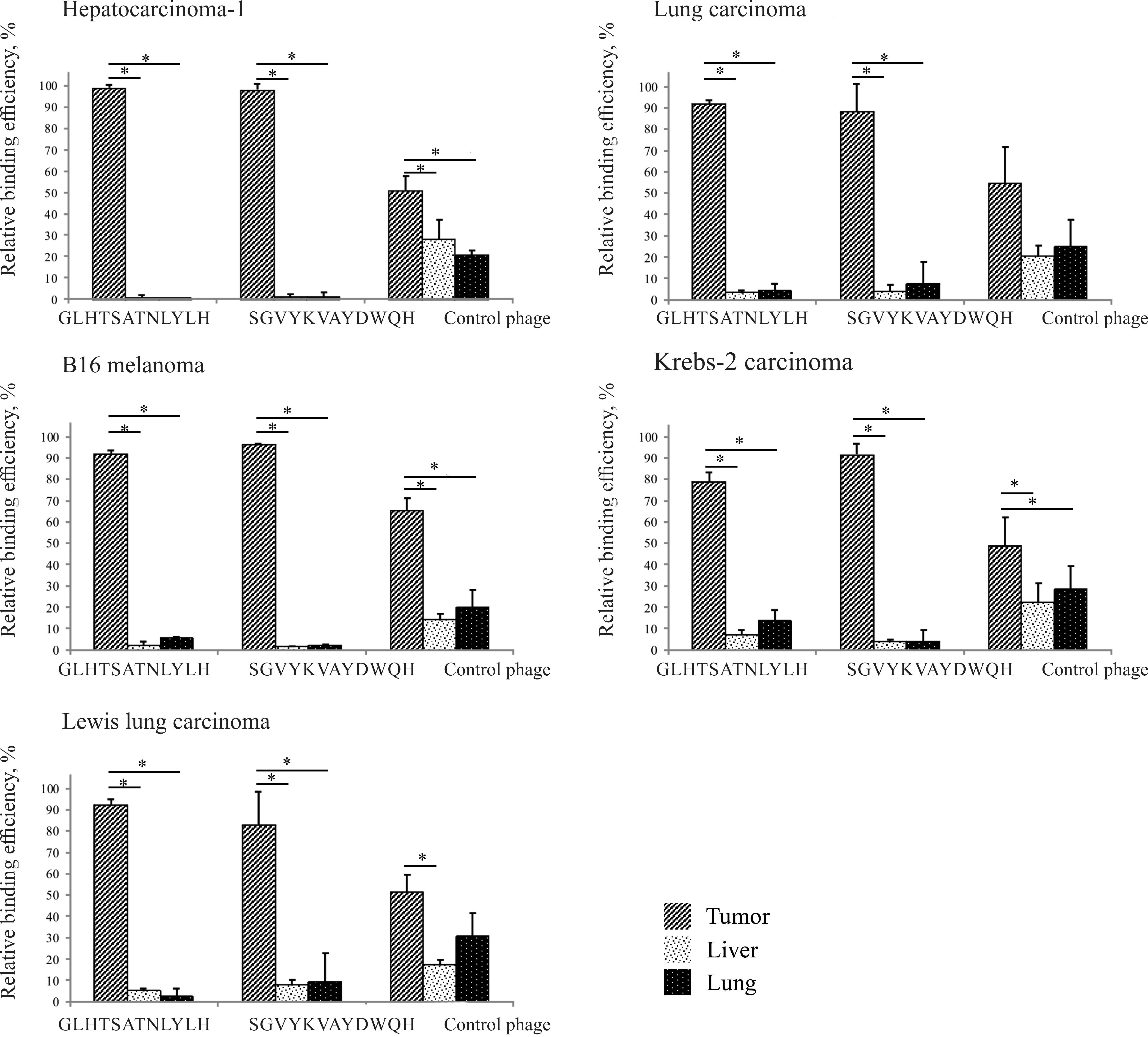
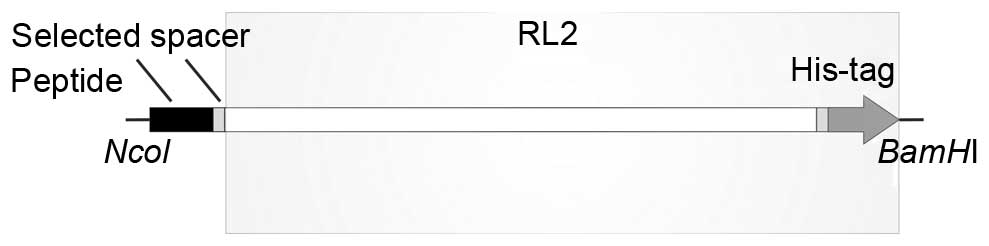
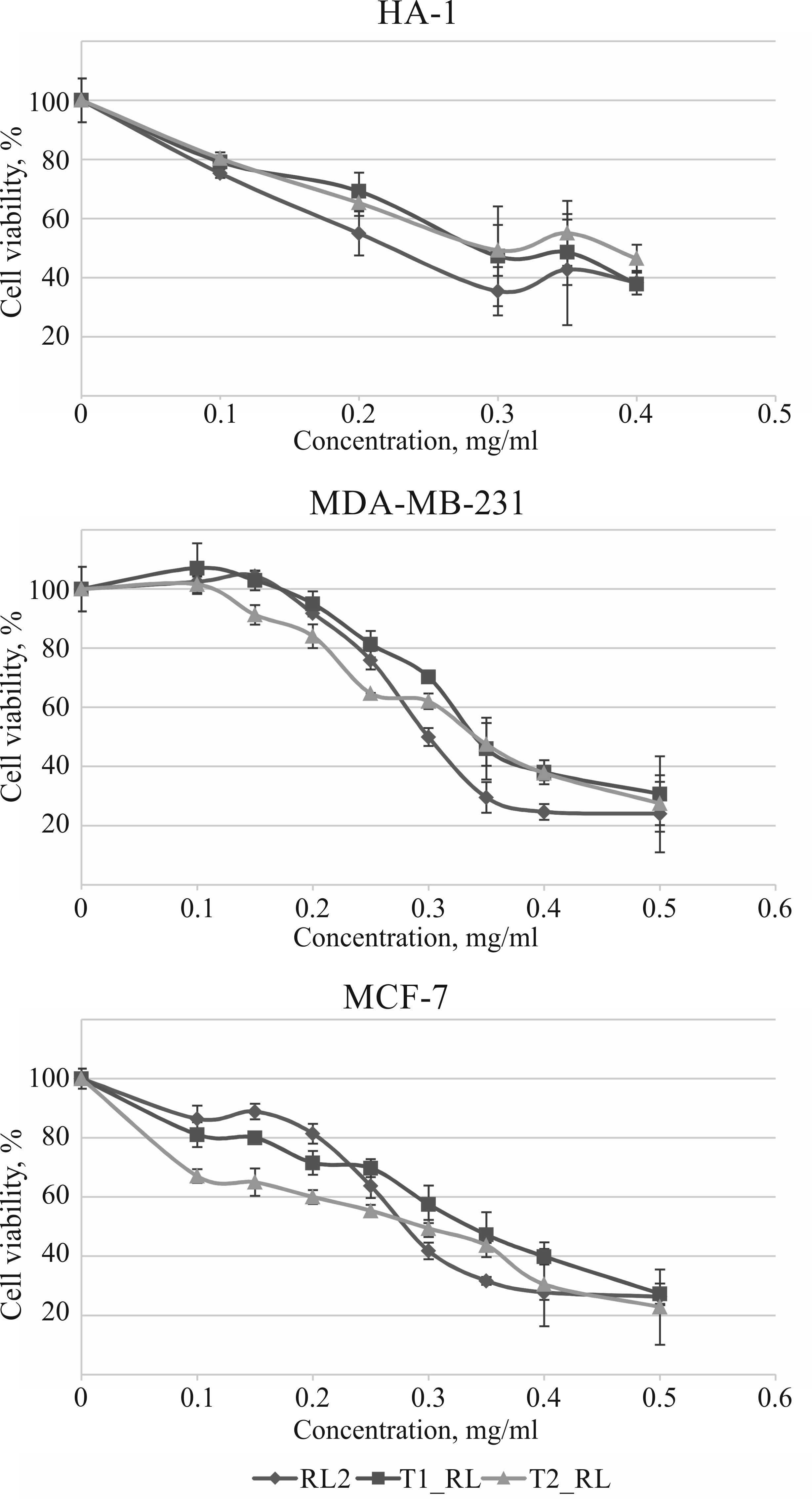
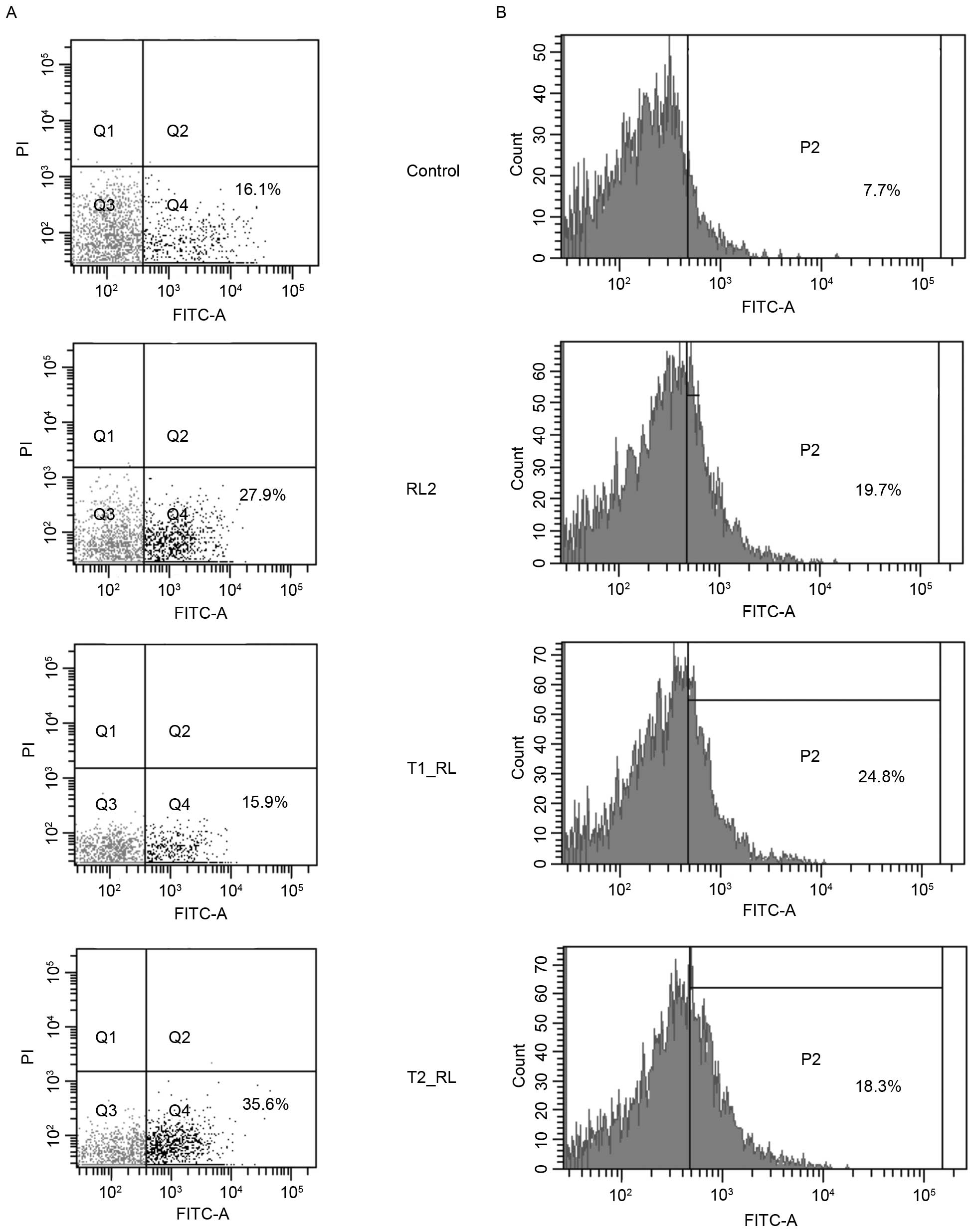
|
1
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apoptosis pathways in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res (Phila).
7:1081–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nekipelaya VV, Semenov DV, Potapenko MO,
Kuligina EV, Kit Yu, Romanova IV and Richter VA: Lactaptin is a
human milk protein inducing apoptosis of MCF-7 adenocarcinoma
cells. Dokl Biochem Biophys. 419:58–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vlassov VV, Richter VA, Semenov DV,
Nekipelaya VV, Kuligina EV and Potapenko MO: Peptide inducing
apoptotic death of human cancer cells. Patent RF N 2317304.
