Introduction
Bovine dialyzable leukocyte extract (bDLE) is a
heterogeneous mixture of low-molecular weight substances released
from disintegrated leukocytes of homogenized bovine spleen
(1). bDLE has been reported to have
various biological activities, including induction of cytotoxic
effects in several cancer cell lines in vitro (1) and in melanoma in vivo (2), as well as modulation of the expression
of transcription factors, including nuclear factor-κB and activator
protein 1 (3), with no effect on
normal cells (1). Furthermore, bDLE
has demonstrated antioxidant activity (4). bDLE has been used as an immunomodulator
and coadjuvant in clinical trials.
Chronic myeloid leukemia (CML) is a malignant
hematological disease of hematopoietic stem/progenitor cells caused
by the t(9;22)(q34;q11) chromosomal translocation and expression of
the Bcr-Abl oncoprotein (1).
Leukaemia is the tenth most common cause of cancer-associated
mortalities, worldwide, accounting for >265,000 mortalities in
2012 (5). CML incidence increases
with age and accounts for 20% of all leukemia cases, with an annual
incidence of 1–1.5 cases per 100,000 individuals (5). in 2012. Currently, CML is treated with
chemotherapeutics agents and specific inhibitors, such as imatinib
or dasutinib. which have demonstrated a high response rate;
however, effects are often short-lived and disease progression is
common (6). An alternative strategy
to treat leukemia, cell differentiation therapy, has been proposed
and consists of forcing leukemia cells toward a process of terminal
differentiation by using biological or chemical agents (7–9). Certain
compounds used with this objective in clinical practice are
all-trans retinoic acid (ATRA) (7)
and 1,25-dihydroxyvitamin D3 (7–9). Certain
substances used may exhibit selective activity against tumor cells
and minimal side effects against normal cells (10). An in vitro model for
investigating cell differentiation has been established using the
human chronic myelogenous leukemia K562 cell line (4), which expresses characteristics of
erythrocytes, monocytes and megakaryocytes. Following exposure to
phorbol myristate acetate (PMA), the K562 cancer cell line is
differentiated toward cells with monocytic and/or megakaryocytic
characteristics (2), while treatment
with imatinib, butyric acid and haemin cause erythroid
differentiation (7,9). The present study investigated the cell
death and differentiation activity induced by bDLE in the human
CML, using K562 as a model cell line.
Materials and methods
bDLE
bDLE was produced by the Laboratory of Immunology
and Virology, Faculty of Biological Sciences, University Autonomous
of Neuvo León (UANL) (San Nicolás de los Garza, Mexico). bDLE is a
mixture of low-molecular weight substances (cut-off of 10–12 kDa)
obtained from the dialysis of disintegrated bovine spleens in
water, subsequently lyophilized and determined to be free of
pyrogens using the Limulus amoebocyte lysate assay
(Endotoxin Detection kit; MP Biomedicals, LLC, Santa Ana, CA, USA),
and confirmed to be free of bacterial contamination by culturing in
various culture media as well as in vivo mouse inoculation.
bDLE obtained from 75×108 leukocytes is defined as five
units (5 U). For the subsequent assays, bDLE was suspended in
RPMI-1640 (Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.). The suspension was filtered
with a 0.2 µm-diameter filter (EMD Milipore, Billerica, MA,
USA).
K562 cell treatments
The K562 cell line was originally established from
the pleural effusions of a patient with CML in terminal blast
crisis. The cell line was obtained from American Type Culture
Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium
supplemented with 10% FBS and 1% antibiotic-antimycotic solution
(Gibco; Thermo Fisher Scientific, Inc.), at 37°C in a humidified
incubator with 5% CO2. To determine the cytotoxic effect
and induction of cell differentiation by bDLE in K562 cells, cells
were seeded onto 6-well plates at a density of 1×105
cells/well and treated with bDLE (0.07, 0.14, 0.21, 0.28, 0.35,
0.5, 0.75 and 1 U/ml). PMA (10 ng/ml; Sigma-Aldrich; EMD Millipore)
and dimethyl sulfoxide (DMSO;1.5% v:v; Sigma-Aldrich; EMD
Millipore) were used as positive controls for the induction of cell
differentiation in the K562 cell line. All treatments (bDLE, PMA
and DMSO) were suspended in RPMI-1640 medium supplemented with 10%
FBS, and plates were incubated for 96 h at 37°C in a 5%
CO2 atmosphere. After trypsinization, adherent and
non-adherent cells were collected. The MOLT-3 (T acute
lymphoblastic leukemia) cell line was obtained from American Type
Culture Collection and was used as a cytotoxic control of the T
cell lineage.
