cells to survive, proliferate and
migrate (1). Vascular endothelial
growth factor-C (VEGF-C), a member of the VEGF family, has been
reported to promote tumor lymphangiogenesis (2). VEGF-C is a secreted homodimeric
glycoprotein with a central VEGF homology domain containing
receptor binding sites for VEGF receptor-2 (VEGFR-2) and VEGFR-3
(2). Transgenic overexpression of
VEGF-C in keratocytes causes lymphatic, but not vascular,
endothelial proliferation and vessel enlargement in mouse skin
(3). Several studies have
demonstrated that cancers with lymph node metastasis usually
express more VEGF-C than the corresponding normal mucosa (4), but this correlation is not universal
(5), since VEGF-C expression is
upregulated in numerous, but not all, human cancers (6,7).
Neuropilin-2 (NRP-2) has been reported to function
as a co-receptor for class 3 semaphorins and several VEGFs,
including VEGF-C (8). NRP-2, a cell
surface glycoprotein, is crucial for repulsive axon guidance,
vascularization and angiogenesis (9,10). Since
it lacks intracellular signaling motifs, NRP-2 forms co-receptor
complexes with plexins and VEGFRs such as VEGFR-3 to mediate signal
transduction (8,11).
The role of NRP-2 in tumor pathogenesis has not been
completely clarified. Expression of NRP-2 has been detected in
breast (12) and pancreatic cancer
(13). Furthermore, the expression
levels of NRP-2 in lung lesions increased from dysplasia to
microinvasive carcinoma (14). In
addition, non-small-cell lung carcinoma patients with upregulated
NRP-2 expression had a significantly worse prognosis than those
without expression of NRP-2 (15).
Thyroid carcinoma is the most frequent malignancy of
the endocrine system, mainly affecting women, with an estimated
60,220 new cases and 1,850 mortalities in the USA in 2013 (16). The majority of thyroid tumors follow
an indolent clinical course with favorable prognosis (17). However, thyroid carcinoma has a
tendency to spread into lymphatic channels and metastasize to
regional lymph nodes at a high frequency (17–19).
Vascular invasion may be an adverse prognostic sign, and thyroid
cancer cells can metastasize via the bloodstream or the lymphatic
vasculature (18,19).
The mechanisms that determine the route of
metastatic spread remain largely unknown. Several studies have
reported that the expression of VEGF-C is correlated with
metastasis in papillary thyroid carcinoma (PTC) (18,19). These
results indicate that VEGF-C expression may play a role in
lymphangiogenesis of thyroid carcinoma and participate in the
molecular regulation of tumor metastasis. In addition, NRP-2
expression was observed in 64.3% of PTC patients (20), and was reported to correlate with
lymph node metastasis and VEGF-D expression in human PTC tissues
(20). These data indicate that NRP-2
may contribute to the regulation of invasion and motility of
thyroid cancer cells. Taken together, these studies suggest that
VEGF-C and its co-receptor NRP-2 may be involved in the regulation
of the metastatic potential of thyroid cancer cells.
In the present study, the role of VEGF-C/NRP-2
signaling in metastasis was characterized in two types of thyroid
cancer cells, including PTC and follicular thyroid carcinoma (FTC),
which represent >90% of all thyroid malignancies (21). The results demonstrate that the
activation of the VEGF-C/NRP-2 axis is mediated at least through
the mitogen-activated protein kinase (MAPK) kinase
(MEK)/extracellular signal-regulated kinase (ERK) and p38 MAPK
signaling cascades in PTC cells. The VEGF-C/NRP-2 axis promotes the
invasiveness and migration of thyroid cancer cells, and this axis
critically requires NRP-2 for tumor invasion.
Materials and methods
Cell lines
The human K1 papillary thyroid cell line was
purchased from the European Collection of Authenticated Cell
Cultures (Salisbury, UK) and maintained in a 2:1:1 mixture medium
of Dulbecco's modified Eagle's medium: Ham's F12: molecular cell
developmental biology 105 medium (Sigma-Aldrich, St. Louis, MO,
USA) supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mM
glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The human
thyroid cancer WRO cell line (provided by Dr Jen-Der Lin, Chang
Gung Memorial Hospital, Taoyuan, Taiwan) was cultured in RPMI
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% (v/v) FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml
penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Both cell lines were maintained at 37°C in a
humidified 5% CO2/95% air atmosphere.
Overexpression of NRP-2
Expression plasmid of human NRP-2 (GenBank:
NM_201266; http://www.ncbi.nlm.nih.gov/nuccore/41872561) was
purchased from OriGene Technologies, Inc. (Rockville, MD, USA).
Transfection was performed using GeneIn™ transfection reagent
according to the manufacturer's protocol (MTI-GlobalStem,
Gaithersburg, MD, USA). In brief, cells were seeded in 6-well
culture plates (0.5–1.0×106 cells/ml/well) and transfected with 8
µg expression plasmid for 36 h. Transfected cells were starved in
4% (v/v) FBS medium for the next 16 h. To stimulate the
VEGF-C/NRP-2 axis, the cells were treated with 100 ng/ml human
recombinant VEGF-C (PeproTech Inc., Rocky Hill, NJ, USA) for the
indicated times. To block the VEGF-C/NRP-2 interaction, the cells
were pre-incubated with an NRP-2 function-blocking antibody (0.2
µg/ml; R&D Systems, Inc., Minneapolis, MN, USA) for 1.5 h
before being stimulated with VEGF-C (22). To determine the signaling pathways,
the transfected cells were pre-incubated with PD98059 (25 µM),
SB203580 (10 µM) or SB202190 (20 µM) (Sigma-Aldrich) alone for 0.5
h, followed by VEGF-C stimulation, as mentioned above. After
incubation, the cells were collected and subjected to western blot
analysis as described previously (23). The antibodies used were directed
against the Myc epitope tag (cat. no. 2276; 1:1,000),
phosphorylated (p)-ERK (cat. no. 4376; 1:2,000), ERK (cat. no.
4695; 1:2,000), p-p38 MAPK (cat. no. 9215; 1:2,000) and p38 MAPK
(cat. no. 9212; 1:2,000) (Cell Signaling Technology, Inc., Danvers,
MA, USA). All assays were performed in triplicate.
