Introduction
Endometrial stromal sarcoma (ESS) is an uncommon
malignancy, accounting for <1% of all uterine carcinomas and
7–15% of all uterine sarcomas (1).
ESS is classified as low-grade endometrial stromal sarcoma (LGESS),
high-grade endometrial stromal sarcoma (HGESS) and undifferentiated
uterine sarcoma (2). LGESS shows
minimal to no cytological atypia and low mitotic activity (usually
<5 mitoses per 10 high-power fields (HPFs). HGESS shows high
mitotic activity (typically >10 per 10 HPFs) and is typically
very striking. LGESS is generally a slow-growing malignancy with an
indolent clinical course, but with a tendency for late recurrence,
while HGESS is more aggressive, frequently metastasizes and has an
extremely poor outcome.
Although no universal staging system exists for ESS,
the International Federation of Gynecology and Obstetrics surgical
staging system for endometrial cancer is typically used (2). Total abdominal hysterectomy and
bilateral salpingo-oophorectomy is recommended as the primary
treatment, with debulking recommended when extrauterine disease is
apparent. The role of chemotherapy, radiation or hormonal treatment
as adjuvant therapy has not yet been established. A number of
studies have demonstrated estrogen and progesterone receptor
expression in LGESS (3,4). Furthermore, LGESS has previously been
shown to be responsive to hormonal therapy, including aromatase
inhibitors and megestrol acetate (3,5,6). Studies have shown that synthesized
progestins, including medroxyprogesterone acetate (MPA), are an
effective conservative treatment for endometrial cancer (7,8).
In our previous study, we reported the cases of 2
patients with metastatic LGESS lesions who experienced prolonged
survival following treatment with MPA (9). However, to the best of our knowledge,
only 5 case reports detailing recurrent LGESS treated with
aromatase inhibitors are reported in the literature. The present
study reports a case of recurrent LGESS that was treated with
surgery, followed by MPA for 2 years as first-line therapy and the
aromatase inhibitor letrozole for 6 years as second-line hormonal
therapy. The patient has survived for 13 years since the initial
surgery.
Case report
A 58-year-old (gravida 2, para 2) woman was referred
to Shimane University School of Medicine (Izumo, Japan) in May 2002
due to persistent abnormal vaginal bleeding. The patient reported a
history of rheumatoid arthritis, but no other significant past
