Introduction
Carcinoma of unknown primary (CUP) is generally
defined as a category of histologically proven malignancy with a
primary tumor that cannot be detected by conventional diagnostic
methods (1). As a type of
heterogeneous disease, it accounts for 0.5–9% of all cases of
malignant diseases (2). Since the
primary cannot be identified effectively, most patients receive
empirical therapy in clinical practice with unsatisfactory outcome
(3,4).
Under these circumstances, although certain individuals with
favorable prognoses benefit from therapy, the majority of such
patients generally have poor outcome with a median life expectancy
of less than 12 months (5,6).
Fluorine-18-2-fluoro-2-deoxy-D-glucose positron
emission tomography/computed tomography (F-18 FDG PET/CT) has high
sensitivity in detecting multiple malignancies, and is routinely
applied clinically for staging, restaging and monitoring treatment
(7–9).
In addition, a number of studies have indicated the crucial role of
F-18 FDG PET/CT in CUP patients (10–13).
Generally, the previous studies indicated that F-18 FDG PET/CT was
able to detect the primary (9.6–47.2%) and guide the choice of
treatment (29.4–47%) in approximately one-third of all patients
(12,14–18).
Although these studies provided useful evidence to guide clinical
practice, their limitations were still significant, and further
improvement is required. First of all, the definition of CUP was
not unified and specific in previous studies. Secondly, certain
studies did not exclude lymphoma and hematological malignant
disease from the samples, which may have led to an underestimation
of the actual detection rate. In addition, not all patients in the
previous studies received biopsy to prove the metastases, or
extensive conventional examination to identify the primaries.
Furthermore, the most relevant studies had a small sample size that
was generally no larger than 200 individuals. Finally, this issue
was seldom investigated with respect to different pathological
types and locations. This study aimed to investigate the value and
indications of F-18 FDG PET/CT in CUP patients. Utilizing a large
sample size, we focused on identifying the primary and the role of
the scan in guiding treatment plans according to the different
metastatic sites and pathological types.
Materials and methods
Patients
The present study was approved by the ethics
committee of Tianjin Cancer Institute and Hospital, China, and
conducted in accordance with the Declaration of Helsinki. Written
informed consent was obtained from the patientsor their family. In
this retrospective study, selected cases were consecutively
included among 26,763 patients who received an F-18 FDG PET/CT scan
between January 2006 and October 2014 in Tianjin Medical University
Cancer Institute and Hospital, China. All individuals had
biopsy-proven malignant metastases. However, prior to receiving the
F-18 FDG PET/CT scan, the primary could not be confirmed using
regular methods, including detailed physical examination, routine
serum tumor marker test and other imaging auxiliary examinations
including chest X-ray, CT, magnetic resonance imaging (MRI),
mammography (in females), cervical, abdominal and breast (in
females) ultrasonography and endoscopy. The biopsy samples of all
patients were stained using immunohistochemistry methods to exclude
hematological malignant disease or lymphoma, and to preliminarily
predict the primary location. Following the F-18 FDG PET/CT scan,
the potential primary carcinoma was routinely confirmed by biopsy
or imaging follow-up. The minimum follow-up period was 9 months,
and patients who did not attend a follow-up appointment were
excluded from the study. Finally, a total of 449 patients were
included.
Imaging
The F-18 FDG PET/CT scan was performed using a
Discovery ST PET/CT scanner or a Discovery PET/CT 710 scanner (GE
Healthcare, Milwaukee, WI, USA). All patients fasted for at least 6
h, and the blood glucose concentration (BGC) of each patient was
monitored prior to intravenous injection of F-18 FDG. For those
poorly controlled diabetic patients with high BGC, 4–12
international units of fast-acting insulin were intravenously
injected prior to tracer administration. The injection dose of
radioactive tracer was calculated as 0.11–0.13 millicuries per
kilogram of body weight. The F-18 FDG PET/CT scan was conducted
according to a standard protocol. A whole-body scan from mid-thigh
to vertex commenced at ~60 min after injection.
Non-contrast-enhanced CT was conducted with a current of 120–170
mA, a voltage of 120 kV, a section thickness of 5 or 3.75 mm and a
reconstruction interval of 5 or 3.75 mm. The attenuation-corrected
PET image was scanned at 2 min per frame and reconstructed using CT
data with iterative algorithms.
Image interpretation
Morphological, metabolic and fused PET/CT images
were inspected in axial, coronal and sagittal view using Xeleris
software from GE Healthcare. Three senior nuclear medicine
physicians independently interpreted the PET/CT images (based on
clinical pathological data, location, shape, CT attenuation and
F-18 FDG uptake), and then reached a consensus to the diagnosis of
all patients.
