Introduction
Among men, hepatocellular carcinoma (HCC) is the
fifth most common cancer worldwide and the second leading cause of
cancer-associated mortalities annually (1). Current curative treatments, including
surgical resection and liver transplantation, are generally
ineffective, and can be applied only during early-stage HCC
(2,3).
In addition, only 10–15% of patients are qualified to be treated
with curative surgery (2,3). Thus, the majority of HCC patients must
be considered for systemic chemotherapies or supportive therapies
(3). However, the majority of
chemotherapeutic agents exhibit poor effectiveness and can only
marginally improve patient survival rates (3).
Cisplatin is one of the most widely used
chemotherapeutic agents for treating various types of malignancies
(4). Cisplatin is primarily
considered as a DNA-damaging agent that forms various types of
bifunctional adducts upon reacting with cellular DNA (4). The final cellular outcome of DNA adduct
formation is generally apoptotic cell death, which is considered to
occur through the disruption of cellular processes such as the
deregulation of signal transduction pathways involved in growth,
differentiation and stress responses (5). Although DNA damage is widely considered
to be responsible for cisplatin-induced apoptosis, the molecular
mechanisms that establish the formation of DNA adducts in response
to cisplatin-induced apoptosis have not been identified
conclusively (6,7). This research gap raises the question of
whether alternative and/or additional mechanisms are involved.
Accumulating evidence has revealed that cisplatin-induced apoptosis
could occur independently of DNA damage through oxidative stress in
various cell types (8–10). Therefore, alternative pathways
affected by cisplatin should be further explored, since they could
yield critical insights for improving the current cisplatin-based
regimens. Despite the fact that the current postulated mechanisms
remain controversial, there is a leading theory that involves the
ability of cisplatin to inhibit sublethal damage repair in
resistant tumor cells (5,6). Thus, further characterization of the
possible underlying mechanism(s) associated with the sensitization
engendered by cisplatin in HCC cells is justified.
With these premises in mind, the aim of the present
study was to identify the molecular signatures of selected HCC cell
lines and to provide mechanistic insight into apoptotic pathways
alternatively activated by cisplatin to elucidate the fundamental
causes of sensitization during endoplasmic reticulum (ER) stress.
In the present study, two p53-dysfunctional HCC models, Hep3B and
Mahlavu (11), were selected, of
which, the former was more differentiated than the latter. It was
noticed that Mahlavu cells were distinctly more sensitive to
cisplatin than Hep3B cells. Our results also revealed a novel
functional attribute of cisplatin involving the robust suppression
of two prominent pathways that regulate the expression of B-cell
lymphoma (Bcl)-2, survivin and glucose-regulated protein (GRP) 78
independently of DNA damage and repair mechanisms. Thus, these
findings provide a crucial missing link in the understanding of the
mechanism of cisplatin-induced ER stress. Critically, this novel
functional attribute of cisplatin may also provide an avenue for
novel therapeutic strategies against chemotherapy-resistant HCC
cells.
Materials and methods
Cell culture and cell viability
assay
The two HCC cell lines used in the present study,
Hep3B cells purchased from Bioresource Collection and Research
Center (Hsinchu, Taiwan) and Mahlavu cells provided by Professor
K.H. Lin (Chang Gung University, Taiwan), were grown in Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine
serum (Biological Industries, Cromwell, CT, USA) in 5%
CO2, in a humidified incubator (37°C). The cells were
treated with cisplatin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) at various concentrations for 24 or 72 h, and cell
viability was measured using a sulforhodamine B (SRB) assay, as
previously described (12). Briefly,
cells were fixed using trichloroacetic acid and then incubated with
SRB (Sigma-Aldrich; Merck Millipore) upon being washed and
air-dried. The precipitate was dissolved in Trizma® base
solution (Sigma-Aldrich; Merck Millipore). The absorbance was
measured at 540 nm by using a microplate reader.
Western blot analysis
A total of 5×105 cells were seeded for 24
h and treated with cisplatin for different periods. Cells were
lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich;
Merck Millipore), and protein concentrations were assayed using the
Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). In total, 40 µg of proteins was separated on 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
electrophoretically to a polyvinylidene difluoride membrane. Upon
blocking, the membrane was hybridized at 4°C overnight with one of
the following primary antibodies: Anti-GRP78 (catalog no. sc-13968;
dilution, 1:500), anti-activating transcription factor (ATF) 3
(catalog no. sc-188; dilution, 1:500), anti-ATF4 (catalog no.
sc-200; dilution, 1:500), anti-ATF6 (50 kDa; catalog no. sc-22799;
dilution, 1:500), anti-C/emopamil binding protein homologous
protein (CHOP; (catalog no. sc-7351; dilution, 1:500),
anti-γ-glutamylcysteine synthetase heavy chain (γ-GCSh;
catalog no. sc-100747; dilution, 1:500), anti-Bcl-2 (ctalog no.
sc-7382; dilution, 1:500), anti-survivin (catalog no. sc-10811;
dilution, 1:500) (all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-β-catenin (catalog no. 06-734; dilution, 1:2,500;
Upstate Biotechnology, Inc., Lake Placid, NY, USA),
anti-poly(adenosine diphosphate-ribose) polymerase 1 (PARP-1;
1074-1; dilution, 1:1,000; Epitomics, Burlingame, CA, USA) and
anti-β-actin (catalog no. A5441; dilution, 1:10,000; Sigma-Aldrich;
Merck Millipore) antibodies, followed by incubation with secondary
antibodies conjugated to horseradish peroxidase. The
antigen-antibody complexes were detected using a Pierce enhanced
chemiluminescence detection system (Thermo Fisher Scientific,
Inc.).
Flow cytometric measurement of
intracellular nitric oxide (NO) and cellular glutathione (GSH)
depletion
All fluorescence reagents were purchased from
Sigma-Aldrich (Merck Millipore), unless otherwise specified.