Migration and invasion assays
To analyze cell migration using in vitro
scratch assay, NRP-2-overexpressing cells were seeded in triplicate
in 24-well plates (1.5–4.0×105 cells/ml/well) and cultured in 4%
(v/v) FBS medium overnight at 37°C (24,25). Upon
washing with phosphate-buffered saline, the cell monolayer was
scraped in a straight line with a pipette tip. After incubation
with culture medium (4% FBS) containing VEGF-C plus or minus
PD98059, SB203580 or SB202190 for 8 h, the cells were observed
using a Nikon inverted microscope (Nikon Corporation, Tokyo, Japan)
and Image Pro-Plus image analysis software (Media Cybernetics,
Inc., Rockville, MD, USA). Images were obtained of ≥4 randomly
selected microscopic fields per well, and four randomly selected
gaps were calculated per photograph. The invasive activity of
NRP-2-overexpressing cells was examined using the
Corning® BioCoat™ Tumor Invasion System (Corning Life
Sciences, Tewksbury, MA, USA) according to the manufacturer's
protocol (25). In brief, 5×104 cells
were resuspended in 200 µl 4% FBS medium and placed in the top
chamber (8-µm insert) in triplicate wells for each group (VEGF-C
plus or minus PD98059, SB203580 or SB202190), while the lower
chamber was coated with Matrigel. After incubation for 24 h, the
cells from the top chamber were removed using a cotton swab, and
the cells on the lower surface of the insert were fixed and stained
using Giemsa stain (Sigma-Aldrich). The number of cells was counted
using a Nikon inverted microscope (Nikon Corporation). All assays
were performed in triplicate.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Group comparisons were performed using analysis of
variance with Tukey's comparison test. All statistical analysis was
performed using GraphPad Prism software (version 6; GraphPad
Software Inc., San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
The VEGF-C/NRP-2 axis activates ERK
and p38 MAPK in thyroid cancer cells
In order to test the aforementioned hypothesis, the
present study first examined whether NRP-2 could be expressed in
different types of human thyroid cancer cells, including K1 (PTC)
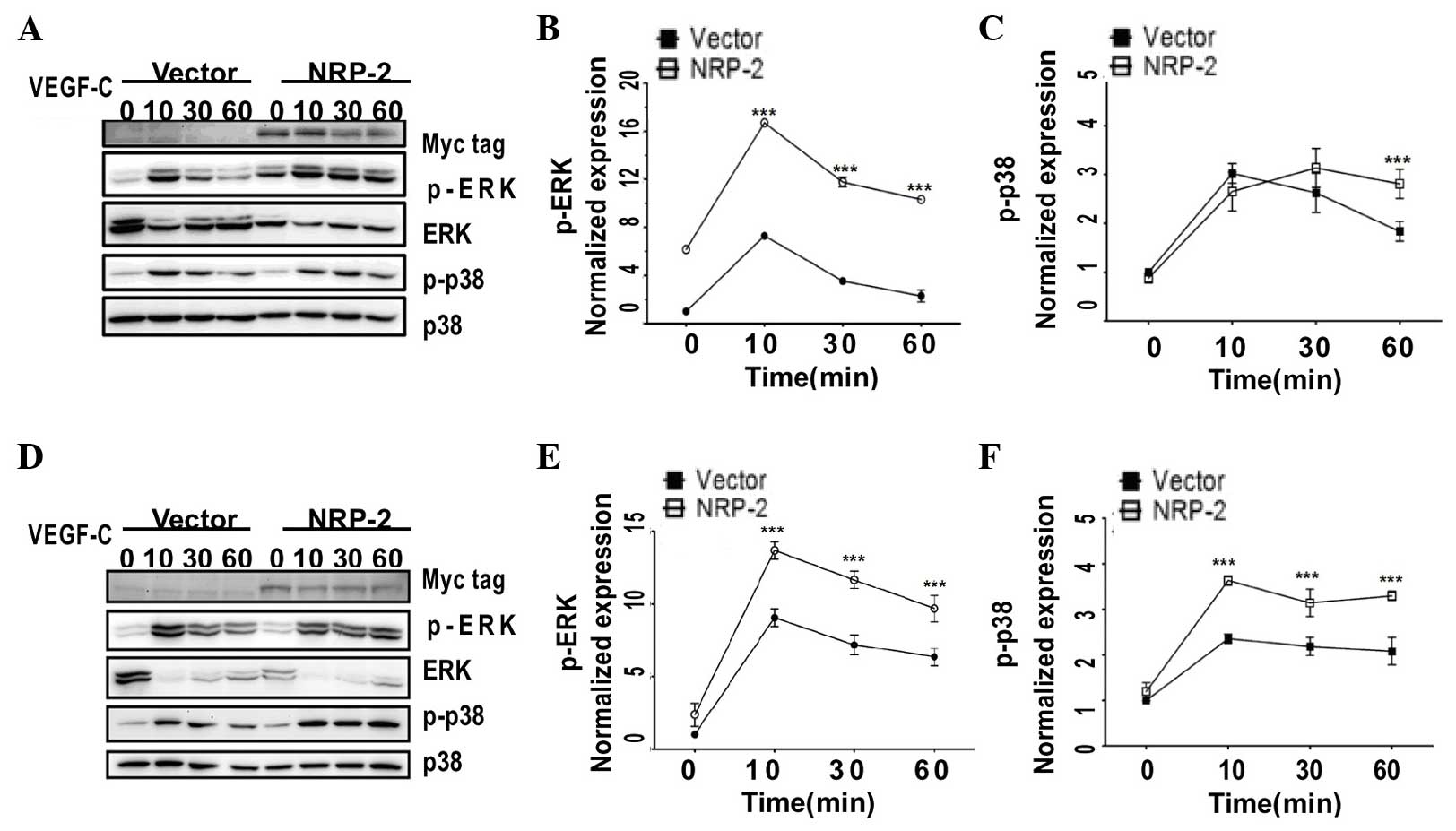
and WRO (FTC). As indicated in Fig.