Statistical analysis
Categorical variables were recorded as numbers and
percentages. The median and range were used to express abnormal
distribution variables. All statistics were analyzed using SPSS
17.0 software (IBM SPSS, Armonk, NY, USA).
Results
Metastases and primary sites
The median age of patients was 58 years old (range,
13–83 years). A total of 261 male cases (58.1%) and 188 female
patients (41.9%) were included in this study. The pathological type
and localization of metastases are summarized in Table I.
 | Table I.Pathological type and localization of
metastases. |
Table I.
Pathological type and localization of
metastases.
|
| Pathological
type | Adenocarcinoma | Squamous cell
carcinoma | Small-cell
carcinoma | Melanoma | Otherc | Undefined |
|---|
| Localization of
metastases | Total (n=449) | 179 (39.9) | 121 (26.9) | 26 (5.8) | 19 (4.2) | 28 (6.2) | 76 (16.9) |
| LN or soft
tissue | 338 (75.2) | 122 (31.6) | 113 (25.2) | 26 (5.8) | 16 (3.6) | 20 (4.4) | 41 (9.1) |
|
Cervical | 169 (37.6) | 37 (8.2) | 89 (19.8) | 10 (2.2) | 5 (1.1) | 6 (1.3) | 22 (4.9) |
|
Supraclavicular | 58 (12.9) | 26 (5.8) | 16 (3.6) | 6 (1.3) | 0 (0.0) | 4 (0.9) | 6 (1.3) |
|
Axillary | 49 (10.9) | 35 (7.8) | 0 (0.0) | 2 (0.4) | 6 (1.3) | 1 (0.2) | 5 (1.1) |
|
Inguinal | 28 (6.2) | 8 (1.8) | 5 (1.1) | 2 (0.4) | 5 (1.1) | 4 (0.9) | 4 (0.9) |
|
Mediastinal | 12 (2.7) | 3 (0.7) | 2 (0.4) | 5 (1.1) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
|
Retroperitoneal | 10 (2.2) | 6 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 2 (0.4) |
|
Abdominal/pelvic | 3 (0.7) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
|
Iliac | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Othera | 8 (1.8) | 5 (1.1) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 2 (0.4) | 0 (0.0) |
| Skeleton | 49 (10.9) | 26 (5.8) | 3 (0.7) | 0 (0.0) | 0 (0.0) | 3 (0.7) | 17 (3.8) |
|
Axial | 36 (8.0) | 19 (4.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 14 (3.1) |
|
Other | 13 (2.9) | 7 (1.6) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 3 (0.7) |
| Brain | 22 (4.9) | 9 (2.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 3 (0.7) | 8 (1.8) |
| Liver | 15 (3.3) | 9 (2.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 3 (0.7) |
| Gland | 7 (1.5) | 1 (0.2) | 4 (0.9) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) |
|
Parotid | 5 (1.1) | 0 (0.0) | 4 (0.9) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) |
|
Adrenal | 2 (0.4) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Effusion | 6 (1.3) | 5 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