Macrophage treatments
A total of 5 male BALB/c mice (6 weeks old; 24–26 g)
were obtained from the Laboratory of Immunology and Virology of the
University Autonomous of Nuevo León) and maintained in a controlled
environment at 25°C (12 h light/dark cycles) with free access to
food and water. The mice were sacrificed by cervical dislocation,
and resident peritoneal macrophages were obtained through repeated
sterile ice-cold RMPI-1640 lavages within the peritoneal cavity,
according to protocols approved by the Institutional Animal Care
and Use Committee of the Laboratory of Immunology and Virology of
the University Autonomous of Nuevo León. Furthermore, human
monocytes were isolated from peripheral blood obtained from normal
donors, the blood was diluted with PBS at a ratio of 1:1 (vol/vol)
and subsequently the blood was centrifuged on a Ficoll-Paque
gradient for 30 min at 500 × g at room temperature. The
interphase layer consisted of peripheral blood mononuclear cells
and was washed three times with culture medium. Murine peritoneal
macrophage and human monocyte cells (2×106 cells/ml)
were seeded in 6-well plates and incubated at 37°C in an atmosphere
of 5% CO2 for 3 h to allow cell adherence to the
plastic. Subsequently, the non-adherent cells were removed, and
adherent cells were obtained by trypsinization. Cell supernatants
were centrifuged at 500 × g at 25°C for 10 min and seeded at
1×105 cells/well. Cells were treated with bDLE (0.5,
0.75 and 1 U/ml) and incubated at 37°C in a 5% CO2
atmosphere for 96 h, followed by evaluation of cell death by
propidium iodide (PI) staining.
Cell viability assessment by PI
staining
To evaluate cell death, cells were centrifuged at
500 × g at 25°C for 10 min and washed with PBS, resuspended
in 0.2 ml PBS and PI (50 µg/ml; BD Biosciences, Franklin Lakes, NJ,
USA) and incubated for 5 min at room temperature (25°C) in the
dark, followed by analysis with an Accuri™ C6 flow cytometer (BD
Biosciences).
Analysis of cell proliferation by
trypan blue staining
K562 cells previously treated for 96 h with bDLE,
PMA or DMSO were harvested, centrifuged at 500 × g at 25°C
for 10 min and resuspended in 0.2 ml PBS. Cell proliferation
percentage was estimated by cell counting following trypan blue
staining for each group. Furthermore, to assess the proliferative
capacity of the differentiated cells, following 96 h of incubation
with bDLE, PMA or DMSO, the cells were collected, centrifuged at
500 × g at 25°C for 10 min, washed with RPMI-1640 and seeded
at 1×105 cells/well. Cells were subsequently incubated
at 37°C in an atmosphere of 5% CO2 for 10 days, and the
cell count was evaluated by trypan blue staining and proliferation
was estimated.
Cell cycle analysis
Cell cycle progression analysis was performed with
the Cycle Test™ Plus DNA Reagent kit (BD Biosciences) according to
manufacturer's protocol. In brief, 1×105 cells/well
previously treated for 96 h (with bDLE, PMA or DMSO) were washed
with PBS and fixed with 1 ml buffer solution (containing sodium
citrate, sucrose and DMSO). Subsequently, 250 µl of solution A
(containing trypsin in a spermine tetrahydrochloride detergent
buffer) was added, and the samples were incubated for 10 min at
room temperature. Following incubation, 200 µl of solution B
(trypsin inhibitor and RNase buffer) was added, followed by the
addition of 200 µl of cold (2–8°C) solution C (containing propidium
iodide staining solution). Samples were gently mixed and incubated
for 10 min in the dark on ice. Data acquisition was performed on a
BD Accuri C6 flow cytometer (10,000 events were analyzed) and data
analysis was performed using ModFit LT version 4.0 software (Verity
Software, Inc., Topsham, ME USA).
