Introduction
Colorectal cancer (CRC) is a worldwide problem for
public health, and is becoming more prevalent in Asian countries,
particularly China (1). CRC is a
prototypic model for the genetic basis of cancer. Alterations in
the DNA mismatch repair (MMR) pathway have been causally linked to
its etiology. The MMR pathway is one of the major DNA repair
pathways; it plays an important role in repairing single-base
mismatches and in insertion-deletion loops, which result from
slippage during DNA replication (2,3). More and
more MMR genes are being found to contain common single nucleotide
polymorphisms (SNPs), which can predispose individuals to
non-familial CRC with low to moderate penetrance (4–6). mutL
homolog 1 (MLH1), which is located on chromosome 3p22.2, is a key
component of the MMR system; it is involved in mismatch strand
excision and subsequent repair (7),
while recruiting other mismatch repair proteins to the mismatch
sites to correct the errors during DNA replication (8,9). The
MLH1-93G/A polymorphism (rs1800734) is located in the core promoter
region, which is essential for maximum transcriptional activity.
Polymorphism variants in this region are predicted to affect MLH1
protein expression (10,11). It was previously reported that the
loss of MLH1 proteins expression had been associated with the
susceptibility of several cancers. Based on these observations,
particularly in view of the importance of MLH1 in colorectal
carcinogenesis, we hypothesized that the polymorphism in the MLH1
gene may modulate the risk of CRC. Thus, the present matched
case-control study was performed to investigate whether any
associations exist between the −93G>A polymorphism and SCRC in
China. In addition, immunohistochemical staining was used to
measure the expression of MLH1 protein in cases with different
alleles, which including CRC and normal control cases, in order to
check the function of the −93G>A polymorphism.
Materials and methods
Approval and consent
The Institutional Review Boards of the Drum Tower
Hospital (Nanjing, Jiangsu, China) approved the study and written
informed consent was obtained from all participants.
Study population
The case-control study included 312 SCRC patients
(age range, 19–89 years; mean age, 60.52±16.38 years; male/female,
193/119) and 300 normal healthy controls (age range, 23–78 years;
mean age, 58.64±12.33 years; male/female, 169/131) recruited from
Wuxi No. 2 People's Hospital (Wuxi, China) between January 2006 and
October 2010. All patients were diagnosed with pathologically
confirmed CRC and 284 patients received surgery. The 300 control
subjects were randomly selected from the Center of Physical
Examination (Wuxi No. 2 People's Hospital) from patients who had no
history of malignancy during the same time period of the case
recruitment. None of the subjects were blood-related. All the
subjects were interviewed to obtain information on their
sociodemographic characteristics, dietary habits, smoking and
drinking status, and their individual and family history of cancer.
SCRC cases and controls were matched for age, gender, and smoking
and drinking history.
Genotyping analysis
The National Center for Biotechnology Information
SNP database (http://www.ncbi.nlm.nih.gov/snp/) and related
literature was searched to identify functional SNPs in human
(h)MLH1. The criteria for SNP selection were as follows: i) A minor
allele frequency of >0.05 in the Chinese population; ii) a
genotype call rate of ≥95%; and iii) SNPs that have been closely
associated with tumorigenesis. The MLH1-93G>A polymorphism (SNP
rs1800734) was selected for genotyping. Genomic DNA was extracted
from the peripheral blood samples of all subjects using a
purification kit (Promega, Madison, WI, USA) according to the
manufacturer's instructions. The MLH1-93G>A polymorphism was
genotyped using the TaqMan assay on an Applied
Biosystems® 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
PCR cycling conditions were as follows: 50 cycles at 95°C for 10
min, 95°C for 15 sec and 60°C for 50 sec. The primer sequences were
as follows: Forward, 5′-ACCCAGCAACCCACAGAGT-3′ and reverse,
5′-GTCTAGATGCTCAACGGAAGTG-3′.
Immunohistochemical staining for
MLH1
CRC tissue samples were collected from 60 CRC
patients who underwent surgery. Normal colon tissue samples were
collected from 56 outpatients via colonic biopsies during
colonoscopy examination. Immunohistochemistry was performed using
the avidin-biotin-peroxidase complex method (12). Immunohistochemical staining was
performed on the 4-µm thick paraffin-embedded tissues, which were
mounted on positively-charged slides. The tissue sections were
incubated with primary mouse monoclonal antibody against MLH1
(dilution, 1:50; cat. no. ab14206; Abcam, CA, USA) overnight at
4°C, followed by the biotin-conjugated goat anti-mouse IgG H&L
secondary antibody (dilution, 1:1,000; cat. no. ab6788; Abcam) for
30 min at 37°C. Images were captured using an Olympus-BX50F4
microscope (Olympus, Tokyo, Japan) and quantitatively analyzed
using the Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda,
MD, USA). The normal colorectal tissues were used as positive
controls for MLH1 staining. Negative controls were run without the
primary antibody.