Intracellular NO production was measured using
4,5-diaminofluorescein (DAF-2), as described previously (13). Briefly, cells were treated with
cisplatin, washed with phosphate-buffered saline (PBS) and then
incubated with 1 µM DAF-2 for 10 min in the dark. The cells were
then washed with PBS, detached by trypsinization, collected by
centrifugation at 0.2 × g at room temperature and
resuspended in PBS. To measure cellular GSH depletion,
cisplatin-treated cells were incubated with 25 µM
5-chloromethylfluorescein (CMF) diacetate for 20 min at 5%
CO2 in a 37°C incubator. The CMF fluorescence intensity
was measured using a BD FACSCalibur™ flow cytometer (version 3.3;
BD Biosciences, San Jose, CA, USA) and analyzed using BD CellQuest
Pro software (BD Biosciences).
Detecting intracellular NO using
confocal microscopy
To measure the production of intracellular NO,
cisplatin- and sham-treated HCC cells were cultured in
poly-L-lysine-coated slides. All fluorescence reagents were
purchased from Sigma-Aldrich (Merck Millipore). Upon reaching 80%
cell density, the cells were incubated with 2 µM
DAF-2′,7′-difluorofluorescein diacetate in
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid for 30 min in
the dark and imaged using a Leica TCS SP2 laser scanning confocal
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Confocal microscopic detection of
mitochondrial and cytosolic calcium (Ca2+)
Cells were stained with 1.5 µM Rhod-2 and 2 mM
Fluo-4 (Invitrogen; Thermo Fisher Scientific, Inc.) to detect
mitochondrial and cytosolic Ca2+, respectively.
Fluorescent images of Rhod-2 and Fluo-4 were obtained prior and
subsequent to treating the cells with cisplatin. At the end of each
experimental period, cells were mounted on the stage of a Leica TCS
SP2 laser scanning confocal microscope. Then, cells were randomly
selected and imaged.
Terminal deoxynucleotidyl transferase
2′-deoxyuridine 5′-triphosphate nick end labeling (TUNEL)
assay
DNA fragmentation was detected using a TUNEL assay
and an Apo-BrdU In Situ DNA Fragmentation Assay kit
(BioVision, Inc., Milpitas, CA, USA), according to the
manufacturer's protocol. A BD FACSCalibur™ was used to perform the
analyses. DNA content was quantified using ModFit LT™ software
(version 3.0; Verity Software House, Inc., Topsham, ME, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation from ≥3 independent experiments, and were analyzed using
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HCC cells exhibit distinct disparity
in response to chemotherapeutic drugs
Two chemotherapeutic drugs, cisplatin and
doxorubicin, were selected to test their cytotoxicity effect on two
HCC cell lines, Mahlavu (a poorly differentiated and highly
Bcl-2-expressing cell line with a p53 mutation at codon 249) and
Hep3B (a well-differentiated and low Bcl-2-expressing subline
formed by p53-null cells) (13–15). As
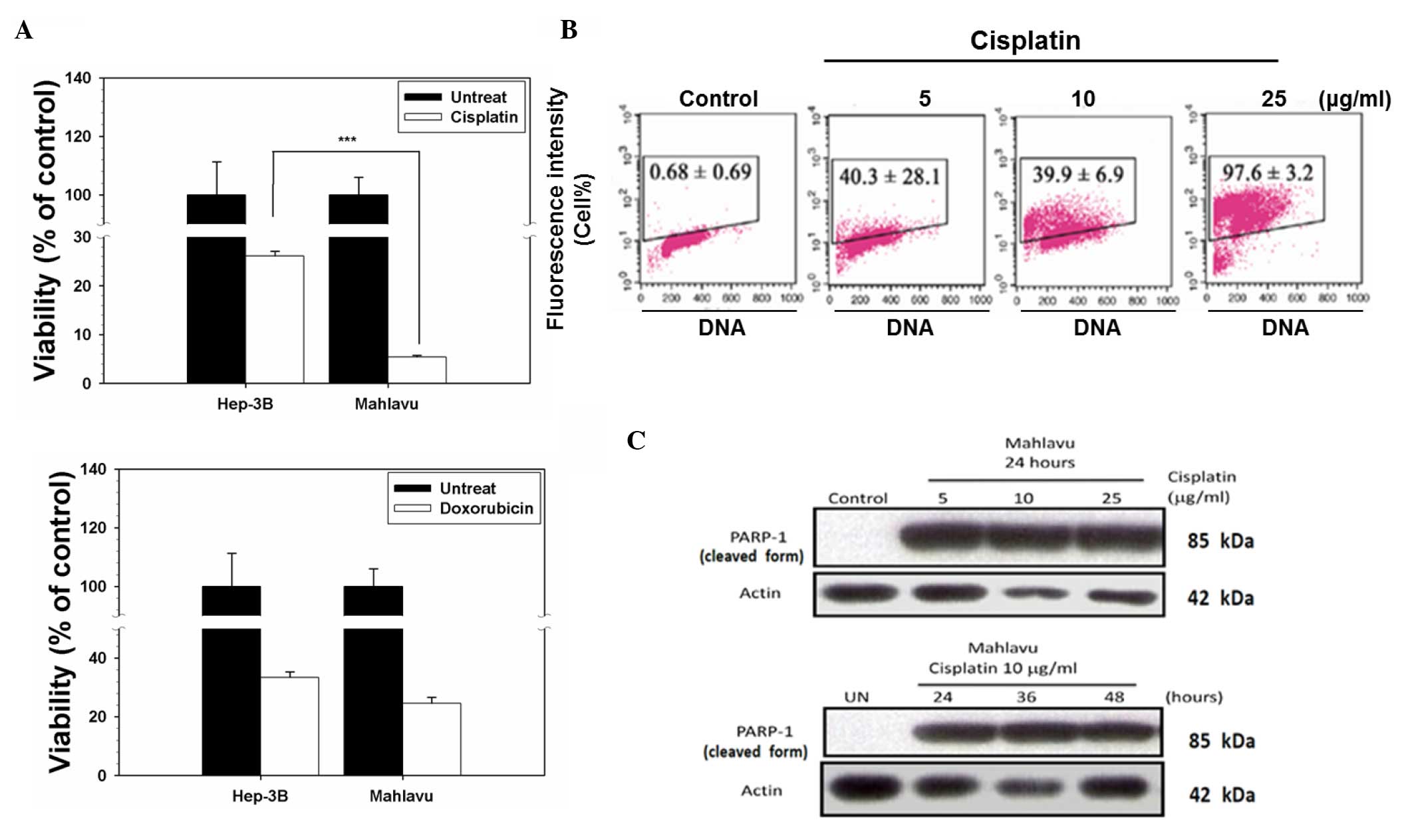
indicated in Fig. 1A, upon treatment
with cisplatin, cell viability decreased to 5% in the Mahlavu
cells, whereas 28% of the Hep3B cells remained viable. However,
there was no significant difference between the Mahlavu and Hep3B
cells upon treatment with doxorubicin (P=0.086). By contrast, the
Mahlavu cells were observed to be distinctly more sensitive to
cisplatin than Hep3B cells. Next, cisplatin-induced apoptosis was
investigated by a TUNEL assay. Cisplatin induced cell apoptotic
death in a concentration-dependent manner in the Mahlavu cells
(Fig. 1B), and it also induced the
cleavage of PARP-1 (a 116-kDa nuclear enzyme downstream of caspase
3) to produce an 85-kDa fragment (Fig.