1, overexpressed NRP-2, which had a C-terminal Myc tag, was
detected as a single major band in the two thyroid cell lines.
Next, to study the VEGF-C/NRP-2 axis in the two
thyroid cancer cells, NRP-2-overexpressing cells were treated with
exogenous recombinant human VEGF-C, and the activities of p-ERK and
p-p38 MAPK were evaluated. In both K1 and WRO cells, when NRP-2 was
overexpressed, an early and transient activation of p-ERK was
observed following VEGF-C induction (100 ng/ml), which peaked at 10
min [2.29-fold for the K1 cells (Fig.1A
and B) and 1.51-fold for the WRO cells (Fig.1D and E)] and remained elevated at 60
min compared with the levels displayed by the
vector-only-transfected cells. Similarly, VEGF-C strongly activated
p-p38 MAPK, which was maximal at 10 min (1.54-fold for WRO cells)
and was sustained for ≤60 min in the NRP-2-overexpressing WRO cells
(Fig.1D and F), while p-p38 MAPK was
markedly activated at 60 min in the NRP-2-overexpressing K1 cells
compared with the levels detected in the vector only-transfected
cells, which were declining toward basal levels (Fig. 1A and C). Consistent with previous
studies reporting that NRP-2 functions as a co-receptor for VEGF
(8), the present data demonstrated
that the VEGF-C/NRP-2 axis is activated with differential kinetics
of kinase activation in the two types of human thyroid cancer cells
evaluated.
To further investigate the downstream pathways of
the VEGF-C/NRP-2 axis in thyroid cancer cells, PD98059, a potent
and selective inhibitor of MEK activation, and SB203580, a
selective and potent inhibitor of p38 MAPK (26), were used in NRP-2-overexpressing cells
(Fig. 2). As expected, treatment with
PD98059 (25 µM) strongly abolished the stimulated ERK-mediated
activation of the VEGF-C/NRP-2 axis in K1 cells (Fig. 2A), but barely exerted any effect on
the activated p-p38 (Fig. 2A).
However, neither PD98059 nor SB203580 (10 µM) produced any
inhibition of ERK and p38 MAPK phosphorylation on the VEGF-C/NRP-2
axis in WRO cells (Fig. 2B and D).
Unexpectedly, SB203580 inhibited both p38 MAPK and ERK
phosphorylation on the VEGF-C/NRP-2 axis in K1 cells (Fig. 2C).
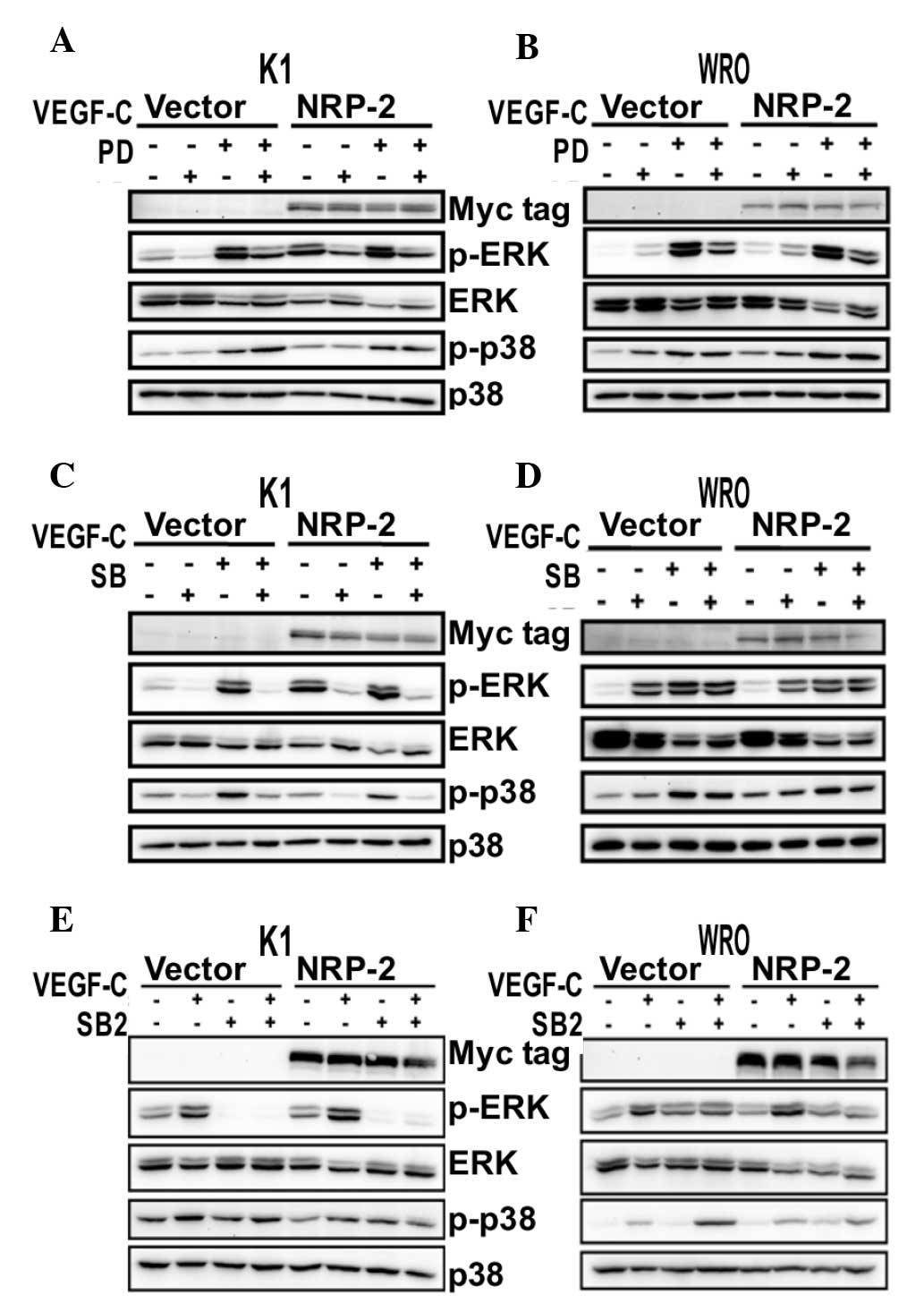 | Figure 2.Effect of PD, SB and SB2 on the
VEGF-C/NRP-2 axis. NRP-2-overexpressing K1 and WRO cells were
either pretreated with (A and B) PD (25 µM), (C and D) SB (10 µM),
(E and F) SB2 (20 µM) or DMSO for 30 min, and then stimulated with
VEGF-C (100 ng/ml) for 10 min. The levels of p-ERK and p-p38 MAPK
were measured using western blot analysis. An anti-Myc tag antibody
was used to detect the expression of NRP-2. Representative western
blots from three independent experiments are shown. VEGF, vascular
endothelial growth factor; NRP-2, neuropilin-2; MAPK,
mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; p-, phosphorylated; PD, PD98059; SB,
SB203580; SB2, SB202190; DMSO, dimethyl sulfoxide. |
To ascertain the effect of p38 MAPK inhibitors on
the VEGF-C/NRP-2 axis, another cell permeable inhibitor of p38
MAPK, SB202190 (26), was used in
NRP-2-overexpressing cells. Similarly, the phosphorylation levels
of p38 MAPK and ERK on the VEGF-C/NRP-2 axis were suppressed by
SB202190 (20 µM) in K1 cells (Fig.