medical or surgical history. Endometrial curettage revealed LGESS,
based on the characteristics of the cells observed, which resembled
the stromal cells of proliferative endometrium. In consequence, a
total abdominal hysterectomy with bilateral salpingo-oophorectomy
was performed in May 2002. The resected specimens were sectioned
(section thickness, 3 µm), and stained with hematoxylin and eosin.
Subsequently, the specimens were immunohistochemically stained with
the following antibodies: Anti-cluster of differentiation (CD) 10
(1:1; pre-diluted rabbit monoclonal; clone SP67; Roche Diagnostics,
Basel, Switzerland); anti-estrogen receptor (1:1; pre-diluted
rabbit monoclonal; clone SP1; Roche Diagnostics); anti-progesteron
receptor (1:1; pre-diluted rabbit monoclonal; clone 1E2; Roche
Diagnostics); anti-h-caldesmon (1:50; mouse monoclonal; clone h-CD;
Dako, Glostrup, Denmark); anti-cytokeratin AE1/AE3/PCK26 (1:1;
pre-diluted; clone AE1/AE3/PCK26; Roche Diagnostics);
anti-cytokeratin Cam5.2 (1:2; mouse monoclonal; clone Cam5.2; Roche
Diagnostics); anti-desmin (1:100; mouse monocolonal; clone D33;
Dako); anti-α-smooth muscle actin (1:100; mouse monoclonal; clone
1A4; Dako); anti-Melan A (1:1; pre-diluted mouse monoclonal; clone
A103; Dako); and anti-human melanoma black-45 (1:50; mouse