Statistical analysis
Standard χ2 tests were used to determine
the differences in allelic and genotypic frequencies between SCRC
patients and control subjects in the case-control study. Student's
t-test was used to compare MLH1 expression between the G and A
alleles. Allele and genotype proportions were tested for
Hardy-Weinberg equilibrium. The genotype data were further
stratified by gender, age, smoking history, alcohol intake, tumor
location and size, differentiation, Duke's stage (13), and lymphatic and distant metastasis
status of CRC. The statistical tests were analyzed by SPSS 16.0
system software (SPSS Inc., Chicago, IL, USA) and a two-tailed
significance level of P<0.05 was used.
Results
Table I shows the
allele and genotype distributions for SCRC patients and controls.
The allele and genotype proportions were in Hardy-Weinberg
equilibrium (P=0.099 and P=0.418, respectively). Meanwhile, the
allele and genotype frequencies between the SCRC patients and
controls were compared, and no significant differences were
observed in either the allele (P=0.879) or genotype (P=0.219)
frequencies. When the CRC patients were stratified by gender, age,
smoking history, alcohol intake, tumor location and size,
differentiation, Duke's stage, and lymphatic and distant
metastasis, no association was found between the rs1800734
polymorphism and the clinical variables of gender, age, smoking,
alcohol intake, tumor location and size, differentiation or distant
metastasis in SCRC patients (all P>0.05). However, stratifying
the samples by Duke's stage and lymphatic metastasis, significant
differences were found in the allele (χ2 test, P=0.004)
and genotype (χ2 test, P=0.015) frequencies (Tables II and III). In addition, there were also a
significant association between the allele (χ2 test,
P=0.010) and genotype (χ2 test, P=0.031) frequencies and
lymphatic metastasis (Tables II and
III). The frequency of the A/A
genotype and allele A was higher in Duke's stage C+D SCRC patients
than in Duke's stage A+B SCRC patients. The frequency of the A/A
genotype and allele A was also higher in the SCRC patients with
lymphatic metastasis than in the SCRC patients without lymphatic
metastasis.
 | Table I.Distribution of alleles and genotypes
for MLH1-93G/A (single nucleotide polymorphism rs1800734) in
sporadic colorectal cancer patients and controls. |
Table I.
Distribution of alleles and genotypes
for MLH1-93G/A (single nucleotide polymorphism rs1800734) in
sporadic colorectal cancer patients and controls.
|
|
| Allele, n (%) |
| Genotype, n (%) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Subjects | Number | G | A | P-value | GG | GA | AA | P-value | HWE-p |
|---|
| All cases | 312 | 271 (43.4) | 353 (56.6) | 0.879 | 66 (21.2) | 139 (44.6) | 107 (34.2) | 0.219 | 0.099 |
| All control | 300 | 258 (43.0) | 342 (57.0) |
| 52 (17.3) | 154 (51.3) | 94 (31.3) |
| 0.418 |
| Female cases | 119 | 107 (45.0) | 131 (55.0) | 0.503 | 27 (22.7) | 53 (44.5) | 39 (32.8) | 0.774 | 0.275 |
| Female controls | 131 | 110 (42.0) | 152 (58.0) |
| 25 (19.1) | 60 (45.8) | 46 (35.1) |
| 0.494 |
| Male cases | 193 | 164 (42.5) | 222 (57.5) | 0.725 | 39 (20.2) | 86 (44.6) | 68 (35.2) | 0.110 | 0.220 |
| Male controls | 169 | 148 (43.8) | 190 (56.2) |
| 27 (16.0) | 94 (55.6) | 48 (28.4) |
| 0.090 |
 | Table II.Stratified analyses between the human
mutL homolog 1 (rs1800734) genotypes and the sporadic CRC risk. |
Table II.