2E), whereas the phosphorylation levels of p38 MAPK and ERK
were not affected by SB202190 in WRO cells (Fig. 2F).
Consistent with previous studies reporting that the
activation of ERK is an important kinase cascade in the regulation
of VEGF-C signaling in cancer cells (27,28), the
present data suggest that the activation of the VEGF-C/NRP-2 axis
is mediated at least through the MEK/ERK and p38 MAPK signaling
cascades in K1 cells. Furthermore, since the present results did
not reveal significant differences in the treatment of WRO cells
with inhibitors, other signaling cascades may be regulated by the
VEGF-C/NRP-2 axis in WRO cells.
The VEGF-C/NRP-2 axis promotes
migration and invasion of thyroid cancer cells
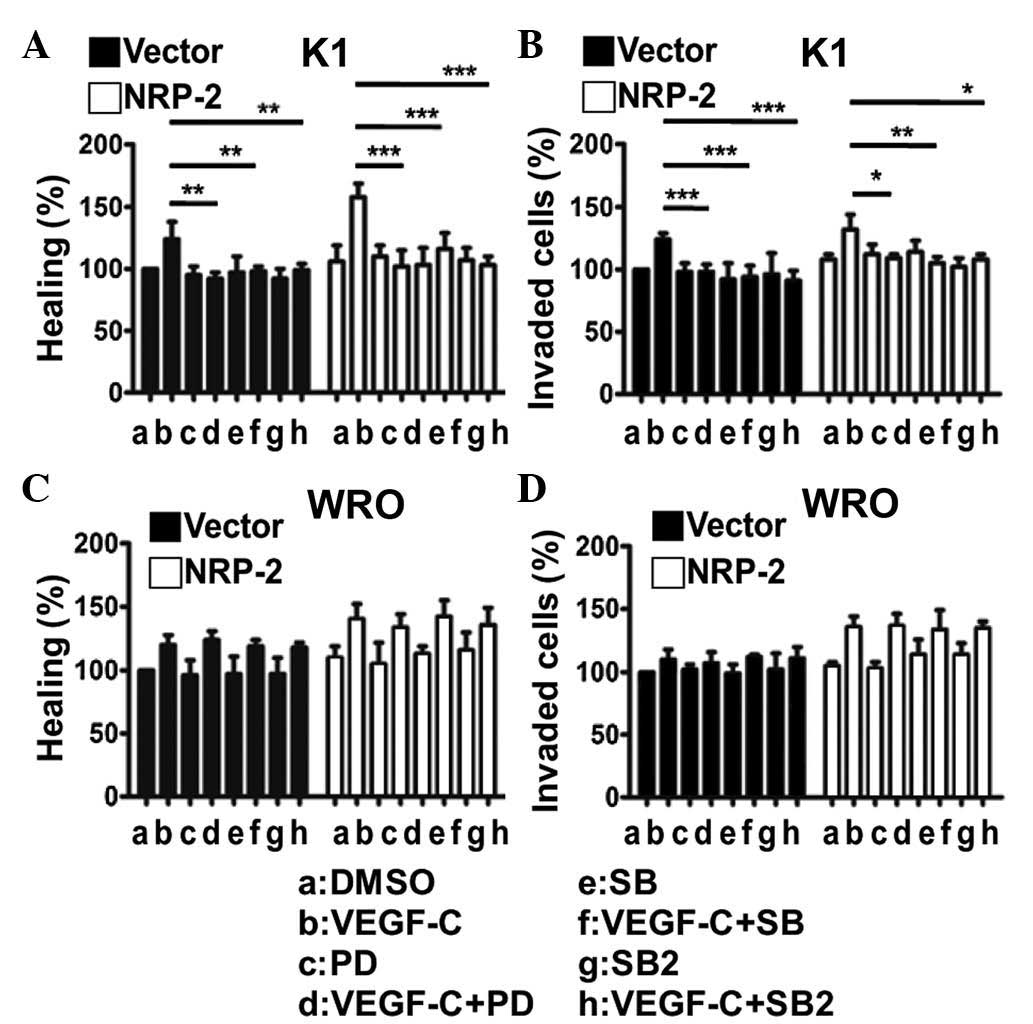
Wound-healing assay and in vitro invasion
assay were used to examine the role of the VEGF-C/NRP-2 axis on the
migration and invasiveness of thyroid cancer cells (Fig. 3). Treatment with VEGF-C significantly
increased the migratory activities (Fig.
3A and C) and invasiveness (Fig. 3B
and D) of the two NRP-2-overexpressing thyroid cancer cells
compared with vector-transfected cells (P<0.05). In addition,
overexpression of NRP-2 did not significantly affect the migratory
ability or invasiveness of the two thyroid cells compared with the
vector-transfected cells (Fig. 3).