1C). These data clearly demonstrated that the cell death
associated with cisplatin treatment was apoptotic in nature.
Cisplatin provokes NO production and
perturbs Ca2+ homeostasis
Accumulating evidence indicates that
cisplatin-induced apoptosis could occur independently of DNA damage
through oxidative stress in various cell types (5–7).
Therefore, it was examined whether cisplatin regulates ER stress
through aggravated nitrosative stress coupled to perturbed
mitochondrial Ca2+ homeostasis. As represented in
Fig. 2A, when Mahlavu cells were
treated with cisplatin, a stimulated overproduction of NO was
observed, according to the data obtained using flow cytometry.
Furthermore, cisplatin triggered an increased influx of cytosolic
Ca2+ into the mitochondria and caused a Ca2+
overload in these organelles, as indicated by the red fluorescence
of Rhod-2 (Fig. 2B). Thus, cisplatin
was demonstrated to induce ER stress response through aggravated
nitrosative stress coupled to perturbed mitochondrial
Ca2+ homeostasis.
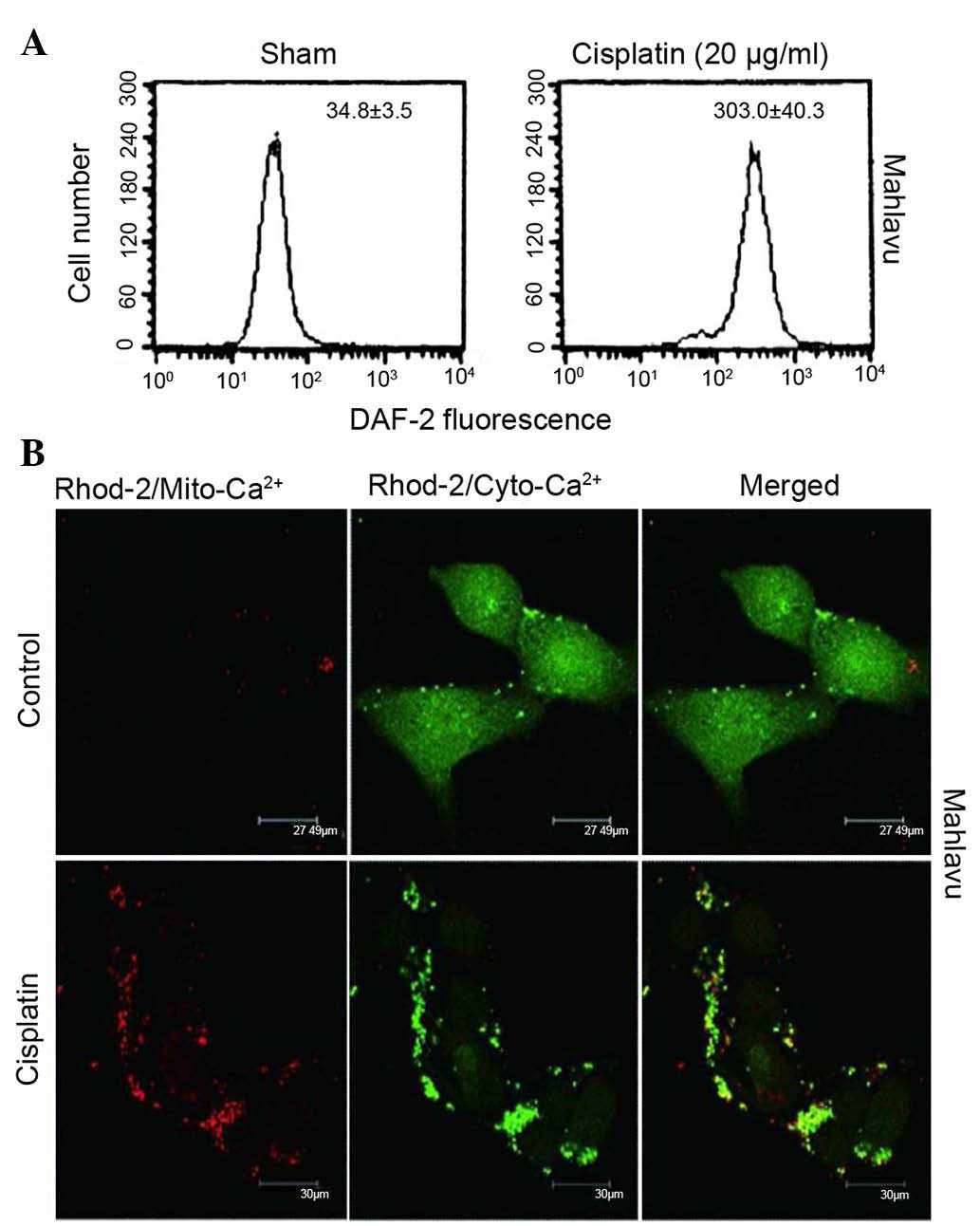 | Figure 2.Cisplatin could provoke increased
production of NO and perturbed Ca2+ homeostasis. (A) A
nearly 10-fold increase in NO production was observed when Mahlavu
cells were treated with 20 µg/ml cisplatin, as demonstrated by flow
cytometry using DAF-2 as a probe. (B) At resting state, Mahlavu