Morphological characteristics by
Romanowsky staining
To determine the effects of treatment on the size
and morphological characteristics of K562 cells, the cells were
harvested and fixed with 1% formaldehyde for 1 min at room
temperature (20–25°C). Slides were dried, and Romanowsky staining
solution was added, rinsed with deionized water, air dried and
observed under a Nikon inverted light microscope (Eclipse TE300;
Nikon Corporation, Tokyo, Japan) at magnification, ×40.
Evaluation of cluster of
differentiation (CD)14+, CD68+,
CD163+ and CD42+ surface markers
For assessment of monocytic differentiation induced
by treatments, K562 cells were harvested and incubated for 30 min
at room temperature simultaneously with phycoerythrin-conjugated
anti-CD14 (cat. no. 340585; 1:20; Invitrogen; Thermo Fisher
Scientific, Inc.), fluorescein isothiocyanate (FITC)-conjugated
anti-CD68 (cat. no. 562117; 1:20; BD Biosciences) and
allophycocyanin-conjugated anti-CD163 (cat. no. 556018; 1:20; BD
Biosciences). To evaluate megakaryocytic lineage the cells were
incubated with peridinin chlorophyll protein complex-conjugated
anti-CD42a (cat. no. 340537; 1:20; BD Biosciences) in PBS with 1%
FBS and 0.1% sodium azide for 30 min at 25°C. Following incubation,
samples were washed and resuspended in PBS and 10,000 events were
recorded by flow cytometry (BD Accuri C6 flow cytometer). The
CD14+ cell population was gated and the CD68+
and CD163+ populations were evaluated.
Phagocytosis assay
K562 cells previously treated for 96 h (with bDLE,
PMA or DMSO) and washed three times with PBS, were incubated with
FITC-Dextran (0.1 mg/ml; molecular weight 70,000; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 60 min. As a negative
control, cells were incubated with FITC-Dextran at 4°C for 60 min.
To stop phagocytosis the cells were washed twice with cold PBS
supplemented with 1% FBS. Phagocytosis was assessed by flow
cytometry (10,000 events were analyzed) and quantified as a
percentage of cellular uptake of FITC-Dextran.
Determination of nitric oxide (NO) by
colorimetric assay
The supernatants of each treatment were used to
determine NO production using the nitrate-nitrite colorimetric
assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) according
to the manufacturer's protocol. Briefly, 40 µl of supernatants were
mixed with 40 µl of assay buffer, 10 µl of enzyme cofactor and 10
µl of nitrate reductase, and incubated at room temperature for 3 h
(to allow the conversion of nitrate to nitrite). Following 10 min
of incubation in Griess reagent at room temperature, the absorbance
was measured at 560 nm in a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Cytokine and chemokine production
assessment by flow cytometry
Cytokine production was analyzed using a BD
Cytometric Bead Array (CBA) Human Inflammatory Cytokines kit (BD
Biosciences). For the evaluation, cell supernatants were harvested
following 96 h incubation, and stored at −20°C until analysis
according to manufacturer's protocol. The cytokines evaluated were
interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α
and the evaluation of chemokine production included: Chemokine
(C-X-C motif) ligand (CXCL)8/IL-8, chemokine (C-C motif) ligand
(CCL)5/regulated on activation, normal T cell expressed and
secreted (RANTES), CXCL9/monokine induced by gamma interferon,
CCL2/monocyte chemoattractant protein-1 (MCP-1) and
CXCL10/interferon gamma-induced protein 10. Cytokine and chemokine
production were measured by flow cytometry (BD Accuri C6) according
to the manufacturer's protocol, and CBA analysis was performed
using FCAP Array™ software version 1.0 (Soft Flow, Inc., St. Louis
Park, MN, USA).