These results indicate that the VEGF-C/NRP-2 axis can promote the
invasiveness and migratory ability of thyroid cancer cells.
In K1 cells, western blot analysis revealed that the
MEK/ERK and p38 MAPK signaling cascades were activated by the
VEGF-C/NRP-2 axis (Fig. 2A-C).
Accordingly, the invasive activities of NRP-2-overexpressing K1
cells were significantly suppressed by PD98059, SB203580 and
SB202190 (Fig. 3B). Together with the
results mentioned above, the present data suggest that the
activation of the VEGF-C/NRP-2 axis is mediated at least through
the MEK/ERK and p38 MAPK signaling cascades, and further regulates
the invasive activities of K1 cells. As for WRO cells, the present
results did not reveal significant differences in the treatment of
cells with MEK or p38 MAPK inhibitors following the activation of
the VEGF-C/NRP-2 axis (Fig. 2D-F). As
a result, these inhibitors did not produce a significant inhibition
of the migratory activity (Fig. 3C)
or invasiveness (Fig. 3D) of
NRP-2-overexpressing WRO cells, which provides additional evidence
that other signaling cascades may be regulated by the VEGF-C/NRP-2
axis in WRO cells.
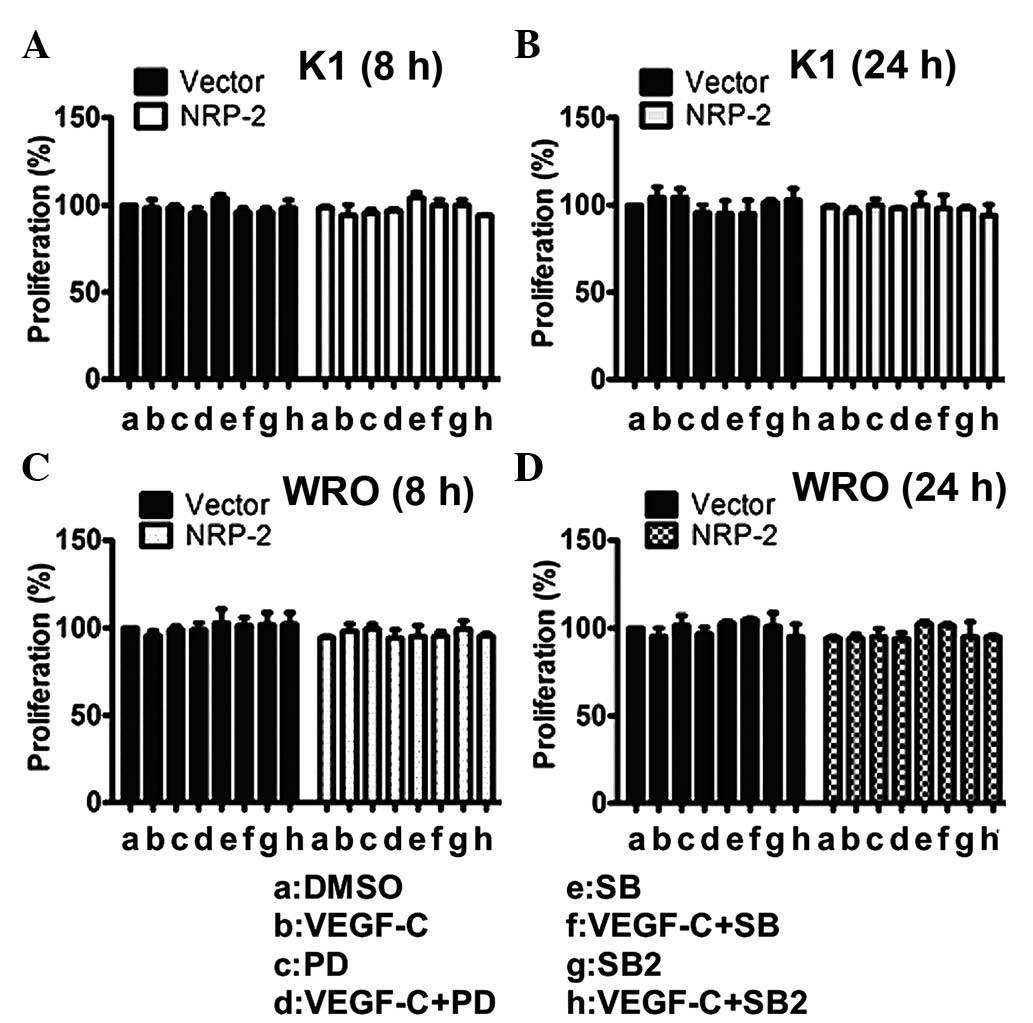
To further rule out the possibility that the effect
of the VEGF-C/NRP-2 axis on invasiveness was caused by different
proliferation rates, the growth rates of the NRP-2-overexpressing
cells were compared (Fig. 4). The
control cells and the NRP-2-overexpressing cells had similar growth
rates after 8 (Fig. 4A and C) and 24
h (Fig. 4B and D) in culture.
Therefore, these results indicate that the increased mobility of
thyroid cancer cells was due to the activation of the VEGF-C/NRP-2
axis. Taken together, these data strongly support the finding that
the VEGF-C/NRP-2 axis is actively involved in regulating the
mobility and invasiveness of thyroid cancer cells.
VEGF-C/NRP-2 axis-mediated migration
and invasion of thyroid cancer cells require NRP-2 signaling
To evaluate more rigorously the role of NRP-2 in the
invasiveness of thyroid cancer cells following the activation of
the VEGF-C/NRP-2 axis, an NRP-2 function-blocking antibody was used
to selectively block the binding of the VEGF family ligands to
NRP-2 (29,30). In NRP-2-overexpressing K1 cells, the
VEGF-C/NRP-2 axis became defective due to the reduction in ERK and
p38 MAPK phosphorylation (Fig. 5A).