Stratified analyses between the human
mutL homolog 1 (rs1800734) genotypes and the sporadic CRC risk.
|
| Genotype, n (%) |
|
|
|---|
|
|
|
|
|
|---|
| Subjects | GG | GA | AA | χ2 | P-value |
|---|
| Controls | 52 (17.3) | 154 (51.3) | 94 (31.3) | 3.036 | 0.219 |
| Patientsa | 66 (21.2) | 139 (44.6) | 107 (34.2) |
|
|
| Gender |
|
|
| 0.344 | 0.842 |
| Male | 39 (20.2) | 86 (44.6) | 68 (35.2) |
|
|
|
Female | 27 (22.7) | 53 (44.5) | 39 (32.8) |
|
|
| Age, years |
|
|
| 4.553 | 0.103 |
|
>60 | 29 (19.6) | 60 (40.5) | 59 (39.9) |
|
|
| ≤60 | 37 (22.6) | 79 (48.2) | 48 (29.2) |
|
|
| Smoking |
|
|
| 0.759 | 0.684 |
| Yes | 36 (23.4) | 65 (42.2) | 53 (34.4) |
|
|
| No | 26 (20.0) | 61 (46.9) | 43 (33.1) |
|
|
| Alcohol intake |
|
|
| 3.971 | 0.137 |
| Yes | 35 (21.4) | 66 (40.2) | 63 (38.4) |
|
|
| No | 27 (22.5) | 60 (50.0) | 33 (27.5) |
|
|
| Tumor location |
|
|
| 6.653 | 0.155 |
|
Proximalb | 18 (19.6) | 46 (50.0) | 28 (30.4) |
|
|
|
Distalc | 16 (18.2) | 34 (38.6) | 38 (43.2) |
|
|
|
Rectal | 28 (26.9) | 46 (44.2) | 30 (28.9) |
|
|
| Tumor size, cm |
|
|
| 1.176 | 0.555 |
|
<5 | 37 (21.3) | 74 (42.5) | 63 (36.2) |
|
|
| ≥5 | 25 (22.7) | 52 (47.3) | 33 (30.0) |
|
|
|
Differentiation |
|
|
| 3.154 | 0.532 |
| Well
and moderately | 47 (21.9) | 90 (41.9) | 78 (36.2) |
|
|
|
Poorly | 8 (19.5) | 22 (53.7) | 11 (26.8) |
|
|
|
Otherd | 7 (25.0) | 14 (50.0) | 7 (25.0) |
|
|
| Duke's stage |
|
|
| 8.360 | 0.015 |
|
A/B | 28 (27.2) | 51 (49.5) | 24 (23.3) |
|
|
|
C/D | 34 (18.8) | 75 (41.4) | 72 (39.8) |
| Lymphatic
metastasis |
|
|
| 6.590 | 0.031 |
|
Yes | 32 (18.6) | 72 (41.9) | 68 (39.5) |
|
|
| No | 30 (26.8) | 54 (48.2) | 28 (25.0) |
| Distant
metastasis |
|
|
| 4.794 | 0.091 |
|
Yes | 10 (21.3) | 27 (57.4) | 10 (21.3) |
|
|
| No | 52 (21.9) | 99 (41.8) | 86 (36.3) |
|
|
 | Table III.Allellic distribution of human mutL
homolog 1–93G/A (single nucleotide polymorphism rs1800734) with
regard to Duke's stage and lymphatic metastasis status among 284
sporadic colorectal cancer cases. |
Table III.
Allellic distribution of human mutL
homolog 1–93G/A (single nucleotide polymorphism rs1800734) with
regard to Duke's stage and lymphatic metastasis status among 284
sporadic colorectal cancer cases.