|
Pleural | 3 (0.7) | 3 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Pericardiac | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
Ascitic | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Lung | 6 (1.3) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.9) |
| Pleural
membrane | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Otherb | 4 (0.9) | 3 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
Using F-18 FDG PET/CT, the primary sites of 115
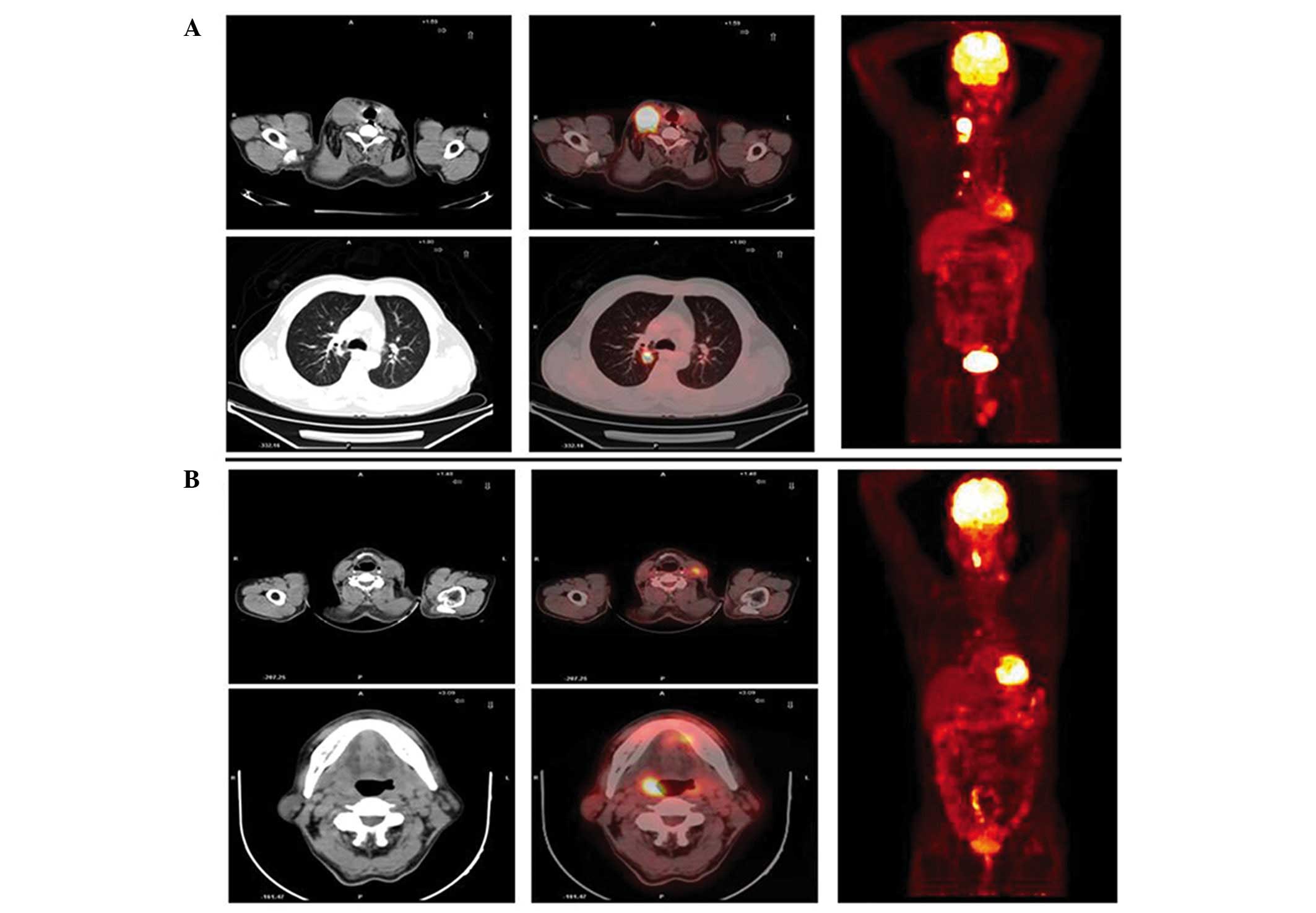
cases were located (115 of 449, 25.6%) (Table II). Representative cases are shown in
Fig. 1. Of these patients, 40
primaries were identified in the lung (34.8%), 16 in the
nasopharynx (13.9%), 13 in the pancreas (11.3%), 11 in the tonsil
(9.6%), 8 in the small intestine (7.0%), 6 in the stomach (5.2%), 4
in the larynx (3.5%), 4 in the ovary (3.5%), 3 in the esophagus
(2.6%), 3 in the colorectum (2.6%), 2 in the kidney (1.7%), 1 in
the male mammary gland (0.9%), 1 in the prostate (0.9%), 1 in the
penis (0.9%), 1 in the cholecyst (0.9%) and 1 in the uterus (0.9%).
In addition to the previously known metastases, F-18 FDG PET/CT
identified additional metastatic foci in 131 of the 449 patients
(29.2%).
 | Table II.Localization of primary sites that
were accurately identified by
fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission
tomography/computed tomography in 115 patients. |
Table II.
Localization of primary sites that
were accurately identified by
fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission
tomography/computed tomography in 115 patients.
| Localization of
primaries | No. of patients
(%) |
|---|
| Lung | 40 (34.8) |
| Nasopharynx | 16 (13.9) |
| Pancreas | 13 (11.3) |
| Tonsil | 11 (9.6) |
| Small
intestine | 8 (7.0) |
| Stomach | 6 (5.2) |
| Larynx | 4 (3.5) |
| Ovary | 4 (3.5) |
| Esophagus | 3 (2.6) |
| Colorectum | 3 (2.6) |
| Kidney | 2 (1.7) |
| Male mammary
gland | 1 (0.9) |
| Prostate | 1 (0.9) |
| Penis | 1 (0.9) |
| Cholecyst | 1 (0.9) |
| Uterus | 1 (0.9) |
Pathologically, F-18 FDG PET/CT identified primary
carcinoma in 29 of the 76 patients with undefined pathological type
(38.2%), 36 of 121 squamous cell carcinoma patients (29.8%), 8 of
28 other pathological type patients (28.6%), 38 of 179
adenocarcinoma patients (21.2%), 3 of 26 small-cell carcinoma
patients (11.5%) and 1 of 19 melanoma patients (5.3%)(Table III).
 | Table III.Stratification analysis of primary
sites according to pathological type. |
Table III.