Of note, the phosphorylation levels of ERK and p38 MAPK were
significantly suppressed in the NRP-2-overexpressing WRO cells
(Fig. 5B). Additionally, treatment
with the NRP-2 function-blocking antibody in NRP-2-overexpressing
thyroid cancer cells also strongly impaired VEGF-C/NRP-2-induced
migratory activity (Fig. 5C) and
invasiveness (Fig. 5D). Taken
together, the present data suggest that the VEGF-C/NRP-2 axis is
important in the invasiveness of thyroid cancer cells, and that
this axis critically requires NRP-2 for cell invasion.
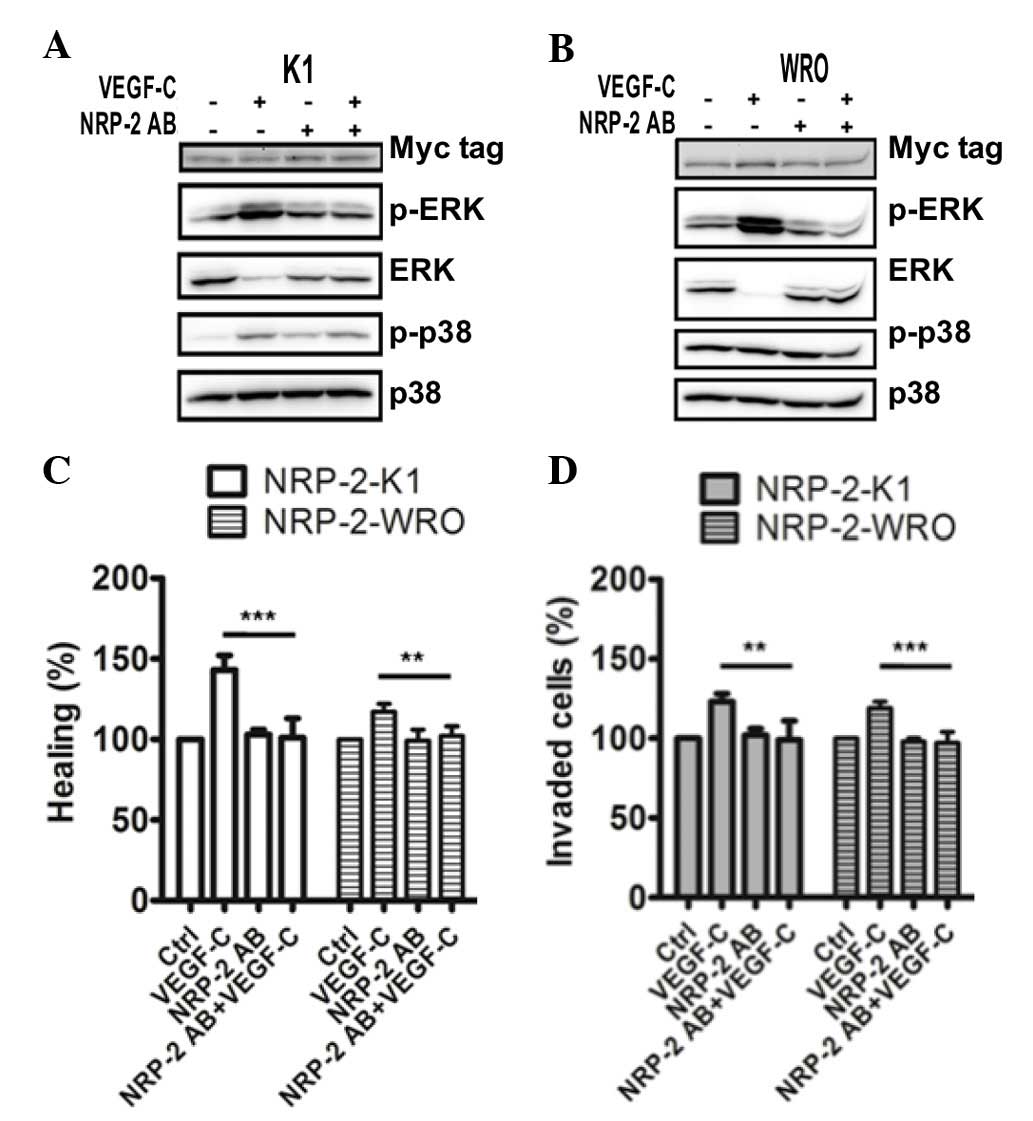 | Figure 5.NRP-2 blocking signals suppress
VEGF-C-induced metastasis. NRP-2-overexpressing (A) K1 and (B) WRO
cells were pretreated with an NRP-2 function-blocking antibody (0.5
µg/ml) for 1.5 h, and then stimulated with VEGF-C (100 ng/ml) for
10 min. The levels of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase phosphorylation were measured
using western blot analysis. An anti-Myc tag antibody was used to
detect the expression of NRP-2. Representative western blots from
three independent experiments are shown. (C) Scratch wound-healing
assay and (D) invasion assay were performed to evaluate the effect
of NRP-2 blocking signals on cell migration and invasion. Bar
graphs represent the mean ± standard deviation of the relative
percentages for each treatment from three independent experiments.
**P<0.01; ***P<0.001. VEGF, vascular endothelial growth
factor; NRP-2, neuropilin-2; MAPK, mitogen-activated protein
kinase; ERK, extracellular signal-regulated kinase; p-,
phosphorylated; PD, PD98059; SB, SB203580; SB2, SB202190; Ctrl,
control; AB, antibody. |
Discussion
As previously reported, regulation of tumor
metastasis by VEGF-C occurs by increasing the migratory ability of
cancer cells to lymph nodes (29).
NRP-2 has been documented to be a co-receptor for VEGF-C (8), and lymph node metastasis of human PTC
has been correlated to NRP-2 expression (12). However, the roles of NRP-2 and its
ligand, VEGF-C, in the metastasis of thyroid cancer cells remain
largely unknown. In the present study, the activation of the
VEGF-C/NRP-2 axis was mediated at least through the MEK/ERK and p38
MAPK signaling cascades, particularly in human PTC (K1) cells. The
migratory activity and invasiveness of thyroid cancer cells were
further upregulated by the activation of the VEGF-C/NRP-2 axis, and
this cell invasion mediated by the VEGF-C/NRP-2 axis was observed
to be NRP-2 dependent.