|
|
| Allele |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Parameter | Cases | G | A | χ2 | P-value | OR (95% CI) |
|---|
| Duke's stage |
|
|
|
| 0.004 |
|
|
A/B | 103 | 107 (51.9) | 99 (48.1) | 8.244 |
| 1.655
(1.172–2.337) |
|
C/D | 181 | 143 (39.5) | 219 (60.5) |
|
|
|
| Lymphatic
metastasis |
|
|
|
| 0.010 |
|
|
Yes | 172 | 136 (39.5) | 208 (60.5) | 6.590 |
| 0.642
(0.458–0.901) |
| No | 112 | 114 (50.9) | 110 (49.1) |
|
|
|
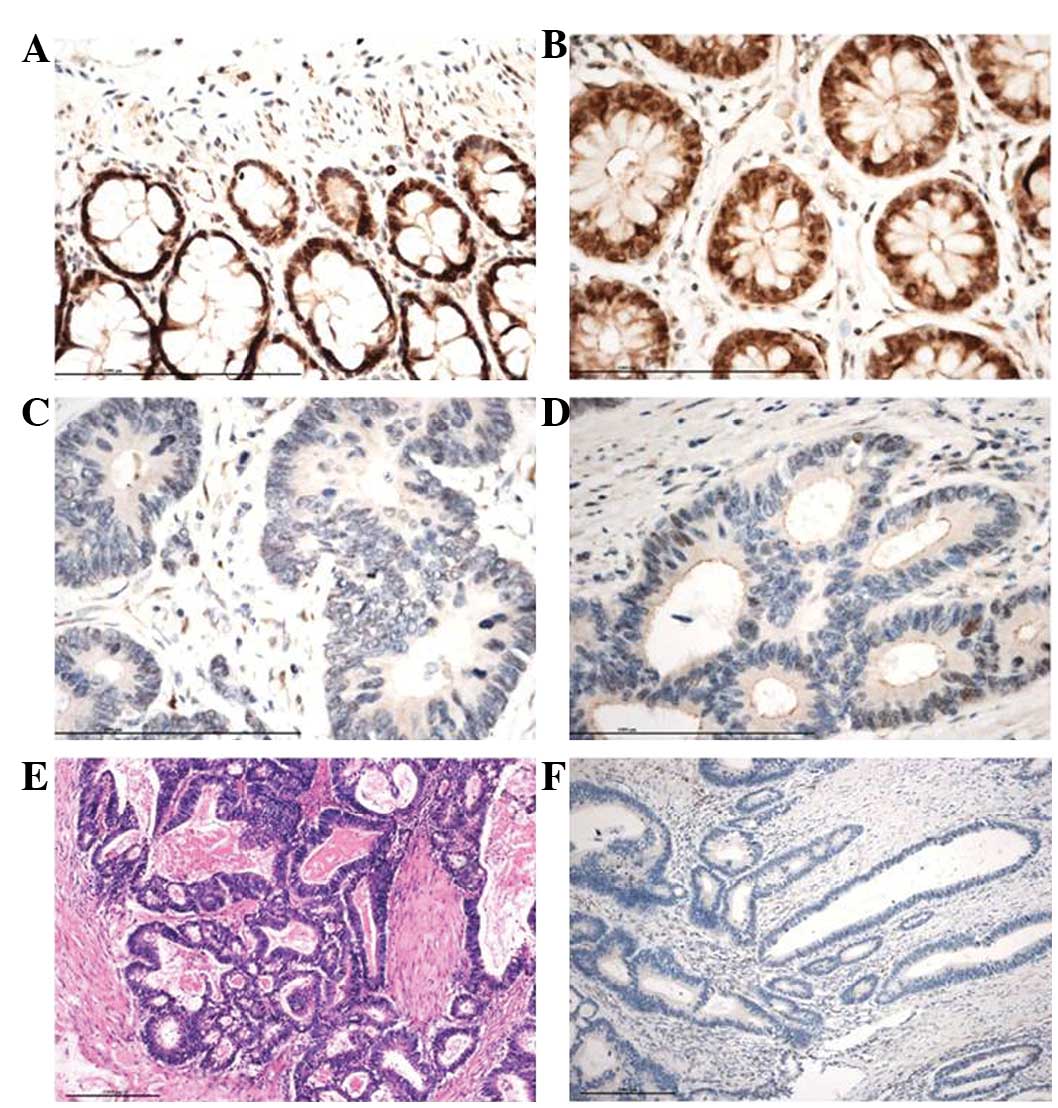
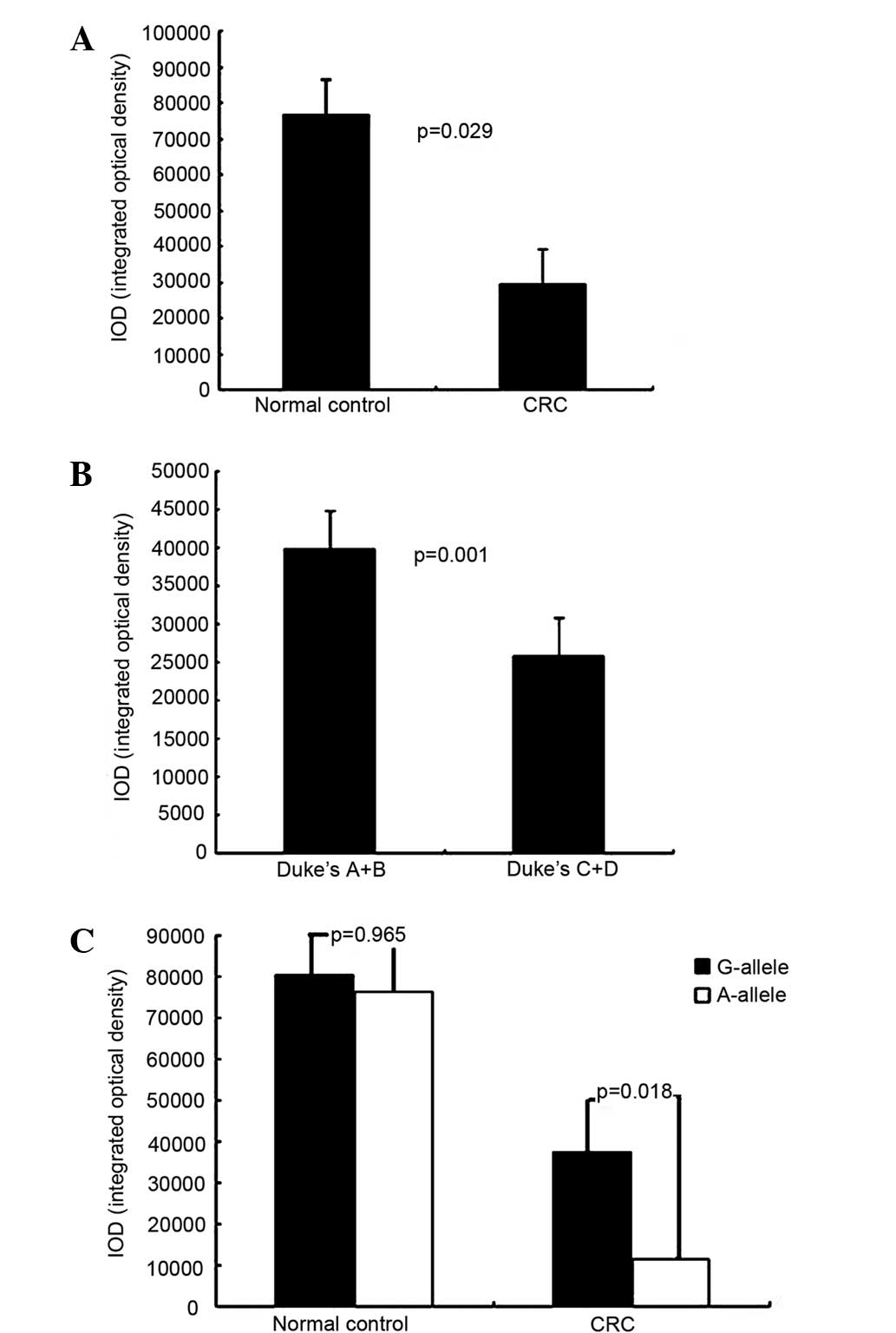
Immunohistochemical staining for MLH1 was evaluated
in 60 SCRC patients (G/G vs. G/A vs. A/A: 17 vs. 26 vs. 17) and 56
normal controls (G/G vs. G/A vs. A/A: 18 vs. 22 vs. 17) (Fig. 1). MLH1 expression levels with the
different alleles were compared between the CRC patients and the
normal controls, as well as between Duke's stage A+B and C+D SCRC
patients (Fig. 2). MLH1 expression
was significantly higher in the normal controls, in Duke's stage
A+B patients and in G allele SCRC patients than in SCRC patients
(P=0.029), Duke's stage C+D patients (P=0.001) and G allele SCRC
patients (P=0.018), respectively. By contrast, there was no
statistically significant difference in hMLH1 expression for the A
and G alleles in the normal controls (P=0.965).
Discussion
Several studies have confirmed that hMLH1 plays an
important role in CRC, while SNPs of mismatch repair genes are
believed to provide important information for the diagnosis of CRC
(14). However, to the best of our
knowledge, few studies on the association between SNPs of MMR genes
and sporadic CRC (SCRC) in China are available. Thus, in the
present study, it was proposed that the SNP of the hMLH1 gene was
linked to CRC.