cells exhibited relatively low levels of mitochondrial
Ca2+ (Rhod-2/sham) (×400 magnification). Upon cisplatin
treatment (25 µg/ml), significantly increased Rhod-2 fluorescence
(mito-Ca2+) (red color) was detected, accompanied by a
concomitantly reduced Fluo-4 fluorescence (cyto-Ca2+)
(green color), indicating that mitochondrial Ca2+
overload had occurred. DAF-2, 4,5-diaminofluorescein; NO, nitric
oxide; Ca2+, calcium; Mito, mitochondrial; Cyto,
cytosolic. |
Cisplatin-induced ER stress response
activates the ATF4- ATF3-CHOP axis
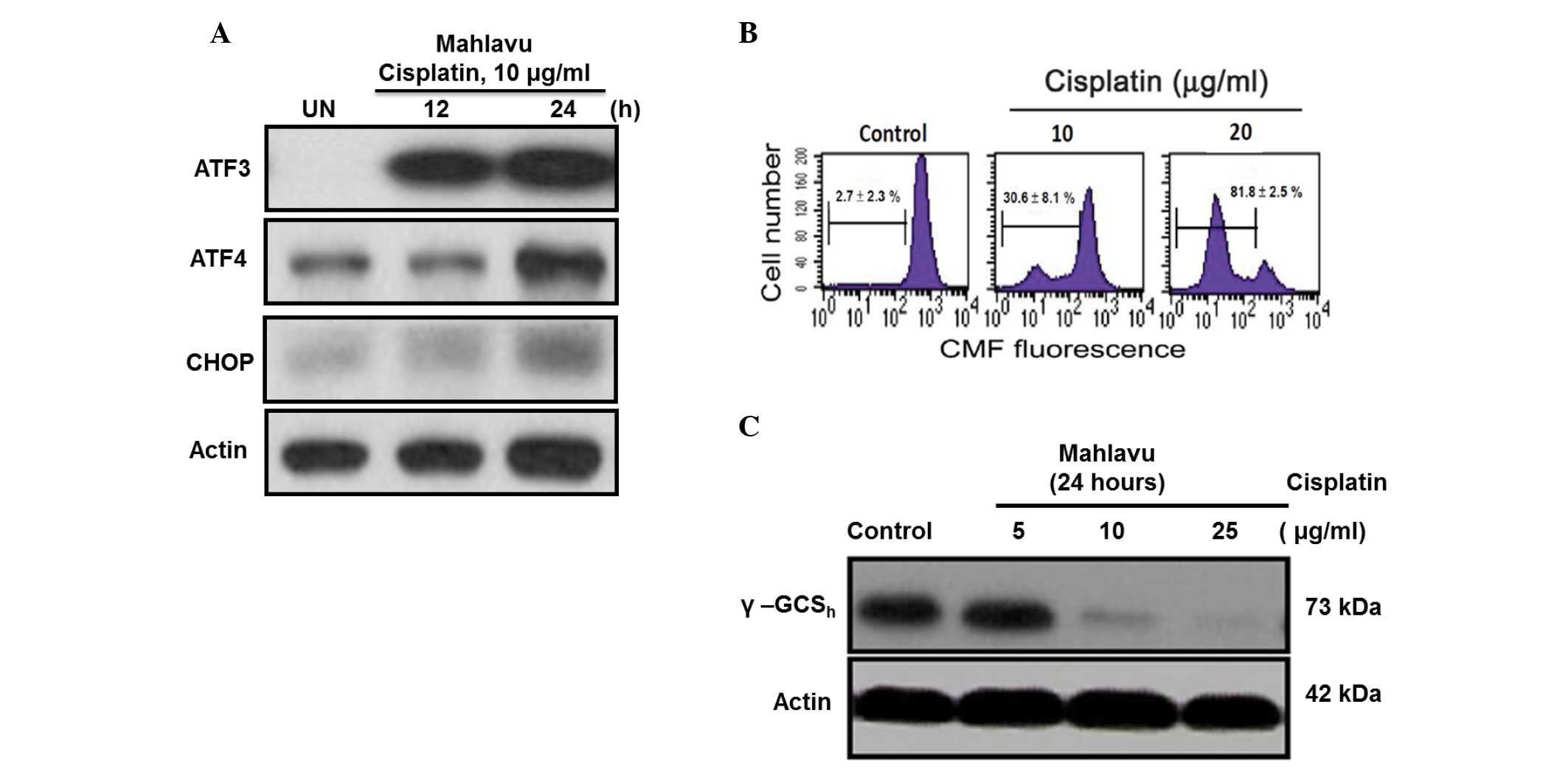
The present study demonstrated that
cisplatin-induced ER stress response could also regulate the
ATF4-ATF3-CHOP axis and its downstream molecules. In Mahlavu cells
treated with cisplatin for 24 h, the ATF4-ATF3-CHOP axis was
concomitantly activated following treatment (Fig. 3A). The CHOP-mediated downstream
molecule GSH may be critical for cell apoptosis (16). In the present study, a
concentration-dependent depletion of intracellular GSH was
identified using flow cytometry (Fig.
3B). Additionally, the current results revealed the first
evidence that CHOP-mediated GSH depletion is at least in part due
to the strong inhibition by cisplatin of γ-GCSh, a
rate-limiting enzyme responsible for cellular GSH biosynthesis
(Fig. 3C) (17).