To the best of our knowledge, the present study is
the only detailed report on the effect of the VEGF-C/NRP-2 axis on
the migratory activities of follicular thyroid cell-derived tumors,
including PTC and FTC. The present report emphasizes that the
presence of VEGF-C has a complex association with
clinicopathological factors such as NRP-2 in the metastasis of
thyroid cancer. Indeed, NRP-2 signaling has been reported to
contribute to focal adhesion kinase (FAK)-mediated signaling
cascade activation, and is further involved in the initiation of
tumorigenesis (30). Inhibition of
FAK activation also resulted in the suppression of proliferation
and migration of breast cancer cells in vitro (31). These studies suggest that FAK
activation may be involved in the metastasis of thyroid cancer
through the regulation of the VEGF-C/NRP-2 axis. However, further
experiments are required to address this possibility.
Activation of nuclear factor-κB (NF-κB) has been
demonstrated to be involved in cell proliferation, resistance to
apoptosis, and promotion of tumor angiogenesis and metastasis,
including those reported in human FTC (32). In the present study, neither MEK or
p38 MAPK inhibitors exhibited a significant inhibition of migratory
activity and invasiveness in WRO FTC cells. A possible explanation
for this observation is that the VEGF-C/NRP-2 axis activates not
only the MEK/ERK and p38 MAPK signaling cascades, but also the
NF-κB signaling cascade. Since NF-κB activation was increased in
VEGF or NRP-mediated cell migration (33,34),
whether activation of NF-κB also contributes to VEGF-C/NRP-2
axis-mediated cell migration requires further investigation.
The present study revealed that SB203580 and
SB202190 inhibited both ERK and p38 MAPK phosphorylation on the
VEGF-C/NRP-2 axis in K1 cells. The proto-oncogene c-Raf, an
upstream serine/threonine kinase of the MEK/ERK signaling cascade,
was reported to be inhibited by SB203580 in vitro (35). Whether the activation of c-Raf is
affected by the VEGF-C/NRP-2 axis in thyroid cancer cells remains
to be determined.
Several studies have reported that the expression of
VEGF-C protein and its messenger RNA are correlated with metastasis
in PTC (18,19). These reports suggest that other
factors are likely to be involved in the regulation of
VEGF-C-mediated metastatic status of thyroid carcinomas. In the
present study, it was observed that NRP-2 was required for the
VEGF-C/NRP-2 axis to promote cell migration and invasiveness of
thyroid cancer cells. In fact, NRP-2 expression was previously
reported to be correlated with VEGF expression and lymph node
status in PTC (20). Furthermore, the
serum levels of VEGF factors were reported to be promising
diagnostic tools in patients with lung cancer (36,37). These
previous studies and the present findings suggest that NRP-2 and
VEGF-C may act as key analytic markers for thyroid cancer
metastases and prognosis. Future experiments are required to
evaluate the clinical application of NRP-2 and VEGF-C expression in
human thyroid cancer specimens.
Acknowledgements
The present study was supported by the Ditmanson
Medical Foundation of Chia-Yi Christian Hospital (Chia-Yi, Taiwan;
grant no. CSMU-CYC-102-02).
Glossary
Abbreviations
Abbreviations:
|
VEGF-C
|
vascular endothelial growth
factor-C
|
|
NRP-2
|
neuropilin-2
|
|
PTC
|
papillary thyroid carcinoma
|
|
FTC
|
follicular thyroid carcinoma
|
References
|
1
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joukov V, Pajusola K, Kaipainen A, Chilov
D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N and Alitalo K: A
novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J. 15:290–298. 1996.PubMed/NCBI
|
|
3
|
Jeltsch M, Kaipainen A, Joukov V, Meng X,
Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK and Alitalo K:
Hyperplasia of lymphatic vessels in VEGF-C transgenic mice.
Science. 276:1423–1425. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amioka T, Kitadai Y, Tanaka S, Haruma K,
Yoshihara M, Yasui W and Chayama K: Vascular endothelial growth
factor-C expression predicts lymph node metastasis of human gastric
carcinomas invading the submucosa. Eur J Cancer. 38:1413–1419.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
George ML, Tutton MG, Janssen F, Arnaout
A, Abulafi AM, Eccles SA and Swift RI: VEGF-A, VEGF-C, and VEGF-D
in colorectal cancer progression. Neoplasia. 3:420–427. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niki T, Iba S, Tokunou M, Yamada T,
Matsuno Y and Hirohashi S: Expression of vascular endothelial
growth factors A, B, C, and D and their relationships to lymph node
status in lung adenocarcinoma. Clin Cancer Res. 6:2431–2439.
2000.PubMed/NCBI
|
|
7
|
Gunningham SP, Currie MJ, Han C, Robinson
BA, Scott PA, Harris AL and Fox SB: The short form of the
alternatively spliced flt-4 but not its ligand vascular endothelial
growth factor C is related to lymph node metastasis in human breast
cancers. Clin Cancer Res. 6:4278–4286. 2000.PubMed/NCBI
|
|
8
|
Favier B, Alam A, Barron P, Bonnin J,
Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, et
al: Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes
human endothelial cell survival and migration. Blood.
108:1243–1250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giger RJ, Cloutier JF, Sahay A, Prinjha
RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh
FS, et al: Neuropilin-2 is required in vivo for selective axon
guidance responses to secreted semaphorins. Neuron. 25:29–41. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan L, Moyon D, Pardanaud L, Bréant C,
Karkkainen MJ, Alitalo K and Eichmann A: Abnormal lymphatic vessel
development in neuropilin 2 mutant mice. Development.
129:4797–4806. 2002.PubMed/NCBI
|
|
11
|
Pellet-Many C, Frankel P, Jia H and
Zachary I: Neuropilins: Structure, function and role in disease.