Stratification analysis of primary
sites according to pathological type.
| Pathological
type | Adenocarcinoma | Squamous cell
carcinoma | Small-cell
carcinoma | Other | Undefined |
|---|
| Primary sites
(successful detection/total number) | 38/179 (21.2%) | 36/121 (29.8%) | 3/26 (11.5%) | 8/28 (28.6%) | 29/76 (38.2%) |
|
Lung | 15 | 6 | 2 | 3 | 14 |
|
Nasopharynx | 1 | 11 | 0 | 0 | 4 |
|
Pancreas | 8 | 1 | 0 | 1 | 3 |
|
Tonsil | 1 | 9 | 0 | 1 | 0 |
| Small
intestine | 3 | 0 | 1 | 0 | 3 |
|
Stomach | 2 | 1 | 0 | 2 | 1 |
|
Larynx | 0 | 2 | 0 | 0 | 2 |
|
Ovary | 4 | 0 | 0 | 0 | 0 |
|
Esophagus | 0 | 3 | 0 | 0 | 0 |
|
Colorectum | 0 | 1 | 0 | 0 | 2 |
|
Kidney | 1 | 0 | 0 | 1 | 0 |
| Mammary
gland (male) | 1 | 0 | 0 | 0 | 0 |
|
Prostate | 1 | 0 | 0 | 0 | 0 |
|
Penis | 0 | 1 | 0 | 0 | 0 |
|
Cholecyst | 0 | 1 | 0 | 0 | 0 |
|
Uterus | 1 | 0 | 0 | 0 | 0 |
We also analyzed the efficiency of F-18 FDG PET/CT
in detecting the primary carcinoma according to different
metastatic sites (sample size more than 20). Metastases initially
identified in the head and neck (cervical and supraclavicular
metastasis) accounted for the majority of patients (227 of 449,
50.6%). F-18 FDG PET/CT identified primary carcinoma in 66 of 227
patients (29.1%). Of these, 20 primaries were located in the lung
(30.3%), 16 in the nasopharynx (24.2%), 11 in the tonsil (16.7%), 4
in the pancreas (6.0%), 3 in the stomach (4.5%), 3 in the larynx
(4.5%), 2 in the small intestine (3.0%), 2 in the esophagus (3.0%),
1 in the ovary (1.5%), 1 in the colorectum (1.5%), 1 in the kidney
(1.5%), 1 in the male mammary gland (1.5%) and 1 in the cholecyst
(1.5%). Among the 49 patients who were initially identified as
having axillary metastases, the primaries were determined by F-18
FDG PET/CT in just 2 patients (pancreas and small intestine; 2/49,
4.1%). In addition, F-18 FDG PET/CT successfully identified the
primary sites in 19 of 49 osseous metastasis patients (38.8%), 5 of
28 inguinal metastasis patients (17.9%) and 5 of 22 cerebral
metastasis patients (22.7%) (Table
IV).
 | Table IV.Stratification analysis of primary
sites according to anatomical position of metastasis. |
Table IV.
Stratification analysis of primary
sites according to anatomical position of metastasis.
| Position of
metastasis | Head and neck | Bone | Inguen | Cerebrum |
|---|
| Primary sites
(successful detection/total number) | 66/227 (29.1%) | 19/49 (38.8%) | 5/28 (17.9%) | 5/22 (22.7%) |
|
Lung | 20 | 12 | 0 | 3 |
|
Nasopharynx | 16 | 0 | 1 | 0 |
|
Pancreas | 4 | 1 | 0 | 0 |
|
Tonsil | 11 | 0 | 0 | 0 |
| Small
intestine | 2 | 1 | 1 | 1 |
|
Stomach | 3 | 2 | 0 | 0 |
|
Larynx | 3 | 0 | 1 | 0 |
|
Ovary | 1 | 0 | 1 | 0 |
|
Esophagus | 2 | 1 | 0 | 0 |
|
Colorectum | 1 | 0 | 0 | 1 |
|
Kidney | 1 | 1 | 0 | 0 |
| Mammary
gland (male) | 1 | 0 | 0 | 0 |
|
Prostate | 0 | 1 | 0 | 0 |
|
Penis | 0 | 0 | 1 | 0 |
|
Cholecyst | 1 | 0 | 0 | 0 |
F-18 FDG PET/CT guided treatment
change
As a result of the F-18 FDG PET/CT scan, the
treatment plans of 130 of the 449 patients (20.9%) required
modification. Fourteen patients canceled surgery due to upstage or
extra metastases being identified by F-18 FDG PET/CT (3.1%). A
total of 121 patients initiated or modified systematic treatment
plans guided by F-18 FDG PET/CT scan, including chemotherapy and
immunotherapy (26.9%). Among these, although without identification
of their primary carcinoma, 11 patients changed their chemotherapy
and immunotherapy plan due to the extra metastases and focal
distribution identified by F-18 FDG PET/CT (2.4%).