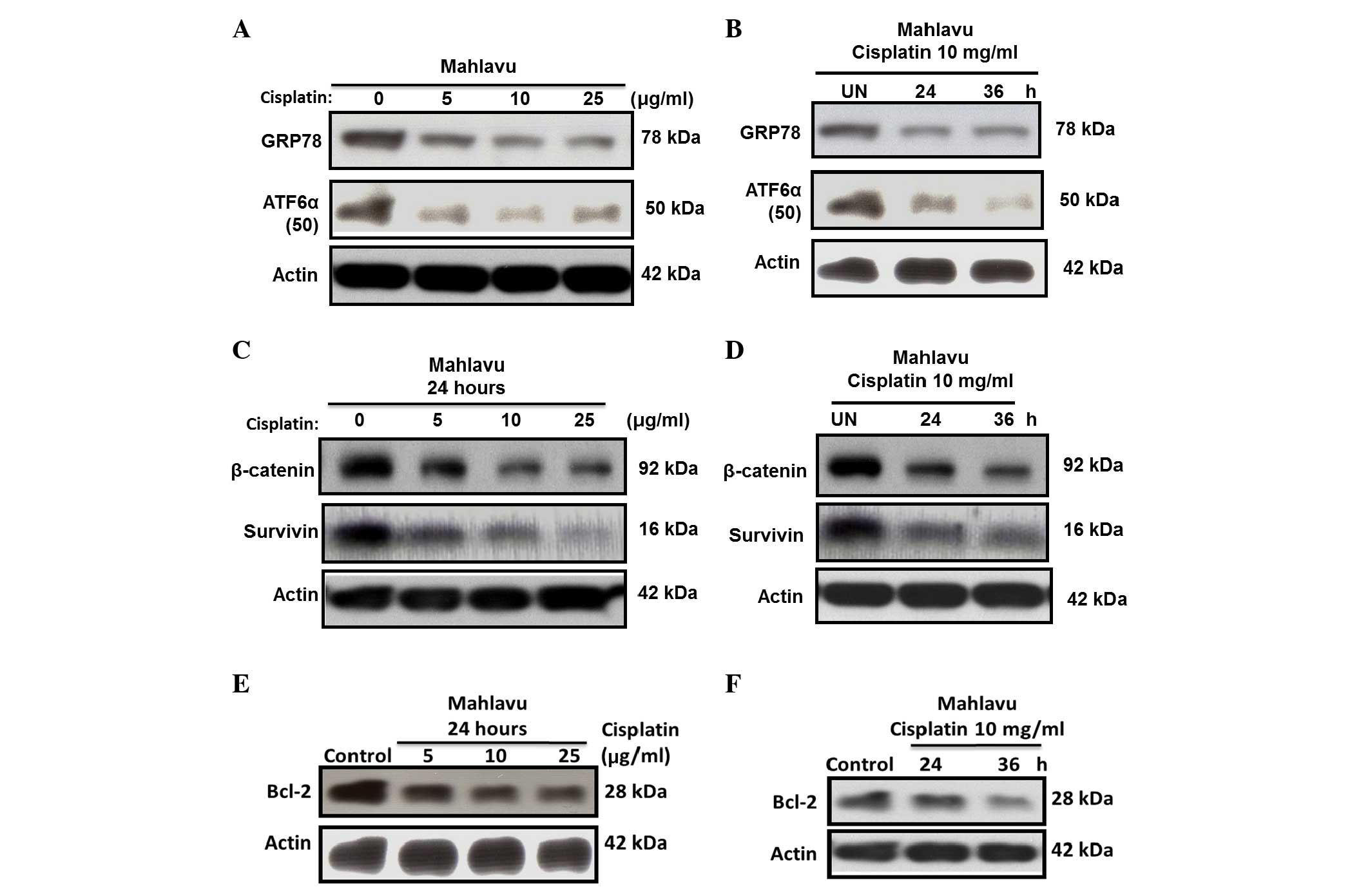
Cisplatin severely downregulates GRP78
via suppressed ATF6α-p50 expression
ER stress can induce transcriptional factor
activation; therefore, the present study sought to determine
whether cisplatin-induced ER stress regulates the activation of the
transcriptional factor ATF6α. At resting state, Mahlavu cells were
observed to be a high GRP78-expressing HCC subline, due to its
constitutive high levels of active ATF6α-p50. However, upon
cisplatin treatment, strong suppression of the active ATF6α-p50
form and downregulation of GRP78 expression was observed (Fig. 4A and B).
Cisplatin induces survivin and Bcl-2
downregulation through inhibiting β-catenin
Since the availability of β-catenin and its nuclear
translocation is key in controlling the transcription of downstream
genes of the Wnt signaling pathway (18,19), the
current study investigated whether cisplatin could also
downregulate the expression of survivin and Bcl-2 through
inhibiting β-catenin. The results revealed that the expression of
survivin (Fig. 4C and D) and Bcl-2
(Fig. 4E and F) in the Mahlavu cells
was substantially inhibited by cisplatin in a time- and
concentration-dependent manner. This phenomenon correlated closely
with the inhibition of β-catenin, suggesting that the expression of
survivin and Bcl-2 through the Wnt signaling pathway was largely
blocked.
Discussion
The present study identified a crucial missing link
in the understanding of the mechanism associated with
cisplatin-induced ER stress. Two HCC model cell lines were
selected, Mahlavu and Hep3B, with different p53 dysfunctions.
Generally, HCC lines were subjected to higher doses of cisplatin
(25–200 µM) in previous platinum-based drug studies (20,21),
according to the literature. For example, Hep3B was observed to be
resistant to p53-mediated growth arrest and apoptosis (22), and received a high dose of cisplatin
(60 µM for 24 h) in a previous study (20). Notably, when comparing the two HCC
cell lines used in the present study, the Mahlavu cells were
observed to be considerably more sensitive to cisplatin than the
Hep3B cells (Fig. 1). This result may
reflect differences in their p53 and molecule expression status
(14,15). The cisplatin response has been
reported to involve ER stress (8,23,24). The ER is the most critical
protein-folding compartment and an intracellular Ca2+
storage organelle in cells (25).