Statistical analysis
All experiments were performed in triplicate, and
statistical analysis was performed using analysis of variance
followed by post hoc Dunnett's tests. SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA) was used for all
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of bDLE on the viability of
K562, MOLT-3, murine peritoneal macrophages and primary human
monocytes
bDLE decreased the viability of K562 [0.5 U/ml
(33.0%), 0.75 U/ml (93.2%) and 1 U/ml (96.8%)] and MOLT-3 [0.21
U/ml (18.8%), 0.28 U/ml (62.0%), 0.35 U/ml (67.1%), 0.5 U/ml
(79.8%), 0.75 U/ml (87.5%) and 1 U/ml (94.7%)] leukemia cell lines
in a dose-dependent manner (P=0.03), demonstrating that bDLE
treatment induced a highly cytotoxic effect on the MOLT-3 cell line
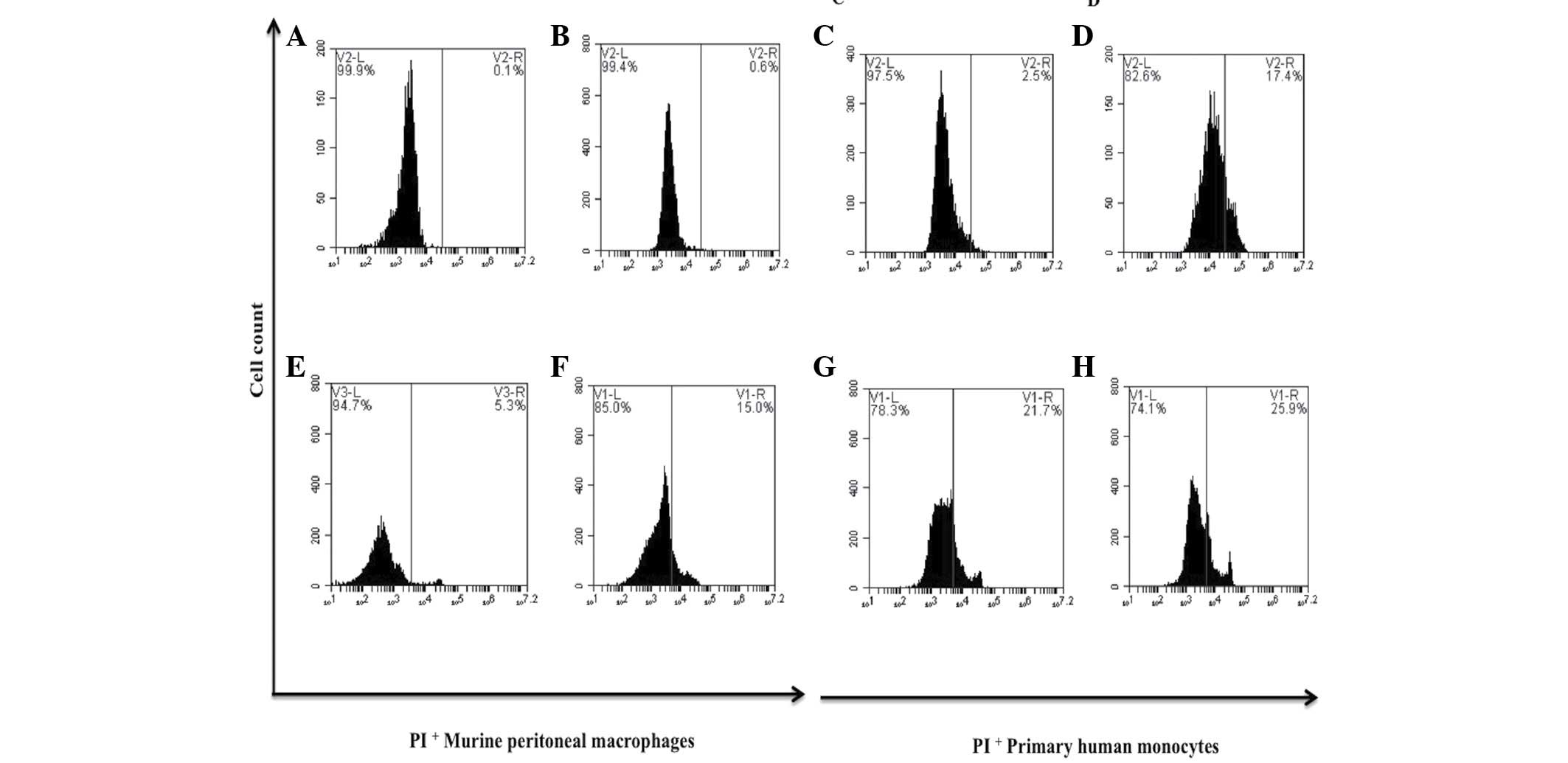
(Figs. 1 and 2). The relative viability of the primary
human monocytes and murine peritoneal macrophages treated with bDLE