Among the 227 patients with metastases initially
identified in the head and neck, 73 changed their treatment
strategy following the F-18 FDG PET/CT scan (32.2%). Of these
patients, 6 canceled their surgical treatment (2.6%) and 69
modified their chemotherapy and immunotherapy strategy (30.4%).
False positive identification of
primary carcinoma
Other than the 115 patients whose primaries were
correctly identified, the primaries of 27 patients were incorrectly
identified, as proven by biopsy or follow-up. Of these patients,
pathological biopsy indicated that 9 cases had benign lesions in
the gastrointestinal tract, 3 patients in the thyroid, 1 patient in
the mammary gland, 7 patients in the head and neck, and 4 patients
in the lung. The primaries of the other 3 patients could not be
confirmed by follow-up.
Discussion
At present, CUP is generally accepted as a type of
malignant disease which differs from other metastatic malignant
carcinomas in that the primary tumor site is not known. Accurate
identification of the primary site and precise staging enhance the
prognosis significantly (19).
Previous studies performed exploratory work on the
use of F-18 FDG PET/CT in CUP and gained instructive conclusions.
However, in certain aspects, there is still a long way to go. For
instance, Pelosi et al are considered to be the first team
that focused on this topic back in 2006 (20). Although their study innovatively
evaluated the application of F-18 FDG PET/CT in CUP patients, with
the small sample size of 68, it would be difficult to avoid bias.
In 2008, a meta-analysis containing a relatively large sample of
430 patients further discussed this issue (21). However, a series of confounding
factors, including the definition of CUP, epidemiology, diagnostic
level, types of auxiliary examination, imaging interpretation and
enrollment standards may hamper the reliability of this study. In
2013, Wang et al analyzed a large sample containing 164
patients in China (12). However, the
study included patients without biopsy-proven metastasis or who had
not had thorough conventional examination. In addition to these
typical limitations, few studies stratified patients according to
the different pathological types and metastatic locations.
Tianjin Cancer Institute and Hospital is one of the
largest cancer centers in China. Between January 2006 and July
2015, a total of 30,063 patients received a F-18 FDG PET/CT scan in
our Department of Molecular Imaging and Nuclear Medicine. In this
study, qualifying individuals were selected from among 26,763
patients who had received a F-18 FDG PET/CT scan between January
2006 and October 2014 using strict criteria. The primary carcinoma
was successfully identified in approximately a quarter of CUP
patients using standard F-18 FDG PET/CT after conventional
examination methods had failed. This number was slightly lower than
expected from referring to previous studies. Previous studies had
indicated that the most frequently identified primaries in CUP
identified by F-18 FDG PET/CT were lung or head and neck carcinoma
(14,22). In this study, the F-18 FDG PET/CT scan
revealed that the most common primary was the lung (40/115, 34.8%),
followed by the nasopharynx, pancreas, tonsil, small intestine,
stomach, larynx, ovary, esophagus, colorectum and kidney. Head and
neck carcinoma, including primaries in the nasopharynx, tonsil and
larynx (31/115, 27.0%), was still one of the most commonly
occurring primaries among CUP patients. In addition, single cases
were identified in the male mammary gland, prostate, penis,
cholecyst and uterus. In contrast, 4,395 individuals exhibited
biopsy-proven metastases initially and received F-18 FDG PET/CT
prior to conventional examination. Of these patients, the primary
sites of the majority (4064/4395, 92.5%) were successfully located
by F-18 FDG PET/CT, which were further confirmed by biopsy, other
auxiliary examination or follow-up. Thus, considering its high
detection efficiency, early application of F-18 FDG PET/CT should
be encouraged for locating the primaries prior to the use of other
conventional examination methods for patients with metastasis. For
patients whose primaries cannot be located by conventional
examination, F-18 FDG PET/CT may also be used as an auxiliary
method, with an expected detection rate of ~1/4. In those patients
whose primary sites were successfully located, the majority
originated from the lung and the head and neck (71/115, 61.7%).