monoclonal; clone HMB-45; Dako). The histopathological result was
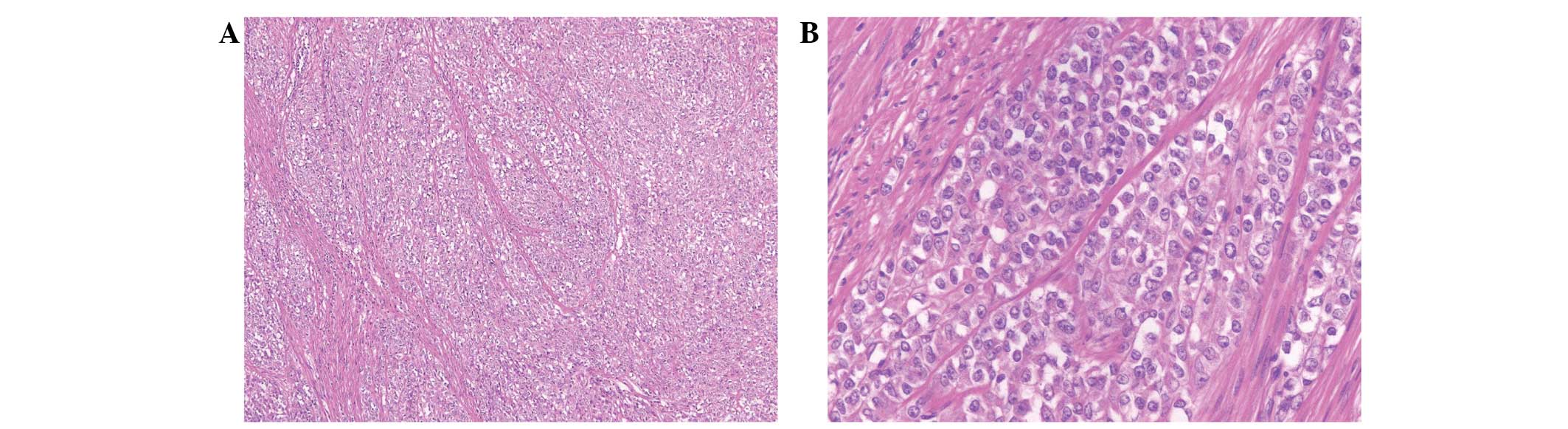
of stage IC, low-grade ESS of the corpus uteri (Fig. 1). In addition, immunostaining revealed
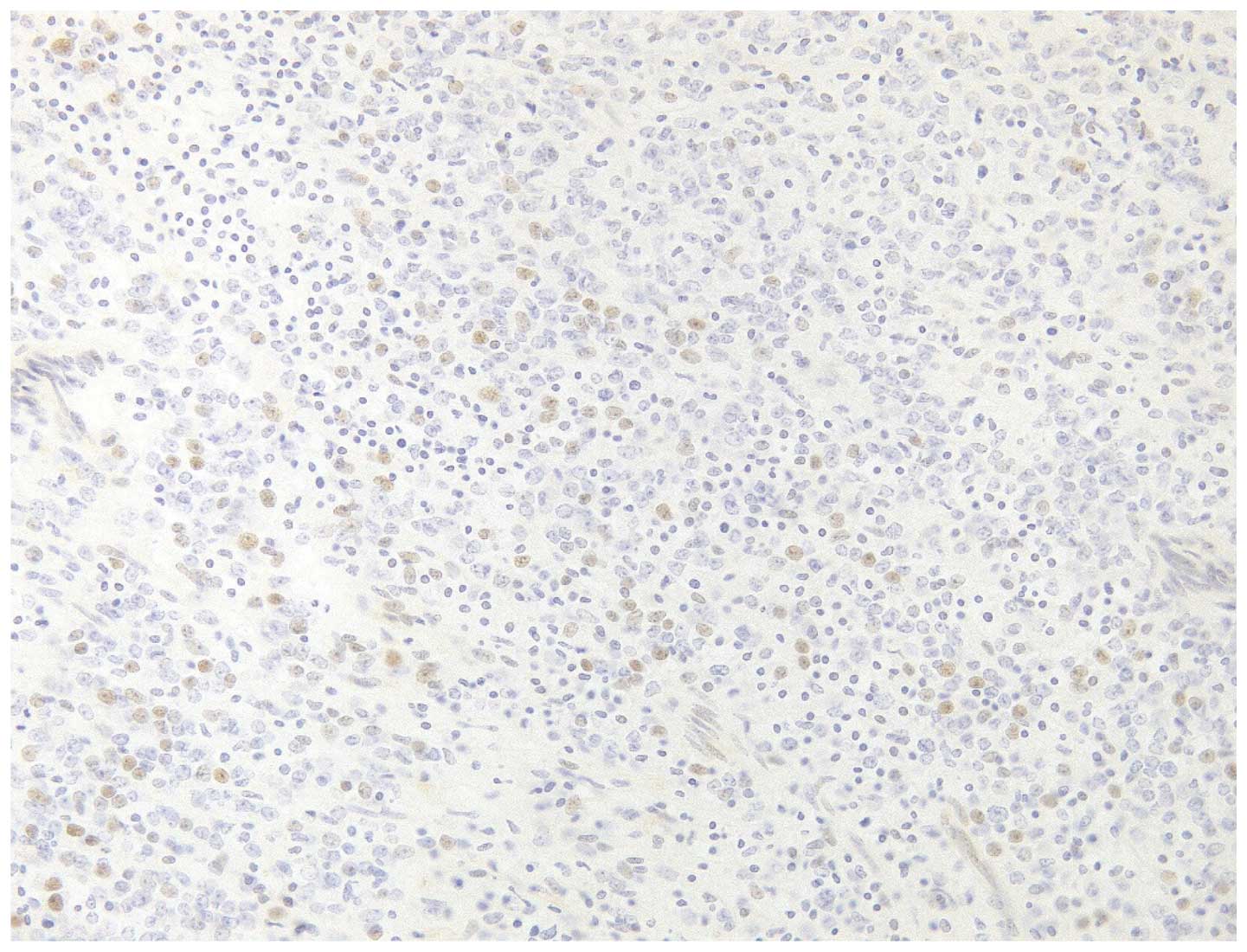
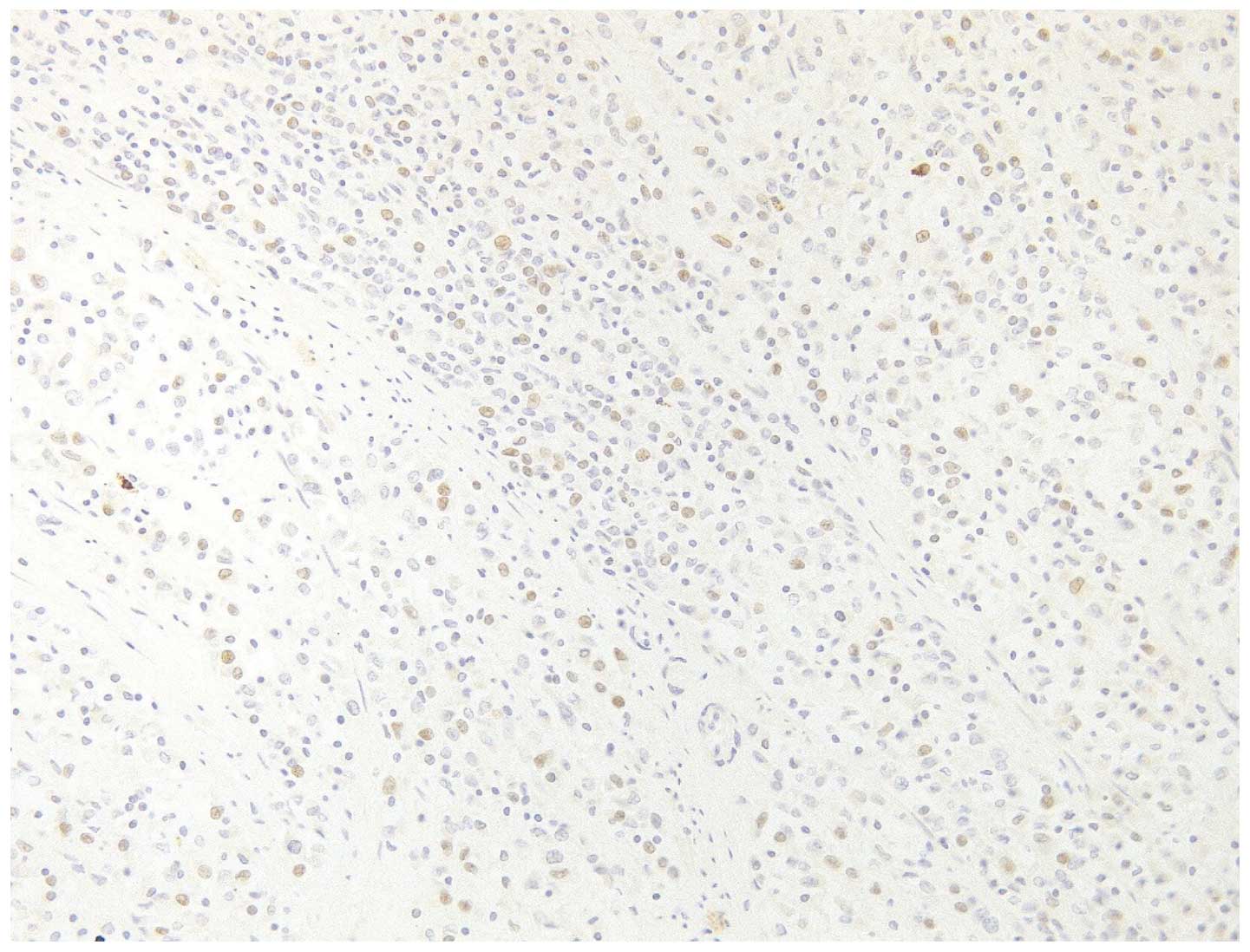
that the tumor tissue was positive for estrogen receptor (Fig. 2), progesterone receptor (Fig. 3) and CD10, and negative for
h-caldesmon, AE1/AE3, Cam5.2, desmin, α-smooth muscle actin, Melan
A and human melanoma black 45.
Post-operatively, the patient was started on 600 mg
daily MPA as adjuvant therapy. The patient experienced no
recurrence for 19 months, but was forced to discontinue MPA at that
time, as it worsened the rheumatoid arthritis symptoms. Another 2
months later, computed tomography (CT) revealed enlargement of the
common iliac lymph nodes. The patient underwent chemotherapy with 6
cycles of doxorubicin (25 mg/m2 on days 1–2) and
ifosfamide (1 mg/m2 on days 1–5) every 3 weeks, along
with lymph-node radiation. Three months after completing
chemotherapy, MPA was restarted as the rheumatoid arthritis
symptoms had improved. The lymph nodes gradually decreased in size
and this partial response was maintained for 3 years.