The genotype distribution of the SNP MLH1-93G/A
(rs1800734) has shown differences among varying ethnic populations.
The frequency of polymorphism −93G/G was found to be higher than
other polymorphisms in European and North-American populations
(15). However, in the present study,
the G/A frequency in normal controls and CRC cases was observed to
be higher than others. This was in agreement with other studies on
Asian populations (16,17). These discrepancies may be explained by
genetic variation in the different ethnic groups of the various
study populations.
It is notable that the MLH1-93G/A variant has been
associated with several cancers. For example, the MLH1-93A allele
has been positively associated with the risk of developing
MMR-deficient CRC, particularly CRC with somatic loss of MLH1
protein expression (18), and the
risk of microsatellite instability (MSI)-positive colon cancer
(19). A previous study also
suggested that the −93A allele was associated with an increased
risk of MSI-high, but not microsatellite-stable, colorectal tumors
(19). It was also reported that the
−93A allele of the MLH1 gene was associated with the risk for
squamous cell lung cancers in Korean patients with a gene-smoking
interaction (16). In the present
study, it was found that the MLH1-93G/A variant was not associated
with the risk of SCRC overall, which is consistent with the results
of a study by Campbell et al (15). However, the frequency of the A allele
was significantly higher in Duke's stage C+D SCRC patients and SCRC
patients with lymphatic metastasis, compared with Duke's stage A+B
SCRC patients and those without lymphatic metastasis, respectively.
Furthermore, MLH1 expression was lower in the Duke's stage C+D SCRC
patients, and the MLH1 expression was lower for the A-allele than
for the G-allele in the CRC patients. Considering these findings,
the MLH1-93G/A polymorphism did not appear to affect MLH1
expression in the normal controls. However, it may play a role in
the expression of MLH1 following SCRC formation, and it may be
associated with the tumor progression of CRC.
The molecular mechanisms responsible for the
involvement of MLH1 in CRC progression remain unclear. The
MLH1-93G/A polymorphism is located in the MLH1 CpG island, at −93
nucleotides from the transcription start site in the core promoter
region (10). There are two
transcription binding sites, nuclear factor for interleukin-6
expression and GT-IIB trihelix transcription factor, harbored in
this region, which are required for maximal transcriptional
activity (10). Based on this
knowledge, it is possible that the −93 A allele is susceptible to
MLH1 abnormal methylation and gene silencing as a result of altered
transcription factor binding. As aforementioned, polymorphism in
this region is predicted to regulate MLH1 protein expression. We
suggest that the −93G to A transition could plausibly reduce MLH1
gene transcription and expression by altering its epigenetic
status, thereby reducing the DNA repair capability. A study by Chen
et al showed an association between the MLH1 −93A allele and
the methylation of the MLH1 promoter in CRC and endometrial cancer
(20). Recent studies have suggested
that site-specific repressors of transcription may recruit DNA
methyltransferases (21,22).
In conclusion, in the present study, an association
was found between the MLH1-93A allele variant and the elevated risk
of Duke's stage C+D CRC. Furthermore, the A-allele may be a
repressive factor for the transcriptional activity of MLH1, and
thereby affect MLH1 expression. However, the manner via which the
variation affects the risk for epigenetic silencing and has a
possible affect on the progression of CRC remains undetermined. In
view of this, further studies and larger sample sizes are required
to confirm these findings.
Acknowledgements
This study was supported by a grant from the Project
of Wuxi Science and Technology Bureau (grant no. CSE01N1217).
References
|
1
|
Fengju S, Guanglin W and Kexin C:
Incidence of colon cancer in Tianjin, China, 1981ߝ2000. Asia Pac J
Public Health. 17:22–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de la Chapelle A: Genetic predisposition
to colorectal cancer. Nat Rev Cancer. 4:769–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kunkel TA and Erie DA: DNA mismatch
repair. Annu Rev Biochem. 74:681–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lipkin SM, Rozek LS, Rennert G, Yang W,
Chen PC, Hacia J, Hunt N, Shin B, Fodor S, Kokoris M, et al: The
MLH1 D132H variant is associated with susceptibility to sporadic
colorectal cancer. Nat Genet. 36:694–699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearnhead NS, Wilding JL, Winney B, Tonks
S, Bartlett S, Bicknell DC, Tomlinson IP, Mortensen NJ and Bodmer
WF: Multiple rare variants in different genes account for
multifactorial inherited susceptibility to colorectal adenomas.