Thus, accumulating reactive nitrogen species could modify proteins
and disrupt Ca2+ homeostasis, which may trigger the
induction of cisplatin-induced ER stress (26,27). Upon
cisplatin treatment, intracellular NO levels increased nearly by
10-fold in the Mahlavu cells (Fig.
2A). This increased NO production could have caused ER
Ca2+ depletion and subsequently increased the influx of
cytosolic Ca2+ into the mitochondria, which could
eventually trigger mitochondrial Ca2+overload (Fig. 2B). These findings support the notion
that NO-dependent mitochondrial disruption can be coupled to the ER
stress response (14,15,20,21). The
present study revealed that cisplatin-induced ER stress response
simultaneously provoked two prominent pathways that may induce
cells apoptosis. The key effectors of ER stress-signaling pathways
may modulate cisplatin-induced cell death (27) through GRP78 (24) or β-catenin (28). Therefore, identifying those molecules
that can be used to predict cisplatin treatment response would
enable therapy to be tailored on an individual HCC patient basis.
GRP78 is an ER-resident protein responsible for protein folding and
assembly, and its expression level has been correlated with the
acquisition of resistance by certain malignancies against diverse
anticancer drugs (29,30). Since the upregulation of GRP78 was
frequently observed in HCC cells (15), the present study investigated how
cisplatin affects the expression of GRP78 in association with the
possible nitrosative stress-mediated mechanism in the Mahlavu cell
model. The uniqueness of the Mahlavu cells was that they
constitutively overexpressed GRP78, likely through a mild,
endogenously generated NO-mediated increase in the active p50 form
of the transcription factor ATF6 (ATF6α-p50), which, by nuclear
translocation, could release GRP78 from its conjugated form
(18,26,27,31). In
the present study, cisplatin was observed to suppress GRP78 through
a mechanism involving the strong inhibition of the expression of
ATF6α-p50. In this situation, the ability of the cells to withstand
ER stress-induced apoptosis could then be considerably weakened.
The present results also revealed that ATF4, ATF3 and CHOP were all
highly induced by cisplatin (Fig.
3A). Cisplatin-induced CHOP expression is mediated through the
cooperative interaction of ATF4 and ATF3 (Fig. 3A). Since ATF3 has been reported to be
induced by a variety of stress-causing agents, including DNA
damages (25), it is expected that
cisplatin, a chemotherapeutic agent regarded as a typical
DNA-damaging agent (Fig. 3A), could
induce ATF3 expression. The upregulation of ATF3 has been
demonstrated to play a pivotal role in the apoptosis induced by
various histone deacetylase inhibitors and certain chemotherapeutic
agents such as cisplatin (32,33). Based
on these observations, it can be concluded that the suppressed
active ATF6α-p50 formed by cisplatin can render GRP78
downregulation. In this situation, the induction of CHOP mediated
through the cross-talk of ATF4 and ATF3 can be triggered (31). Consistent with the findings reported
in the literature, the present authors previously observed that
cisplatin-induced ER stress response and CHOP expression could
trigger GSH depletion (31,34). Whether cisplatin-induced CHOP
induction can also be triggered by a similar mechanism is currently
under investigation by the present authors. Furthermore, the
mechanism by which GSH depletion occurs during ER stress-mediated
CHOP induction has not previously been addressed. The present study
provides the first evidence that CHOP-mediated cellular GSH
depletion is primarily due to the robust inhibition by cisplatin of
γ-GCSh, a rate-limiting enzyme responsible for the
synthesis of cellular GSH (Fig. 2C)
(17).
The activation of the Wnt/β-catenin pathway has been
reported in HCC cells (18,19). β-catenin, a central effector molecule,
works with the T-cell factor family of transcription factors to
activate the expression of specific oncogenes, including cyclin D1,
c-Myc, vascular endothelial growth factor, Bcl-2 and survivin
(35–37). Mechanistically, determining the extent
to which cisplatin is involved in modulating the transcriptional
activity of the Wnt/β-catenin signaling pathway that could
eventually lead to the downregulation of survivin is warranted.
Using confocal microscopy imaging, the present study determined
that the majority of β-catenin was localized in the nucleus, thus
leading to an increased expression of survivin (data not shown).
Since β-catenin was overexpressed in Mahlavu cells, the present
study observed that, upon cisplatin treatment, the β-catenin
expression of the Mahlavu cells was substantially suppressed. This
suppression could in turn markedly reduce the nuclear translocation
of β-catenin, thus leading to the suppressed oncogenic expression
of survivin (Fig. 4C and D) and Bcl-2
(Fig. 4E and F).
In conclusion, the present study provides the first
evidence that the cisplatin-triggered apoptosis of HCC Mahlavu
cells involves the robust inhibition of three prominent effector
molecules, including GRP78, Bcl-2 and survivin, through the
dualistic modulation of nitrosative stress-mediated ER stress
response via activation of the ATF4-ATF3-CHOP axis and the
downregulation of the Wnt/β-catenin signaling pathway (Fig. 5). The current results suggest that
cisplatin could be clinically useful in eradicating cancer cells
that are resistant to chemotherapeutic agents due to the enhanced
expression of GRP78, Bcl-2 or survivin.