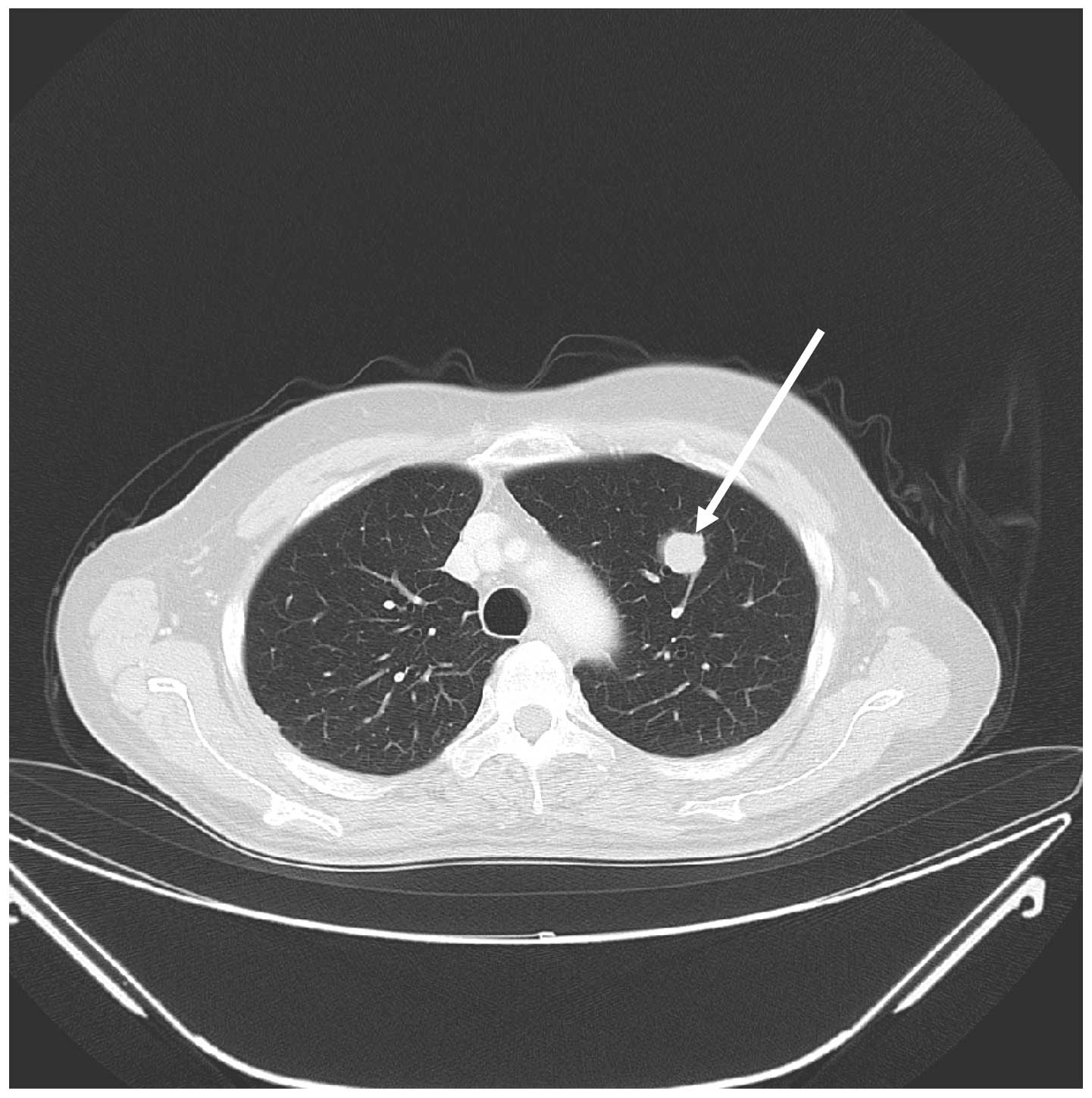
In January 2008, CT revealed a mass in the left lung
measuring 19×13 mm (Fig. 4) and a
para-aortic lymph node enlarged to 20×12 mm, compressing the right
common iliac vein. MPA treatment was discontinued at this point and
the patient underwent a secondary complete resection of the lung
tumor. Similarly to the endometrial curettage result, the
histopathological result confirmed disease metastasis.
Post-operatively, informed consent was obtained for treatment with
2.5 mg daily letrozole. The patient has continued letrozole
treatment to this date, and has remained asymptomatic and
progression-free for 7 years.
