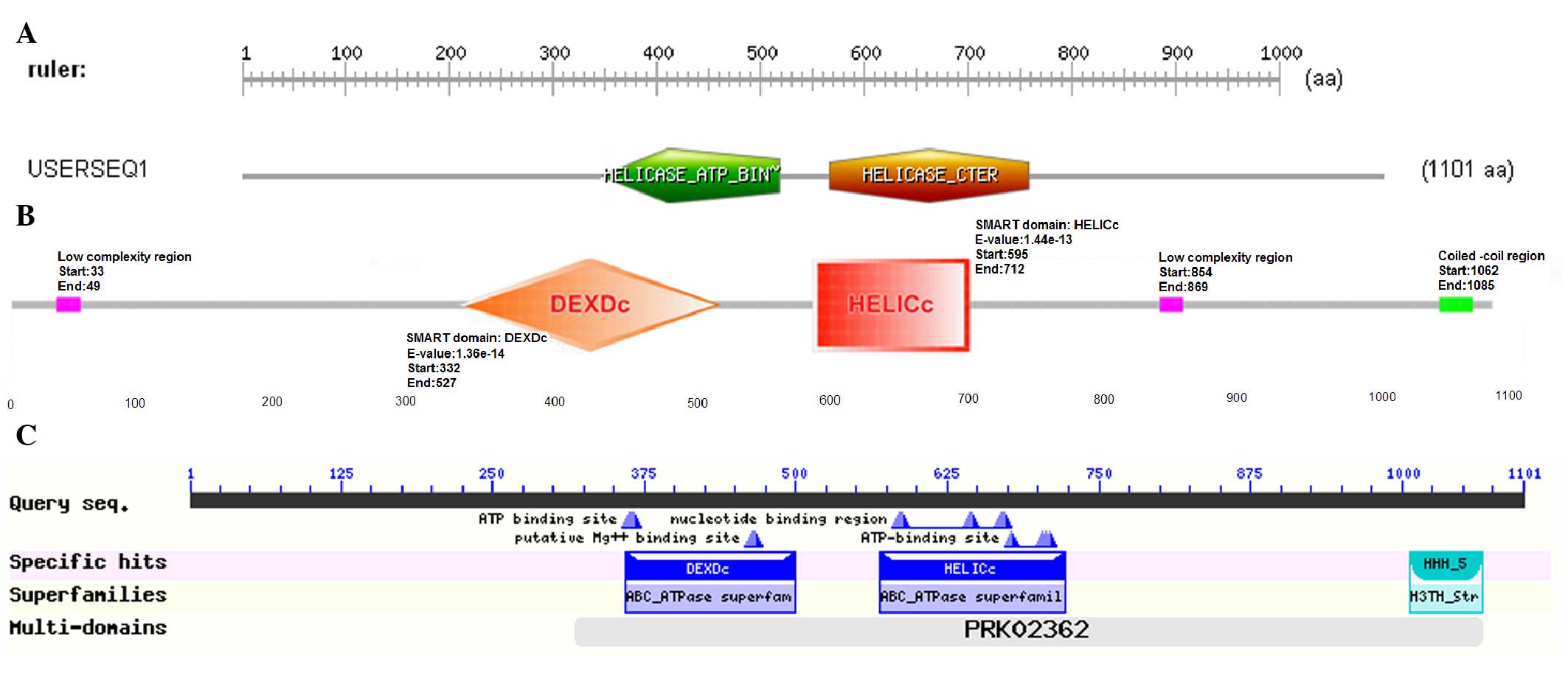
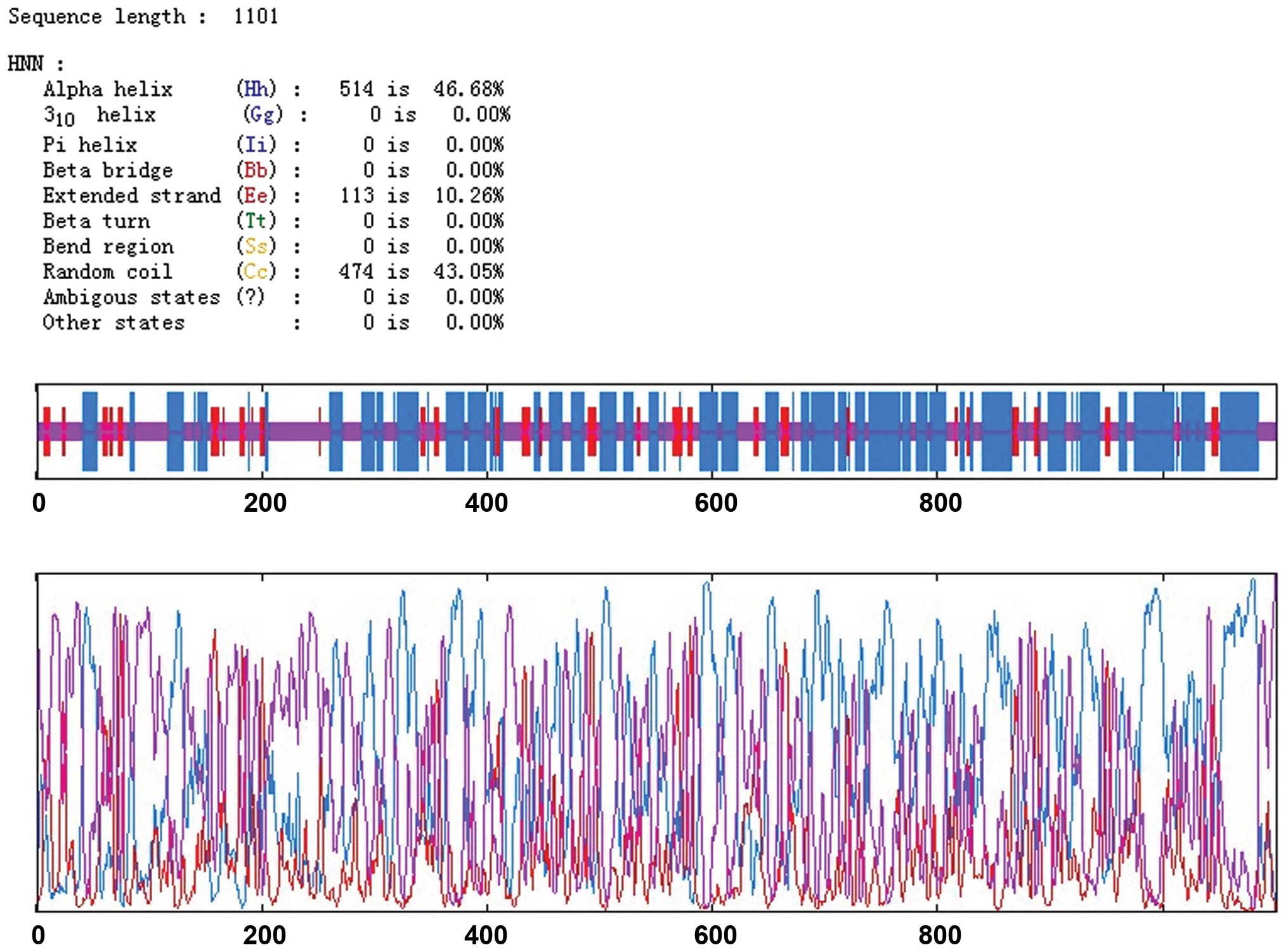
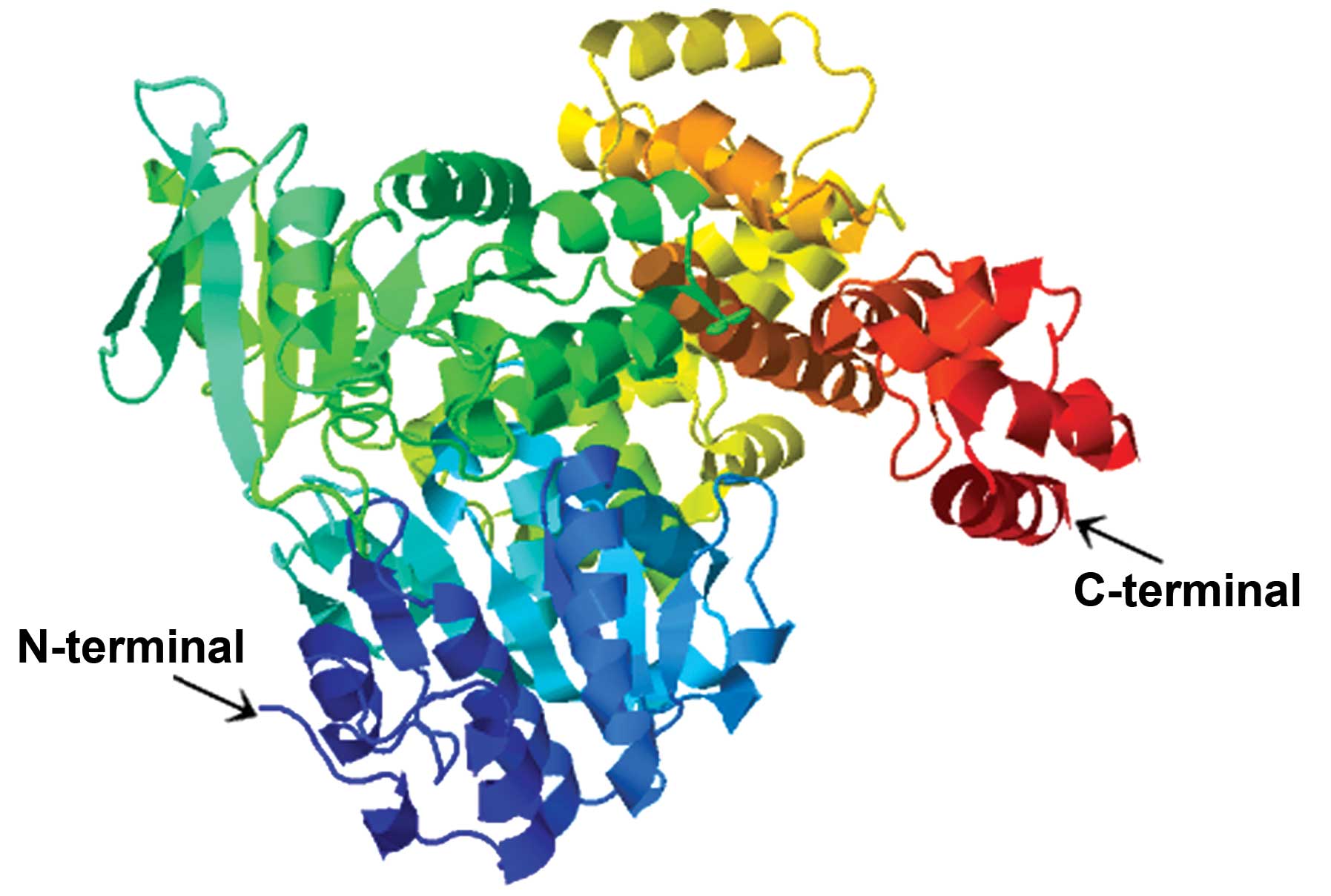
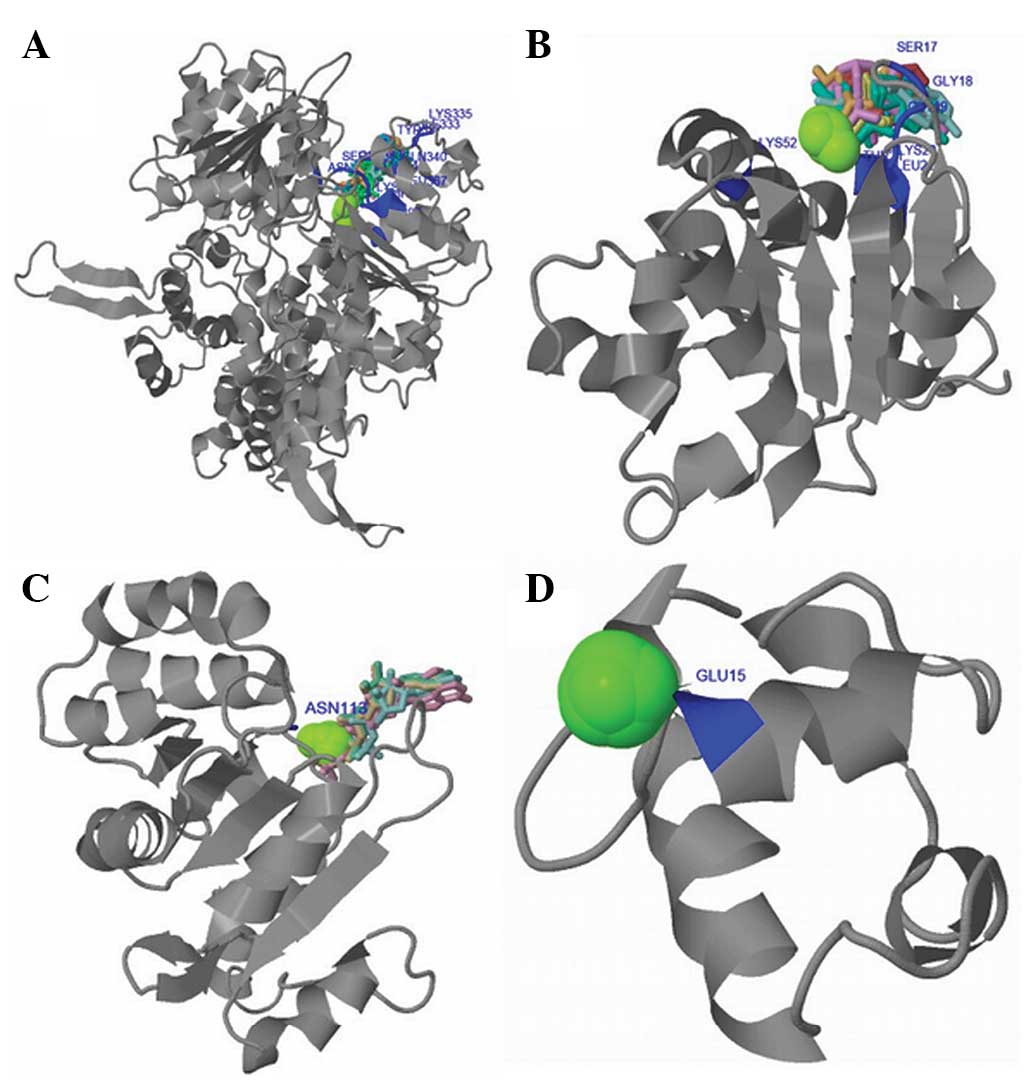
|
1
|
Thomson CA, E Crane T, Wertheim BC,
Neuhouser ML, Li W, Snetselaar LG, Basen-Engquist KM, Zhou Y and
Irwin ML: Diet quality and survival after ovarian cancer: Results
from the Women's health initiative. J Natl Cancer Inst.
106:dju3142014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adelman CA, Lolo RL, Birkbak NJ, Murina O,
Matsuzaki K, Horejsi Z, Parmar K, Borel V, Skehel JM, Stamp G, et
al: HELQ promotes RAD51 paralogue-dependent repair to avert germ
cell loss and tumorigenesis. Nature. 502:381–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marini F and Wood RD: A human DNA helicase
homologous to the DNA cross-link sensitivity protein Mus308. J Biol
Chem. 277:8716–8723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyd JB, Sakaguchi K and Harris PV: mus308
mutants of Drosophila exhibit hypersensitivity to DNA cross-linking
agents and are defective in a deoxyribonuclease. Genetics.
125:813–819. 1990.PubMed/NCBI
|
|
5
|
Leonhardt EA, Henderson DS, Rinehart JE
and Boyd JB: Characterization of the mus308 gene in Drosophila
melanogaster. Genetics. 133:87–96. 1993.PubMed/NCBI
|
|
6
|
Dolgova EV, Alyamkina EA, Efremov YR,
Nikolin VP, Popova NA, Tyrinova TV, Kozel AV, Minkevich AM,
Andrushkevich OM, Zavyalov EL, et al: Identification of cancer stem
cells and a strategy for their elimination. Cancer Biol Ther.