2008.
|
|
4
|
Tikunova NV, Semenov DV, Babkina IN,
Kuligina EV, Koval OA, et al: Recombinant plasmid DNA pFK2,
providing synthesis of the recombinant peptide which is the analog
of human kappa-casein and recombinant peptide-the analog of human
kappa-casein fragment, with the apoptotic activity against human
tumor cells. Patent RF N 2401307. 2010.
|
|
5
|
Semenov DV, Fomin AS, Kuligina EV, Koval
OA, Matveeva VA, Babkina IN, Tikunova NV and Richter VA:
Recombinant analogs of a novel milk pro-apoptotic peptide,
lactaptin, and their effect on cultured human cells. Protein J.
29:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koval OA, Fomin AS, Kaledin VI, Semenov
DV, Potapenko MO, Kuligina EV, Nikolin VP, Nikitenko EV and Richter
VA: A novel pro-apoptotic effector lactaptin inhibits tumor growth
in mice models. Biochimie. 94:2467–2474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koval OA, Tkachenko AV, Fomin AS, Semenov
DV, Nushtaeva AA, Kuligina EV, Zavjalov EL and Richter VA:
Lactaptin induces p53-independent cell death associated with
features of apoptosis and autophagy and delays growth of breast
cancer cells in mouse xenografts. PLoS One. 9:e939212014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bondarenko DA, Richter VA, Kuligina EV,
Koval OA, Fomin AS, et al: Toxicity studies and pharmacokinetics of
Lactaptin. Russian Journal of Biopharmaceuticals. 7:40–47.
2015.
|
|
9
|
Nemudraya AA, Richter VA and Kuligina EV:
Phage peptide libraries as a source of targeting ligands. Acta
Naturae. 8:48–57. 2016.PubMed/NCBI
|
|
10
|
Tamura K, Dudley J, Nei M and Kumar S:
MEGA4: Molecular evolutionary genetics analysis (MEGA) software
version 4.0. Mol Biol Evol. 24:1596–1599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bakhshinejad B and Sadeghizadeh M:
Bacteriophages as vehicles for gene delivery into mammalian cells:
Prospects and problems. Expert Opin Drug Deliv. 11:1561–1574. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fomin AS, Koval' OA, Semenov DV, Potapenko
MO, Kuligina EV, Kit IuIa and Rikhter VA: The Analysis of
biochemical markers of MCF-7 cells apoptosis induced by recombinant
analog of lactaptin. Bioorg Khim. 38:92–98. 2012.PubMed/NCBI
|
|
14
|
Svensen N, Walton JG and Bradley M:
Peptides for cell-selective drug delivery. Trends Pharmacol Sci.
33:186–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venditto VJ and Szoka FC Jr: Cancer
nanomedicines: So many papers and so few drugs! Adv Drug Deliv Rev.
65:80–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamzeh-Mivehroud M, Alizadeh AA, Morris
MB, Church WB and Dastmalchi S: Phage display as a technology
delivering on the promise of peptide drug discovery. Drug Discov
Today. 18:1144–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krumpe LR and Mori T: The use of
phage-displayed peptide libraries to develop tumor-targeting drugs.
Int J Pept Res Ther. 12:79–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wicki A, Witzigmann D, Balasubramanian V
and Huwyler J: Nanomedicine in cancer therapy: Challenges,
opportunities, and clinical applications. J Control Release.
200:138–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruoslahti E: Specialization of tumour
vasculature. Nat Rev Cancer. 2:83–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li ZJ and Cho CH: Peptides as targeting
probes against tumor vasculature for diagnosis and drug delivery. J
Transl Med. 10:(Suppl 1). S12012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown KC: Peptidic tumor targeting agents:
The road from phage display peptide selections to clinical
applications. Curr Pharm Des. 16:1040–1054. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parks SK, Cormerais Y, Marchiq I and
Pouyssegur J: Hypoxia optimises tumour growth by controlling
nutrient import and acidic metabolite export. Mol Aspects Med
47–48. 3–14. 2016. View Article : Google Scholar
|
|
23
|
Wahba HA and El-Hadaad HA: Current
approaches in treatment of triple-negative breast cancer. Cancer
Biol Med. 12:106–116. 2015.PubMed/NCBI
|
|
24
|
Bose S: Triple-negative breast carcinoma:
Morphologic and molecular subtypes. Adv Anat Pathol. 22:306–313.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noren KA and Noren CJ: Construction of
high-complexity combinatorial phage display peptide libraries.
Methods. 23:169–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn KY, Ko HK, Lee BR, Lee EJ, Lee JH,
Byun Y, Kwon IC, Kim K and Lee J: Engineered protein nanoparticles
for in vivo tumor detection. Biomaterials. 35:6422–6429. 2014.
View Article : Google Scholar : PubMed/NCBI
|