Discussion
ESS is classified as low grade, high grade and
undifferentiated based on morphology and mitotic rate. Although
LGESS exhibits a relatively indolent behavior, the possibility of
late recurrences and distant metastases exists (10). The risk of recurrence is believed to
be as much as 50%, although such tumors usually grow slowly and the
recurrence occurs late (10). In a
previous large case series, the time between diagnosis or
hysterectomy and recurrence was reported as between 3 months and 23
years, with a median time of 3 years (10). In the largest clinical study to date
on LGESS, the median time between hysterectomy and relapse was
recorded as 5.4 years for stage I disease and 9 months for disease
at stages III–IV (11). In our
previous series, the median disease-free time was 50 months
(12).
Lymphadenectomy has not been determined to confer
long-term survival in patients with LGESS (13,14). The
patient in the present study was diagnosed with LGESS following
surgery, which did not include either pelvic or para-aortic
lymphadenectomy. Although there has been no systematic study on the
advantages of adjuvant chemotherapy in LGESS, a number of
retrospective analyses have shown that doxorubicin and ifosfamide
combination chemotherapy exhibit a certain degree of efficacy
(15–18). The present patient experienced a
partial response to doxorubicin and ifosfamide-containing
chemotherapy and MPA following the first recurrence.
There are few reports on the effectiveness of
aromatase inhibitors in patients with recurrent LGESS due to the
rarity of the disease. To the best of our knowledge, there are only
5 case reports describing aromatase inhibitors as either first- or
second-line treatment for recurrent LGESS (5,19–22). Table I
shows the demographic features of the patients in these cases,
including the patient featured in the present study.
 | Table I.Previous cases of recurrent low-grade
endometrial stromal sarcoma treated with aromatase inhibitors. |
Table I.
Previous cases of recurrent low-grade
endometrial stromal sarcoma treated with aromatase inhibitors.
| First author,
year | Patient age at
diagnosis, years | Tumor stage | Immunostaining | Interval from
diagnosis to recurrence, months | Site of recurrent
lesion | First-line treatment
for recurrence | Second-line treatment
for recurrence | Survival since
initial diagnosis, years | Survival since use of
aromatase inhibitor, years | (Ref.) |
|---|
| Leunen et al
2004 | 76 | – | ER(+), PR(+) | 300 | Pelvis | Aromatase inhibitor
(letrozole) | – | 28 | 3 | (5) |
| Spano et al
2003 | 44 | – | ER(+), PR(+) | 3 | Lung, rectum | HRT | Aromatase inhibitor
(aminoglutethimide) | 16 | 8 | (19) |
|
| 34 | – | ER(+), PR(+) | 12 | Lung | HRT | Aromatase inhibitor
(letrozole) | 11 | 2 |
|
| Leiser et al
2004 | 48 | I | ER(+), PR(+) | 18 | Pelvis | Chemotherapy
(BEP) | Megestrol acetate +
aromatase inhibitor (anastrozole) | 4.5 | 2 | (20) |
| Maluf et al
2001 | 51 | – | ER(+), PR(+) | 60 | Pelvis, subcutaneous
nodules, subcapsular liver implant | MPA | Aromatase inhibitor
(letrozole) | 8 | 0.75 | (21) |
| Shoji et al
2011 | 34 | I | ER(+), PR(+) | 60 | Pelvis, ovary,
peritoneum | MPA | Aromatase inhibitor
(anastrozole) | 21 | 2 | (22) |
| Current patient | 58 | IC | ER(+), PR(+) | 19 | Lung, para-aortic
lymph node | MPA and chemotherapy
(ICA) | Aromatase inhibitor
(letrozole) | 13 | 7 |
|
Several studies have described estrogen and
progesterone receptor expression in ESS tumors, and have evaluated
the efficacy of progestins as a treatment modality (2,23–26). In all previous patients treated with
aromatase inhibitors, immunostaining was positive for estrogen and
progesterone receptors; this also applied to the present patient.