15:1378–1394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy S: Maintenance of genome stability in
plants: Repairing DNA double strand breaks and chromatin structure
stability. Front Plant Sci. 5:4872014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WQ, Hu N, Hyland PL, Gao Y, Wang ZM, Yu
K, Su H, Wang CY, Wang LM, Chanock SJ, et al: Genetic variants in
DNA repair pathway genes and risk of esophageal squamous cell
carcinoma and gastric adenocarcinoma in a Chinese population.
Carcinogenesis. 34:1536–1542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Y, He Y, Xu J, Xu L, Du J, Zhu C, Gu
H, Ma H, Hu Z, Jin G, et al: Genetic variants at 4q21, 4q23 and
12q24 are associated with esophageal squamous cell carcinoma risk
in a Chinese population. Human Genet. 132:649–656. 2013. View Article : Google Scholar
|
|
10
|
Liang C, Marsit CJ, Houseman EA, Butler R,
Nelson HH, McClean MD and Kelsey KT: Gene-environment interactions
of novel variants associated with head and neck cancer. Head Neck.
34:1111–1118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McKay JD, Truong T, Gaborieau V, Chabrier
A, Chuang SC, Byrnes G, Zaridze D, Shangina O, Szeszenia-Dabrowska
N, Lissowska J, et al: A genome-wide association study of upper
aerodigestive tract cancers conducted within the INHANCE
consortium. PLoS Genet. 7:e10013332011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Käll L, Krogh A and Sonnhammer EL: A
combined transmembrane topology and signal peptide prediction
method. J Mol Biol. 338:1027–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scott L, Chahine J and Ruggiero J:
Prediction of Protein Structures using a Hopfield NetworkSixth
Brazilian Symposium on Neural Networks 2000. IEEE Computer Society
Press; Washington, DC: pp. 284. 2000, View Article : Google Scholar
|
|
14
|
McGlynn P: Helicases at the replication
fork. Adv Exp Med Biol. 767:97–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsang E and Carr AM: Replication fork
arrest, recombination and the maintenance of ribosomal DNA
stability. DNA repair (Amst). 7:1613–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McGlynn P and Lloyd RG: Recombinational
repair and restart of damaged replication forks. Nat Rev Mol Cell
Biol. 3:859–870. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singleton MR and Wigley DB: Modularity and
specialization in superfamily 1 and 2 helicases. J Bacteriol.
184:1819–1826. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moldovan GL, Madhavan MV, Mirchandani KD,
McCaffrey RM, Vinciguerra P and D'Andrea AD: DNA polymerase POLN
participates in cross-link repair and homologous recombination. Mol
Cell Biol. 30:1088–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tafel AA, Wu L and McHugh PJ: Human HEL308
localizes to damaged replication forks and unwinds lagging strand
structures. J Biol Chem. 286:15832–15840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vannier JB, Sandhu S, Petalcorin MI, Wu X,
Nabi Z, Ding H and Boulton SJ: RTEL1 is a replisome-associated
helicase that promotes telomere and genome-wide replication.
Science. 342:239–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coleman KA and Greenberg RA: The
BRCA1-RAP80 complex regulates DNA repair mechanism utilization by
restricting end resection. J Biol Chem. 286:13669–13680. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y and Parvin JD: Small ubiquitin-like
modifier (SUMO) isoforms and conjugation-independent function in
DNA double-strand break repair pathways. J Biol Chem.
289:21289–21295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward JD, Muzzini DM, Petalcorin MI,
Martinez-Perez E, Martin JS, Plevani P, Cassata G, Marini F and
Boulton SJ: Overlapping mechanisms promote postsynaptic RAD-51
filament disassembly during meiotic double-strand break repair. Mol
Cell. 37:259–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marini F, Kim N, Schuffert A and Wood RD:
POLN, a nuclear PolA family DNA polymerase homologous to the DNA
cross-link sensitivity protein Mus308. J Biol Chem.
278:32014–32019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luebben SW, Kawabata T, Akre MK, Lee WL,
Johnson CS, O'Sullivan MG and Shima N: Helq acts in parallel to
Fancc to suppress replication-associated genome instability.
Nucleic Acids Res. 41:10283–10297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelley MR and Fishel ML: DNA repair
proteins as molecular targets for cancer therapeutics. Anticancer
Agents Med Chem. 8:417–425. 2008. View Article : Google Scholar : PubMed/NCBI
|