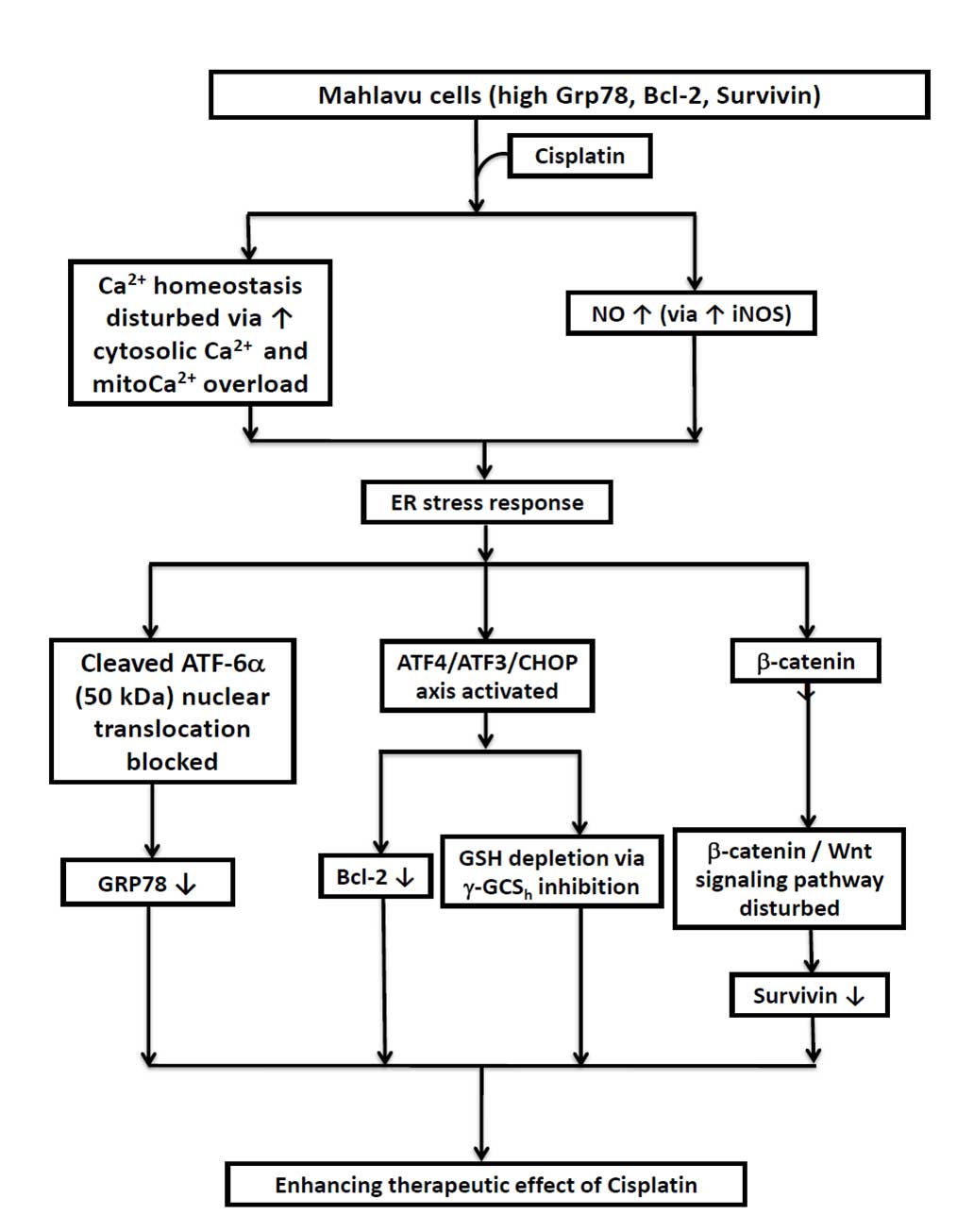 | Figure 5.Diagrammatic illustration of the
three critical cascades of events that negatively modulate the
expression of three prominent cisplatin-associated anti-apoptotic
effectors of hepatocellular carcinoma Mahlavu cells. RT,
radiotherapy; Ca2+, calcium; NO, nitric oxide; iNOS,
inducible nitric oxide synthase; ER, endoplasmic reticulum; ATF,
activating transcription factor; CHOP, C/emopamil binding protein
homologous protein; CMF, 5-chloromethylfluorescein; GSH,
glutathione; γ-GCSh, γ-glutamylcysteine synthetase heavy
chain; GRP, glucose-regulated protein; Bcl-2, B-cell lymphoma
2. |
Acknowledgements
The present study was supported by grants from the
Taiwan National Science Council (Taipei, Taiwan; grant no. NSC
99-2314-B-038-026-MY3), the Ministry of Science and Technology of
the R.O.C (Tapei, Taiwan; grant no. MOST 103-2314-B-038-058) and a
Chi Mei Medical Center (CM) - Taipei Medical University (TMU) joint
grant (Taipei, Taiwan; grant no. 98 CM-TMU 01-02).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim YS, Han S, Heo NY, Shim JH, Lee HC and
Suh DJ: Mortality, liver transplantation, and hepatocellular
carcinoma among patients with chronic hepatitis B treated with
entecavir vs lamivudine. Gastroenterology. 147:152–161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tabrizian P, Roayaie S and Schwartz ME:
Current management of hepatocellular carcinoma. World J
Gastroenterol. 20:10223–10237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaisman A, Varchenko M, Said I and Chaney
SG: Cell cycle changes associated with formation of Pt-DNA adducts
in human ovarian carcinoma cells with different cisplatin
sensitivity. Cytometry. 27:54–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burger H, Nooter K, Boersma AW, Kortland
CJ and Stoter G: Lack of correlation between cisplatin-induced
apoptosis, p53 status and expression of Bcl-2 family proteins in
testicular germ cell tumour cell lines. Int J Cancer. 73:592–599.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS
and Kim YK: Differential roles of hydrogen peroxide and hydroxyl
radical in cisplatin-induced cell death in renal proximal tubular
epithelial cells. J Lab Clin Med. 142:178–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Y, Zhou S, Qui L, Wu J and Xu C:
Effects of zinc gluconate on nephrotoxicity and glutathione
metabolism disorder induced by cis-platin in mice. Drug Metabol
Drug Interact. 14:41–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo TC, Chang PY, Huang SF, Chou CK and
Chao CC: Knockdown of HURP inhibits the proliferation of
hepacellular carcinoma cells via downregulation of gankyrin and
accumulation of p53. Biochem Pharmacol. 83:758–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH,
Lu FJ, Lin YS, Kuo SH and Chen CH: 6-shogaol (alkanone from ginger)
induces apoptotic cell death of human hepatoma p53 mutant Mahlavu
subline via an oxidative stress-mediated caspase-dependent
mechanism. J Agric Food Chem. 55:948–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matouk IJ, Mezan S, Mizrahi A, Ohana P,
Abu-Lail R, Fellig Y, Degroot N, Galun E and Hochberg A: The
oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim
Biophys Acta. 1803:443–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CH, Uen YH, Ho CT, Tseng YT, Liu TZ,
Chiou JF and Leung SW: Constitutive overexpression of Bcl-2,
Survivin and ER stress chaperone GRP-78 confers intrinsic
radioresistance in human hepatocellular carcinoma cells: Insight
into the mechanistic pathways involved*. J Cancer Ther. 4:3992013.