All studies in the present literature review have suggested the
effectiveness of aromatase inhibitors, including letrozole and
anastrozole, in the treatment of recurrent LGESS (5,19–22). No definitive conclusions about
treatment with aromatase inhibitors can be drawn, but this option
should be taken into consideration for patients with recurrent
LGESS and positive immunostaining for estrogen and progesterone
receptors. We recommend that immunostaining be performed when the
tumor is first determined to be ESS.
Aromatase inhibitors were used as second-line
treatment in 3 previous studies and as first-line treatment in only
1 study by Leunen et al (5).
Therefore, no conclusions can be drawn as to the priority of MPA or
aromatase inhibitors as first-line treatment. Due to their
efficacy, further studies are warranted to evaluate aromatase
inhibitors as first-line hormonal therapy in these neoplasms.
In summary, the present case reported a recurrent
LGESS that responded to treatment with the aromatase inhibitor
letrozole, and our experience suggests that aromatase inhibitor
treatment may be effective for patients with recurrent LGESS. A
number of additional case studies will be necessary to confirm
these findings and support the suggested treatment.
References
|
1
|
Echt G, Jepson J, Steel J, Langholz B,
Luxton G, Hernandez W, Astrahan M and Petrovich Z: Treatment of
uterine sarcomas. Cancer. 66:35–39. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurman RJ, Carcangiu ML, Herrington S and
Young RH: World Health Organization Classification of Tumours of
Female Reproductive Organs. 4th. IARC Press; Lyon, France:
|
|
3
|
Tsukamoto N, Kamura T, Matsukuma K, Imachi
M, Uchino H, Saito T and Ono M: Endolymphatic stromal myosis: A
case with positive estrogen and progesterone receptors and good
response to progestins. Gynecol Oncol. 20:120–128. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sabini G, Chumas JC and Mann WJ: Steroid
hormone receptors in endometrial stromal sarcomas. A biochemical
and immunohistochemical study. Am J Clin Pathol. 97:381–386. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leunen M, Breugelmans M, De Sutter P,
Bourgain C and Amy JJ: Low-grade endometrial stromal sarcoma
treated with the aromatase inhibitor letrozole. Gynecol Oncol.
95:769–771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pink D, Lindner T, Mrozek A, Kretzschmar
A, Thuss-Patience PC, Dörken B and Reichardt P: Harm or benefit of
hormonal treatment in metastatic low-grade endometrial stromal
sarcoma: Single center experience with 10 cases and review of the
literature. Gynecol Oncol. 101:464–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaku T, Yoshikawa H, Tsuda H, Sakamoto A,
Fukunaga M, Kuwabara Y, Hataeg M, Kodama S, Kuzuya K, Sato S, et
al: Conservative therapy for adenocarcinoma and atypical
endometrial hyperplasia of the endometrium in young women: Central
pathologic review and treatment outcome. Cancer Lett. 167:39–48.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ushijima K, Yahata H, Yoshikawa H, Konishi
I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, et
al: Multicenter phase II study of fertility-sparing treatment with
medroxyprogesterone acetate for endometrial carcinoma and atypical
hyperplasia in young women. J Clin Oncol. 25:2798–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishibashi M, Nakayama K, Shamima Y,
Katagiri A, Iida K, Nakayama N and Miyazaki K: Two cases of
endometrial stromal sarcoma (ESS) in which survival was prolonged
by administration of MPA. Gan To Kagaku Ryoho. 35:857–861. 2008.(In
Japanese). PubMed/NCBI
|
|
10
|
Stadsvold JL, Molpus KL, Baker JJ, Michael
K and Remmenga SW: Conservative management of a myxoid endometrial
stromal sarcoma in a 16-year-old nulliparous woman. Gynecol Oncol.