remained at 75–80% (Fig. 3). PMA
treatment weakly affected the viability of K562 cells, and did not
affect the MOLT-3 cell line (P=0.75). DMSO treatment did not affect
the leukemia cell line viability (P=0.85; Figs. 1 and 2).
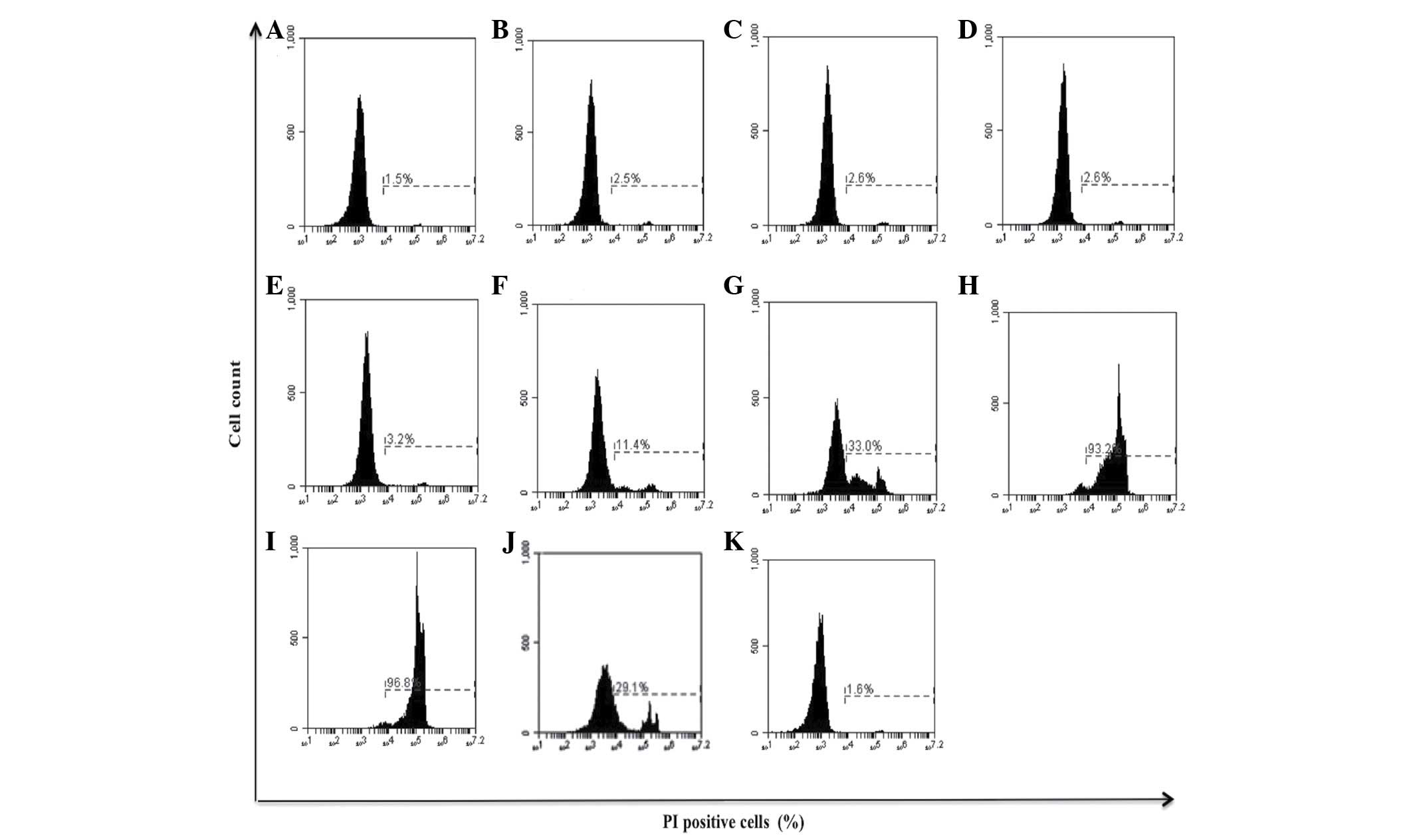 | Figure 1.Effect of bDLE on the viability of
K562 cells. K562 cells were seeded into plates with or without
treatment. (A) Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14 U/ml
bDLE, (D) 0.21 U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml bDLE,
(G) 0.5 U/ml bDLE, (H) 0.75 U/ml bDLE, (I) 1 U/ml bDLE, (J) 10
ng/ml phorbol myristate acetate or (K) dimethyl sulfoxide (1.5%,
v:v) were incubated for 96 h. Cells were harvested and cell death
was detected by PI staining and analyzed by flow cytometry. Flow
cytometry data show representative results. PI, propidium iodide;
bDLE, bovine dialyzable leukocyte extract. |