Proc Natl Acad Sci USA. 101:15992–15997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomlinson IP, Webb E, Carvajal-Carmona L,
Broderick P, Howarth K, Pittman AM, Spain S, Lubbe S, Walther A,
Sullivan K, et al: A genome-wide association study identifies
colorectal cancer susceptibility loci on chromosomes 10p14 and
8q23.3. Nat Genet. 40:623–630. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christmann M, Tomicic MT, Roos WP and
Kaina B: Mechanisms of human DNA repair: An update. Toxicology.
193:3–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynch HT and de la Chapelle A: Hereditary
colorectal cancer. N Engl J Med. 348:919–932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hampel H, Frankel WL, Martin E, Arnold M,
Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J,
et al: Screening for the Lynch syndrome (hereditary nonpolyposis
colorectal cancer). N Engl J Med. 352:1851–1860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito E, Yanagisawa Y, Iwahashi Y, Suzuki Y,
Nagasaki H, Akiyama Y, Sugano S, Yuasa Y and Maruyama K: A core
promoter and a frequent single-nucleotide polymorphism of the
mismatch repair gene hMLH1. Biochem Biophys Res Commun.
256:488–494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arita M, Zhong X, Min Z, Hemmi H and
Shimatake H: Multiple sites required for expression in 5′-flanking
region of the hMLH1 gene. Gene. 306:57–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpkins SB, Bocker T, Swisher EM, Mutch
DG, Gersell DJ, Kovatich AJ, Palazzo JP, Fishel R and Goodfellow
PJ: MLH1 promoter methylation and gene silencing is the primary
cause of microsatellite instability in sporadic endometrial
cancers. Hum Mol Genet. 8:661–666. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou XP, Dai WJ and Cao J: CDH1 promoter
polymorphism (−347G->GA) is a possible prognostic factor in
sporadic colorectal cancer. World J Gastroenterol. 15:5340–5345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mei Q, Yan HL, Ding FX, Xue G, Huang JJ,
Wang YZ and Sun SH: Single-nucleotide polymorphisms of mismatch
repair genes in healthy Chinese individuals and sporadic colorectal
cancer patients. Cancer Genet Cytogenet. 171:17–23. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campbell PT, Curtin K, Ulrich CM, Samowitz
WS, Bigler J, Velicer CM, Caan B, Potter JD and Slattery ML:
Mismatch repair polymorphisms and risk of colon cancer, tumour
microsatellite instability and interactions with lifestyle factors.
Gut. 58:661–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SH, Lee GY, Jeon HS, Lee SJ, Kim KM,
Jang SS, Kim CH, Lee WK, Kam S, Park RW, et al: -93G->A
polymorphism of hMLH1 and risk of primary lung cancer. Int J
Cancer. 112:678–682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo YL, Hsiao CF, Jou YS, Chang GC, Tsai
YH, Su WC, Chen KY, Chen YM, Huang MS, Hsieh WS, et al:
Polymorphisms of MLH1 and MSH2 genes and the risk of lung cancer
among never smokers. Lung Cancer. 72:280–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allan JM, Shorto J, Adlard J, Bury J,
Coggins R, George R, Katory M, Quirke P, Richman S, Scott D, et al:
MLH1-93G>A promoter polymorphism and risk of mismatch repair
deficient colorectal cancer. Int J Cancer. 123:2456–2459. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raptis S, Mrkonjic M, Green RC, Pethe VV,
Monga N, Chan YM, Daftary D, Dicks E, Younghusband BH, Parfrey PS,
et al: MLH1-93G>A promoter polymorphism and the risk of
microsatellite-unstable colorectal cancer. J Natl Cancer Inst.
99:463–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Taylor NP, Sotamaa KM, Mutch DG,
Powell MA, Schmidt AP, Feng S, Hampel HL, de la Chapelle A and
Goodfellow PJ: Evidence for heritable predisposition to epigenetic
silencing of MLH1. Int J Cancer. 120:1684–1688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brenner C, Deplus R, Didelot C, Loriot A,
Viré E, De Smet C, Gutierrez A, Danovi D, Bernard D, Boon T, et al:
Myc represses transcription through recruitment of DNA
methyltransferase corepressor. EMBO J. 24:336–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brenner C and Fuks F: DNA
methyltransferases: Facts, clues, mysteries. Curr Top Microbiol
Immunol. 301:45–66. 2006.PubMed/NCBI
|