99:243–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amant F, Moerman P, Cadron I, Neven P,
Berteloot P and Vergote I: The diagnostic problem of endometrial
stromal sarcoma: Report on six cases. Gynecol Oncol. 90:37–43.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakayama K, Ishikawa M, Nagai Y, Yaegashi
N, Aoki Y and Miyazaki K: Prolonged long-term survival of low-grade
endometrial stromal sarcoma patients with lung metastasis following
treatment with medroxyprogesterone acetate. Int J Clin Oncol.
15:179–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah JP, Bryant CS, Kumar S, Ali-Fehmi R,
Malone JM Jr and Morris RT: Lymphadenectomy and ovarian
preservation in low-grade endometrial stromal sarcoma. Obstet
Gynecol. 112:1102–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas MB, Keeney GL, Podratz KC and Dowdy
SC: Endometrial stromal sarcoma: Treatment and patterns of
recurrence. Int J Gynecol Cancer. 19:253–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haberal A, Kayikçioğlu F, Boran N,
Calişkan E, Ozgül N and Köse MF: Endometrial stromal sarcoma of the
uterus: Analysis of 25 patients. Eur J Obstet Gynecol Reprod Biol.
109:209–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omura GA, Blessing JA, Major F, Lifshitz
S, Ehrlich CE, Mangan C, Beecham J, Park R and Silverberg S: A
randomized clinical trial of adjuvant adriamycin in uterine
sarcomas: A gynecologic oncology group study. J Clin Oncol.
3:1240–1245. 1985.PubMed/NCBI
|
|
17
|
Pautier P, Genestie C, Fizazi K, Morice P,
Mottet C, Haie-Meder C, Le Cesne A and Lhommé C: Cisplatin-based
chemotherapy regimen (DECAV) for uterine sarcomas. Int J Gynecol
Cancer. 12:749–754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim WY, Lee JW, Choi CH, Kang H, Kim TJ,
Kim BG, Lee JH and Bae DS: Low-grade endometrial stromal sarcoma: A
single center's experience with 22 cases. Int J Gynecol Cancer.
18:1084–1089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spano JP, Soria JC, Kambouchner M,
Piperno-Neuman S, Morin F, Morere JF, Martin A and Breau JL:
Long-term survival of patients given hormonal therapy for
metastatic endometrial stromal sarcoma. Med Oncol. 20:87–93. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leiser AL, Hamid AM and Blanchard R:
Recurrence of prolactin-producing endometrial stromal sarcoma with
sex-cord stromal component treated with progestin and aromatase
inhibitor. Gynecol Oncol. 94:567–571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maluf FC, Sabbatini P, Schwartz L, Xia J
and Aghajanian C: Endometrial stromal sarcoma: Objective response
to letrozole. Gynecol Oncol. 82:384–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shoji K, Oda K, Nakagawa S, Kawana K,
Yasugi T, Ikeda Y, Takazawa Y, Kozuma S and Taketani Y: Aromatase
inhibitor anastrozole as a second-line hormonal treatment to a
recurrent low-grade endometrial stromal sarcoma: A case report. Med
Oncol. 28:771–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu MC, Mor G, Lim C, Zheng W, Parkash V
and Schwartz PE: Low-grade endometrial stromal sarcoma: Hormonal
aspects. Gynecol Oncol. 90:170–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piver MS, Rutledge FN, Copeland L, Webster
K, Blumenson L and Suh O: Uterine endolymphatic stromal myosis: A
collaborative study. Obstet Gynecol. 64:173–178. 1984.PubMed/NCBI
|
|
25
|
Katz L, Merino MJ, Sakamoto H and Schwartz
PE: Endometrial stromal sarcoma: A clinicopathologic study of 11
cases with determination of estrogen and progestin receptor levels
in three tumors. Gynecol Oncol. 26:87–97. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scribner DR Jr and Walker JL: Low-grade
endometrial stromal sarcoma preoperative treatment with Depo-Lupron
and Megace. Gynecol Oncol. 71:458–460. 1998. View Article : Google Scholar : PubMed/NCBI
|