CUP patients with metastases in the head and neck
were separately analyzed in certain previous studies (13,23,24). CUP
accounts for ~2 to 9% of all patients with head and neck carcinoma
(23). Patients may receive accurate
surgery and radiation therapy, and benefit from the precise
identification of primary carcinoma (13). In the present study, metastases
initially presenting in the head and neck accounted for the
majority of patients. The performance of F-18 FDG PET/CT in
detecting primary carcinoma in this subgroup (29.1%) was
comparative with its potency across all CUP patients. Of the
patients whose primary sites were located, the lung (30.3%) and the
head and neck (nasopharynx, tonsil and larynx) (45.5%) were the
most common primaries. In addition, we performed stratification
analysis according to the various pathological types and locations
(patient number more than 20). In CUP patients with squamous cell
carcinoma metastasis (30.6%), adenocarcinoma metastasis (20.7%),
small-cell carcinoma metastasis (11.5%), osseous metastasis
(38.8%), inguinal metastasis (17.9%) and cerebral metastasis
(22.7%), we suggest that F-18 FDG PET/CT may be selectively applied
clinically.
In the present study, we observed that F-18 FDG
PET/CT had an extremely low detection rate in locating the primary
carcinoma of metastatic melanoma and axillary metastasis in CUP
patients. Melanoma derives from melanocytes, and is a type of
lethal malignant disease (25). The
survival rate of patients and their treatment strategy are closely
related to the extent and stage of disease at the first visit to
the clinic (26). However,
large-sample studies have indicated that in ~2–3% of melanoma
cases, metastases were initially observed without detecting the
primary carcinoma (27), which often
led to difficulty in treatment. Particularly in patients with only
metastatic lymph nodes or cutis, it is difficult to distinguish
between distant and regional metastases, which is critical to the
treatment strategy. F-18 FDG PET/CT is a useful tool and a
particularly significant method in staging cutaneous and
noncutaneous melanoma, respectively (28,29). Also,
although argument existed, it demonstrated a positive performance
in monitoring relapse and judging the prognosis of cutaneous
melanoma patients (29).
Currently, although F-18 FDG PET/CT has proven its
potent diagnostic effect, only a few case reports have focused on
its diagnostic role in metastatic melanoma of unknown primary. As
far as we know, this study with a sample size of 19 metastatic
melanoma patients is one of the largest studies on this topic. In
this study, among the 19 patients with metastatic melanoma, the
primary site (small intestine) of only one patient was identified
by F-18 FDG PET/CT (1/19, 5.3%). In addition, F-18 FDG PET/CT only
led to treatment modification for one patient (canceled surgery),
due to identification of further malignancy (1/19, 5.3%). Thus,
considering the low detection rate, we do not recommend regular
application of F-18 FDG PET/CT for the sole purpose of locating the
primary site in metastatic melanoma patients, if conventional
methods have failed to identify this.
Clinically, malignant diseases that initially
present with axillary metastasis are most commonly observed in
breast malignancies (30). Due to the
high diagnostic performance of mammography, breast ultrasonography
and MRI (31,32), the primaries of almost all patients
with breast malignancies were detected initially, and so such
patients were not included in our study. Thus, in the present
study, CUP patients presenting with axillary metastasis usually had
extramammary malignant diseases or occult breast malignancies that
are difficult to detect with the commonly used methods. As far as
we know, there is little relevant research in this field, with the
exception of the study of Bertozzi et al (30). Based on our data, F-18 FDG PET/CT has
an extremely low detection rate in CUP patients with axillary
metastasis (4.1%). As a result, we have serious concerns as to the
clinical benefit of wide application of F-18 FDG PET/CT in
detecting primary carcinoma of CUP patients with axillary
metastasis in cases where conventional examination has failed to
identify the primaries.
Previous studies have indicated that F-18 FDG PET/CT
is able to detect primaries and extra metastases that were
previously undetected, which leads to treatment strategy
modification (14). According to
previous data, approximately one-third of all patients (29.4 to
33.8%) changed their treatment strategy, guided by F-18 FDG PET/CT
(12,14,16). This
study was consistent with previous studies, and our data confirmed
that F-18 FDG PET/CT led to treatment modification in 29% of all
patients and 32.2% of head and neck metastasis patients. Thus, for
the purpose of treatment instruction, we recommend active
application of F-18 FDG PET/CT, and certain patients are likely to
benefit from it.
In conclusion, to the best of our knowledge, the
present study currently has the largest sample size in this field
of research. F-18 FDG PET/CT has proven its clinical value in CUP
patients. Its use is expected to identify the primaries in certain
patients, and such patients are likely to benefit from its
application. The most common primary sites appear at the lung and
head and neck. Clinically, the use of F-18 FDG PET/CT should be
encouraged earlier in CUP patients, or it should be applied as a
complementary radiological method if conventional examination
fails, except in cases of metastatic melanoma and in patients with
axillary metastasis. For CUP patients with a pathological type of
melanoma or a metastatic location in the axilla, we doubt the
effectiveness and clinical benefit of the F-18 FDG PET/CT scan, in
view of its extremely low positive rate.