Biochem J. 411:211–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasuoka H, Kodama R, Tsujimoto M,
Yoshidome K, Akamatsu H, Nakahara M, Inagaki M, Sanke T and
Nakamura Y: Neuropilin-2 expression in breast cancer: Correlation
with lymph node metastasis, poor prognosis, and regulation of CXCR4
expression. BMC Cancer. 9:2202009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen T, Herzog Y, Brodzky A, Greenson JK,
Eldar S, Gluzman-Poltorak Z, Neufeld G and Resnick MB: Neuropilin-2
is a novel marker expressed in pancreatic islet cells and endocrine
pancreatic tumours. J Pathol. 198:77–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lantuéjoul S, Constantin B, Drabkin H,
Brambilla C, Roche J and Brambilla E: Expression of VEGF,
semaphorin SEMA3F, and their common receptors neuropilins NP1 and
NP2 in preinvasive bronchial lesions, lung tumours, and cell lines.
J Pathol. 200:336–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakami T, Tokunaga T, Hatanaka H, Kijima
H, Yamazaki H, Abe Y, Osamura Y, Inoue H, Ueyama Y and Nakamura M:
Neuropilin 1 and neuropilin 2 co-expression is significantly
correlated with increased vascularity and poor prognosis in
nonsmall cell lung carcinoma. Cancer. 95:2196–2201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang BH, Lo CY, Chan WF, Lam KY and Wan
KY: Prognostic factors in papillary and follicular thyroid
carcinoma: Their implications for cancer staging. Ann Surg Oncol.
14:730–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salajegheh A, Pakneshan S, Rahman A,
Dolan-Evans E, Zhang S, Kwong E, Gopalan V, Lo CY, Smith RA and Lam
AK: Co-regulatory potential of vascular endothelial growth factor-A
and vascular endothelial growth factor-C in thyroid carcinoma. Hum
Pathol. 44:2204–2212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu XM, Lo CY, Lam AK, Lang BH, Leung P and
Luk JM: The potential clinical relevance of serum vascular
endothelial growth factor (VEGF) and VEGF-C in recurrent papillary
thyroid carcinoma. Surgery. 144:934–940; discussion 940–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yasuoka H, Kodama R, Hirokawa M, Takamura
Y, Miyauchi A, Inagaki M, Sanke T and Nakamura Y: Neuropilin-2
expression in papillary thyroid carcinoma: Correlation with VEGF-D
expression, lymph node metastasis, and VEGF-D-induced aggressive
cancer cell phenotype. J Clin Endocrinol Metab. 96:E1857–E1861.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romitti M, Ceolin L, Siqueira DR, Ferreira
CV, Wajner SM and Maia AL: Signaling pathways in follicular
cell-derived thyroid carcinomas (review). Int J Oncol. 42:19–28.
2013.PubMed/NCBI
|
|
22
|
Goel HL, Bae D, Pursell B, Gouvin LM, Lu S
and Mercurio AM: Neuropilin-2 promotes branching morphogenesis in
the mouse mammary gland. Development. 138:2969–2976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tai CK, Wang W, Lai YH, Logg CR, Parker
WB, Li YF, Hong JS, Sorscher EJ, Chen TC and Kasahara N: Enhanced
efficiency of prodrug activation therapy by tumor-selective
replicating retrovirus vectors armed with the Escherichia coli
purine nucleoside phosphorylase gene. Cancer Gene Ther. 17:614–623.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su JL, Yang PC, Shih JY, Yang CY, Wei LH,
Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Issbrücker K, Marti HH, Hippenstiel S,
Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC,
Suttorp N and Clauss M: p38 map kinase - a molecular switch between
VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J.
17:262–264. 2003.PubMed/NCBI
|
|
27
|
Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang
S, Wang W, Zhang J and Zhang Y: RNAi-mediated silencing of VEGF-C
inhibits non-small cell lung cancer progression by simultaneously
down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent
axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer.
47:2353–2363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Affolter A, Fruth K, Brochhausen C,
Schmidtmann I, Mann WJ and Brieger J: Activation of
mitogen-activated protein kinase extracellular signal-related
kinase in head and neck squamous cell carcinomas after irradiation
as part of a rescue mechanism. Head Neck. 33:1448–1457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoshida T, Isaka N, Hagendoorn J, di
Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP and Jain RK:
Imaging steps of lymphatic metastasis reveals that vascular
endothelial growth factor-C increases metastasis by increasing
delivery of cancer cells to lymph nodes: Therapeutic implications.
Cancer Res. 66:8065–8075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goel HL, Pursell B, Chang C, Shaw LM, Mao
J, Simin K, Kumar P, Kooi CW Vander, Shultz LD, Greiner DL, et al:
GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine
pathway that contributes to breast cancer initiation. EMBO Mol Med.
5:488–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurio N, Shimo T, Fukazawa T, Takaoka M,
Okui T, Hassan NM, Honami T, Hatakeyama S, Ikeda M, Naomoto Y and
Sasaki A: Anti-tumor effect in human breast cancer by TAE226, a
dual inhibitor for FAK and IGF-IR in vitro and in vivo. Exp Cell
Res. 317:1134–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J and Brown RE: Morphoproteomic
confirmation of an activated nuclear factor-кBp65 pathway in
follicular thyroid carcinoma. Int J Clin Exp Pathol. 5:216–223.
2012.PubMed/NCBI
|
|
33
|
Liu W, Parikh AA, Stoeltzing O, Fan F,
McCarty MF, Wey J, Hicklin DJ and Ellis LM: Upregulation of
neuropilin-1 by basic fibroblast growth factor enhances vascular
smooth muscle cell migration in response to VEGF. Cytokine.
32:206–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Castresana MR and Newman WH:
Reactive oxygen and NF-kappaB in VEGF-induced migration of human
vascular smooth muscle cells. Biochem Biophys Res Commun.
285:669–674. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hall-Jackson CA, Goedert M, Hedge P and
Cohen P: Effect of SB 203580 on the activity of c-Raf in vitro and
in vivo. Oncogene. 18:2047–2054. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu P, Liu W, Wang L, Yang M and Du J: High
circulating VEGF level predicts poor overall survival in lung
cancer. J Cancer Res Clin Oncol. 139:1157–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Meng X, Zeng H, Guan Y, Zhang Q,
Guo S, Liu X and Guo Q: Serum vascular endothelial growth factor-C
levels: A possible diagnostic marker for lymph node metastasis in
patients with primary non-small cell lung cancer. Oncol Lett.
6:545–549. 2013.PubMed/NCBI
|