View Article : Google Scholar
|
|
16
|
Hsu HC, Chiou JF, Wang YH and Chen CH, Mau
SY, Ho CT, Change PJ, Liu TZ and Chen CH: Folate deficiency
triggers an oxidative-nitrosative stress-mediated apoptotic cell
death and impedes insulin biosynthesis in RINm5F pancreatic islet
beta-cells: Relevant to the pathogenesis of diabetes. PLoS One.
8:e779312013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griffith OW and Meister A: Potent and
specific inhibition of glutathione synthesis by buthionine
sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem.
254:7558–7560. 1979.PubMed/NCBI
|
|
18
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takigawa Y and Brown AM: Wnt signaling in
liver cancer. Curr Drug Targets. 9:1013–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim Y, Jang M, Lim S, Won H, Yoon KS, Park
JH, Kim HJ, Kim BH, Park WS, Ha J and Kim SS: Role of cyclophilin B
in tumorigenesis and cisplatin resistance in hepatocellular
carcinoma in humans. Hepatology. 54:1661–1678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim SC, Choi JE, Kang HS and Han SI:
Ursodeoxycholic acid switches oxaliplatin-induced necrosis to
apoptosis by inhibiting reactive oxygen species production and
activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma.
Int J Cancer. 126:1582–1595. 2010.PubMed/NCBI
|
|
22
|
Friedman SL, Shaulian E, Littlewood T,
Resnitzky D and Oren M: Resistance to p53-mediated growth arrest
and apoptosis in Hep 3B hepatoma cells. Oncogene. 15:63–70. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rabik CA, Fishel ML, Holleran JL, Kasza K,
Kelley MR, Egorin MJ and Dolan ME: Enhancement of cisplatin
[cis-diammine dichloroplatinum (II)] cytotoxicity by
O6-benzylguanine involves endoplasmic reticulum stress. J Pharmacol
Exp Ther. 327:442–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmad M, Hahn IF and Chatterjee S: GRP78
up-regulation leads to hypersensitization to cisplatin in A549 lung
cancer cells. Anticancer Res. 34:3493–3500. 2014.PubMed/NCBI
|
|
25
|
Xu W, Liu L, Charles IG and Moncada S:
Nitric oxide induces coupling of mitochondrial signalling with the
endoplasmic reticulum stress response. Nat Cell Biol. 6:1129–1134.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao S, Xiong Z, Mao X, Meng D, Lei Q, Li
Y, Deng P, Chen M, Tu M, Lu X, et al: Atmospheric pressure room
temperature plasma jets facilitate oxidative and nitrative stress
and lead to endoplasmic reticulum stress dependent apoptosis in
HepG2 cells. PLoS One. 8:e736652013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Y, Wang C and Li Z: A new strategy of
promoting cisplatin chemotherapeutic efficiency by targeting
endoplasmic reticulum stress. Mol Clin Oncol. 2:3–7.
2014.PubMed/NCBI
|
|
28
|
Verras M, Papandreou I, Lim AL and Denko
NC: Tumor hypoxia blocks Wnt processing and secretion through the
induction of endoplasmic reticulum stress. Mol Cell Biol.
28:7212–7224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li
YL, Wang HM, Chang JT and Cheng AJ: Grp78 as a therapeutic target
for refractory head-neck cancer with CD24(−)CD44(+) stemness
phenotype. Cancer Gene Ther. 20:606–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin JA, Fang SU, Su CL, Hsiao CJ, Chang
CC, Lin YF and Cheng CW: Silencing glucose-regulated protein 78
induced renal cell carcinoma cell line G1 cell-cycle arrest and
resistance to conventional chemotherapy. Urol Oncol. 32:29.e1–e11.
2014. View Article : Google Scholar
|
|
31
|
St Germain C, O'Brien A and Dimitroulakos
J: Activating Transcription Factor 3 regulates in part the enhanced
tumour cell cytotoxicity of the histone deacetylase inhibitor M344
and cisplatin in combination. Cancer Cell Int. 10:322010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang N, Zhang H, Si-Ma H, Fu Y, Zhao W, Li
D and Yang G: Dexamethasone decreases hepatocellular carcinoma cell
sensitivity to cisplatin-induced apoptosis. Hepatogastroenterology.
58:1730–1735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu G, Su L, Hao X, Zhong N, Zhong D,
Singhal S and Liu X: Salermide up-regulates death receptor 5
expression through the ATF4-ATF3-CHOP axis and leads to apoptosis
in human cancer cells. J Cell Mol Med. 16:1618–1628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong D, Ni M, Li J, Xiong S, Ye W, Virrey
JJ, Mao C, Ye R, Wang M, Pen L, et al: Critical role of the stress
chaperone GRP78/BiP in tumor proliferation, survival, and tumor
angiogenesis in transgene-induced mammary tumor development. Cancer
Res. 68:498–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Altieri DC: Molecular circuits of
apoptosis regulation and cell division control: The survivin
paradigm. J Cell Biochem. 92:656–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tien LT, Ito M, Nakao M, Niino D, Serik M,
Nakashima M, Wen CY, Yatsuhashi H and Ishibashi H: Expression of
beta-catenin in hepatocellular carcinoma. World J Gastroenterol.
11:2398–2401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thompson MD and Monga SP: WNT/beta-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007. View Article : Google Scholar : PubMed/NCBI
|