Acknowledgements
This project was supported by grants from the
National Natural Science Funds (81501984), Tianjin Municipal Bureau
of Health Science and Technology Fund (2013KZ088), the National
Science and Technology Major Project (2013ZX09303001) and the
Health Bureau of Tianjin (2013KZ090).
Glossary
Abbreviations
Abbreviations:
|
CUP
|
carcinoma of unknown primary
|
|
F-18 FDG PET/CT
|
fluorine-18-2-fluoro-2-deoxy-D-glucose
positron emission tomography/computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
BGC
|
blood glucose concentration
|
|
mCi
|
millicurie
|
|
LN
|
lymph node
|
References
|
1
|
Pavlidis N, Briasoulis E, Hainsworth J and
Greco FA: Diagnostic and therapeutic management of cancer of an
unknown primary. Eur J Cancer. 39:1990–2005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le Chevalier T, Cvitkovic E, Caille P,
Harvey J, Contesso G, Spielmann M and Rouesse J: Early metastatic
cancer of unknown primary origin at presentation. A clinical study
of 302 consecutive autopsied patients. Arch Intern Med.
148:2035–2039. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hainsworth JD, Spigel DR, Clark BL,
Shipley D, Thompson DS, Farley C, West-Osterfield K, Lane CM,
Cescon T, Bury MJ and Greco FA: Paclitaxel/carboplatin/etoposide
versus gemcitabine/irinotecan in the first-line treatment of
patients with carcinoma of unknown primary site: a randomized,
phase III Sarah Cannon Oncology Research Consortium Trial. Cancer
J. 16:70–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Møller AK, Pedersen KD, Gothelf A and
Daugaard G: Paclitaxel, cisplatin and gemcitabine in treatment of
carcinomas of unknown primary site, a phase II study. Acta Oncol.
49:423–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandez-Cotarelo MJ, Guerra-Vales JM,
Colina F and de la Cruz J: Prognostic factors in cancer of unknown
primary site. Tumori. 96:111–116. 2010.PubMed/NCBI
|
|
6
|
Hainsworth JD, Daugaard G, Lesimple T,
Hübner G, Greco FA, Stahl MJ, Büschenfelde CM, Allouache D, Penel
N, Knoblauch P and Fizazi KS: Paclitaxel/carboplatin with or
without belinostat as empiric first-line treatment for patients
with carcinoma of unknown primary site: a randomized, phase 2
trial. Cancer. 121:1654–1661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang SW, Hsu CM, Jeng WJ, Yen TC, Su MY
and Chiu CT: A comparison of positron emission tomography and
colonoscopy for the detection of advanced colorectal neoplasms in
subjects undergoing a health check-up. PLoS One. 8:e691112013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun N, Zhao J, Qiao W and Wang T:
Predictive value of interim PET/CT in DLBCL treated with R-CHOP:
meta-analysis. Biomed Res Int. 2015:6485722015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Kligerman S, Goel R, Sajedi P,
Suntharalingam M and Chuong MD: State-of-the-art molecular imaging
in esophageal cancer management: implications for diagnosis,
prognosis, and treatment. J Gastrointest Oncol. 6:3–19.
2015.PubMed/NCBI
|
|
10
|
Os AA, Fischbein NJ, Caputo GR, Kaplan MJ,
Price DC, Singer MI, Dillon WP and Hawkins RA: Metastatic head and
neck cancer: role and usefulness of FDG PET in locating occult
primary tumors. Radiology. 210:177–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nassenstein K, Veit-Haibach P, Stergar H,
Gutzeit A, Freudenberg L, Kuehl H, Fischer M, Barkhausen J,
Bockisch A and Antoch G: Cervical lymph node metastases of unknown
origin: primary tumor detection with whole-body positron emission
tomography/computed tomography. Acta Radiol. 48:1101–1108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Wu Y, Zhang W, Li J, Wu P and Xie
C: Clinical value of whole-body F-18 fluorodeoxyglucose positron
emission tomography/computed tomography in patients with carcinoma
of unknown primary. J Med Imaging Radiat Oncol. 57:65–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JR, Kim JS, Roh JL, Lee JH, Baek JH,
Cho KJ, Choi SH, Nam SY and Kim SY: Detection of occult primary
tumors in patients with cervical metastases of unknown primary
tumors: comparison of (18)F FDG PET/CT with contrast-enhanced CT or
CT/MR imaging-prospective study. Radiology. 274:764–771. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elboga U, Kervancioglu S, Sahin E,
Basibuyuk M, Celen YZ and Aktolun C: Utility of F-18
fluorodeoxyglucose positron emission tomography/computed in
carcinoma of unknown primary. Int J Clin Exp Pathol. 7:8941–8946.
2014.PubMed/NCBI
|
|
15
|
Breuer N, Behrendt FF, Heinzel A, Mottaghy
FM, Palmowski M and Verburg FA: Prognostic relevance of (18)F-FDG
PET/CT in carcinoma of unknown primary. Clin Nucl Med. 39:131–135.
2014.PubMed/NCBI
|
|
16
|
Hu M, Zhao W, Zhang PL, Ju GF, Fu Z, Zhang
GL, Kong L, Yang YQ, Ma YD and Yu JM: Clinical applications of
18F-fluorodeoxyglucose positron emission tomography/computed
tomography in carcinoma of unknown primary. Chin Med J (Engl).
124:1010–1014. 2011.PubMed/NCBI
|
|
17
|
Pak K, Kim SJ, Kim IJ, Nam HY, Kim BS, Kim
K and Kim YK: Clinical implication of (18)F-FDG PET/CT in carcinoma
of unknown primary. Neoplasma. 58:135–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yabuki K, Tsukuda M, Horiuchi C, Taguchi T
and Nishimura G: Role of 18F-FDG PET in detecting primary site in
the patient with primary unknown carcinoma. Eur Arch
Otorhinolaryngol. 267:1785–1792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chorost MI, Lee MC, Yeoh CB, Molina M and
Ghosh BC: Unknown primary. J Surg Oncol. 87:191–203. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pelosi E, Pennone M, Deandreis D,
Douroukas A, Mancini M and Bisi G: Role of whole body positron
emission tomography/computed tomography scan with
18F-fluorodeoxyglucose in patients with biopsy proven tumor
metastases from unknown primary site. Q J Nucl Med Mol Imaging.
50:15–22. 2006.PubMed/NCBI
|
|
21
|
Dong MJ, Zhao K, Lin XT, Zhao J, Ruan LX
and Liu ZF: Role of fluorodeoxyglucose-PET versus
fluorodeoxyglucose-PET/computed tomography in detection of unknown
primary tumor: a meta-analysis of the literature. Nucl Med Commun.
29:791–802. 2008.PubMed/NCBI
|
|
22
|
Wu ZJ, Zhang YX, Wei H and Jia Q: The role
of whole body 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron
emission tomography/computed tomography in the management of
unknown primary tumors. Zhonghua Yi Xue Za Zhi. 87:2253–2256.
2007.(In Chinese). PubMed/NCBI
|
|
23
|
Ryu IS, Choi SH, Kim do H, Han MW, Roh JL,
Kim SY and Nam SY: Detection of the primary lesion in patients with
cervical metastases from unknown primary tumors with narrow band
imaging endoscopy: preliminary report. Head Neck. 35:10–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao K, Luo XM, Zhou SH, Liu JH, Yan SX,
Lu ZJ, Yang SY, Lin LL and Dong MJ:
18F-fluorodeoxyglucose positron emission
tomography/computed tomography as an effective diagnostic workup in
cervical metastasis of carcinoma from an unknown primary tumor.
Cancer Biother Radiopharm. 27:685–693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Vries E and Coebergh JW: Melanoma
incidence has risen in Europe. BMJ. 331:6982005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rohren EM: PET/Computed tomography and
patient outcomes in melanoma. PET Clin. 10:243–254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katz KA, Jonasch E, Hodi FS, Soiffer R,
Kwitkiwski K, Sober AJ and Haluska FG: Melanoma of unknown primary:
experience at Massachusetts General Hospital and Dana-Farber Cancer
Institute. Melanoma Res. 15:77–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krug B, Crott R, Lonneux M, Baurain JF,
Pirson AS and Borght T Vander: Role of PET in the initial staging
of cutaneous malignant melanoma: systematic review. Radiology.
249:836–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwenzer NF and Pfannenberg AC: PET/CT,
MR, and PET/MR in lymphoma and melanoma. Semin Nucl Med.
45:322–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bertozzi S, Londero AP, Petri R and
Bernardi S: Isolated axillary nodal swelling and cancer of unknown
primary. Eur J Gynaecol Oncol. 36:131–137. 2015.PubMed/NCBI
|
|
31
|
Tardivon AA, Athanasiou A, Thibault F and
El Khoury C: Breast imaging and reporting data system (BIRADS)
magnetic resonance imaging illustrated cases. Eur J Radiol.
61:216–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahoney MC, Gatsonis C, Hanna L, DeMartini
WB and Lehman C: Positive predictive value of BI-RADS MR imaging.
Radiology. 264:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|