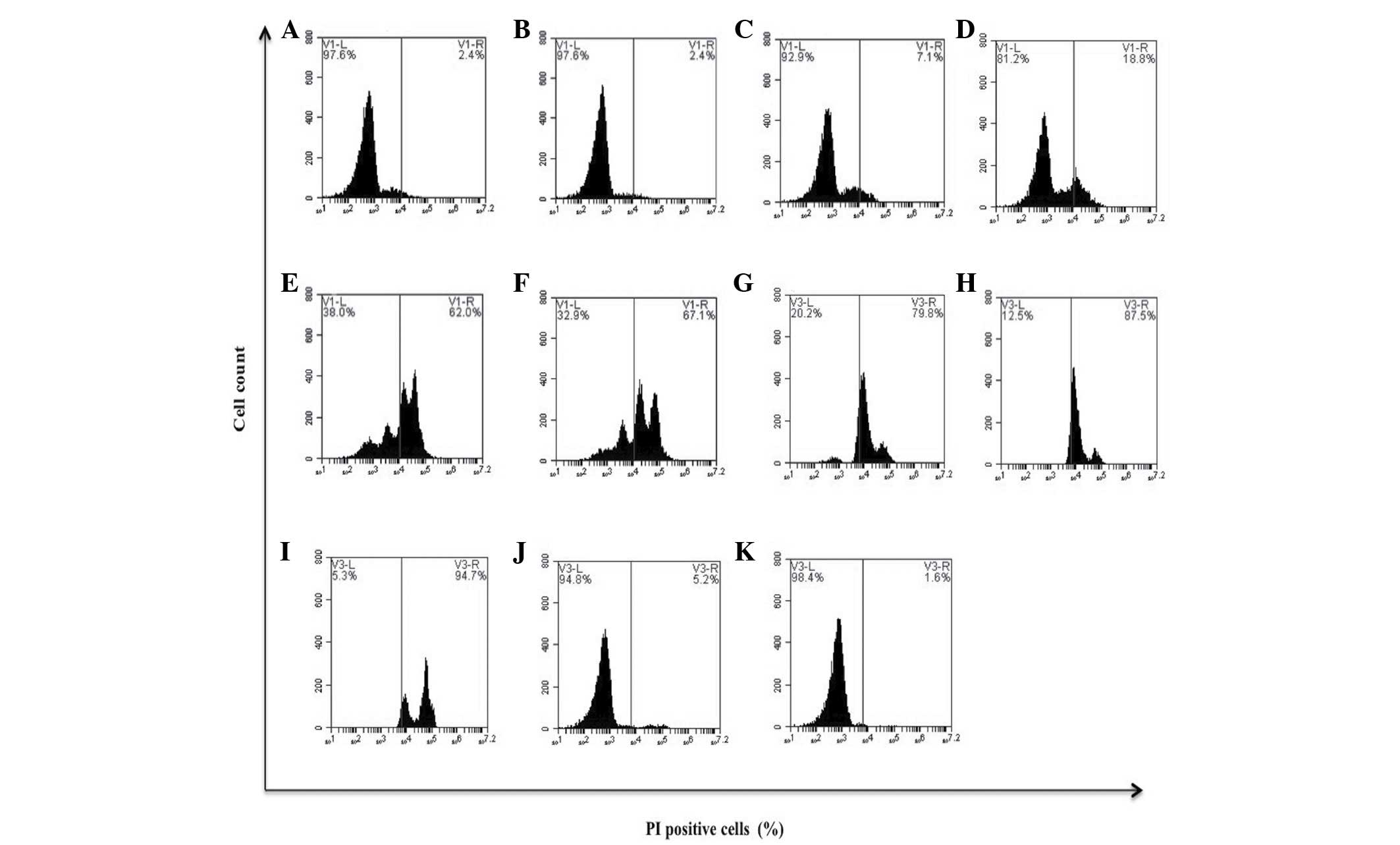 | Figure 2.Effect of bDLE on the viability of
MOLT-3 cells. MOLT-3 cells were seeded into plates with or without
treatments. (A) Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14 U/ml
bDLE, (D) 0.21 U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml bDLE,
(G) 0.5 U/ml bDLE, (H) 0.75 U/ml bDLE, (I) 1 U/ml bDLE, (J) 10
ng/ml phorbol myristate acetate or (K) dimethyl sulfoxide (1.5%,
v:v) were incubated for 96 h. Cells were harvested and cell death
was detected by PI staining and analyzed by flow cytometry. Flow
cytometry data show representative results. PI, propidium iodide;
bDLE, bovine dialyzable leukocyte extract. |
bDLE induces arrest in the S and
G2/M phases of the cell cycle and inhibits cellular
proliferation rate in the K562 cell line
bDLE treatments (0.07–0.35 U/ml) induced alterations
in cell cycle progression, as shown in Fig. 4 and Table
I. bDLE caused S phase arrest at all doses tested [0.07 U/ml
(57.28%), 0.14 U/ml (59.62%), 0.21 U/ml (64.45%), 0.28 U/ml
(58.68%) and 0.35 U/ml (58.64%)]. PMA or DMSO treatments decreased
the percentage of cells in the S phase (16.09 and 45.77%,
respectively), compared with the untreated cells (52.34%). bDLE or
PMA treatments increased the percentage of cells in the
G2/M phase [0.07 U/ml (5.11%), 0.14 U/ml (9.57%), 0.21
U/ml (8.19%), 0.28 U/ml (9.46%), 0.35 U/ml (8.27%) and PMA
(33.07%)]. Untreated cells and cells treated with DMSO showed no
affect on the percentage of cells in the G2/M phase
(3.24 and 4.81%, respectively). Furthermore, a significant decrease
in the percentage of cells in the G0/G1 phase
was detected following bDLE treatment [0.07 U/ml (37.61%), 0.14
U/ml (30.80%), 0.21 U/ml (27.36%), 0.28 U/ml (31.86%) and 0.35 U/ml
(33.09%)] compared with PMA (50.84%), DMSO (49.42%) and untreated
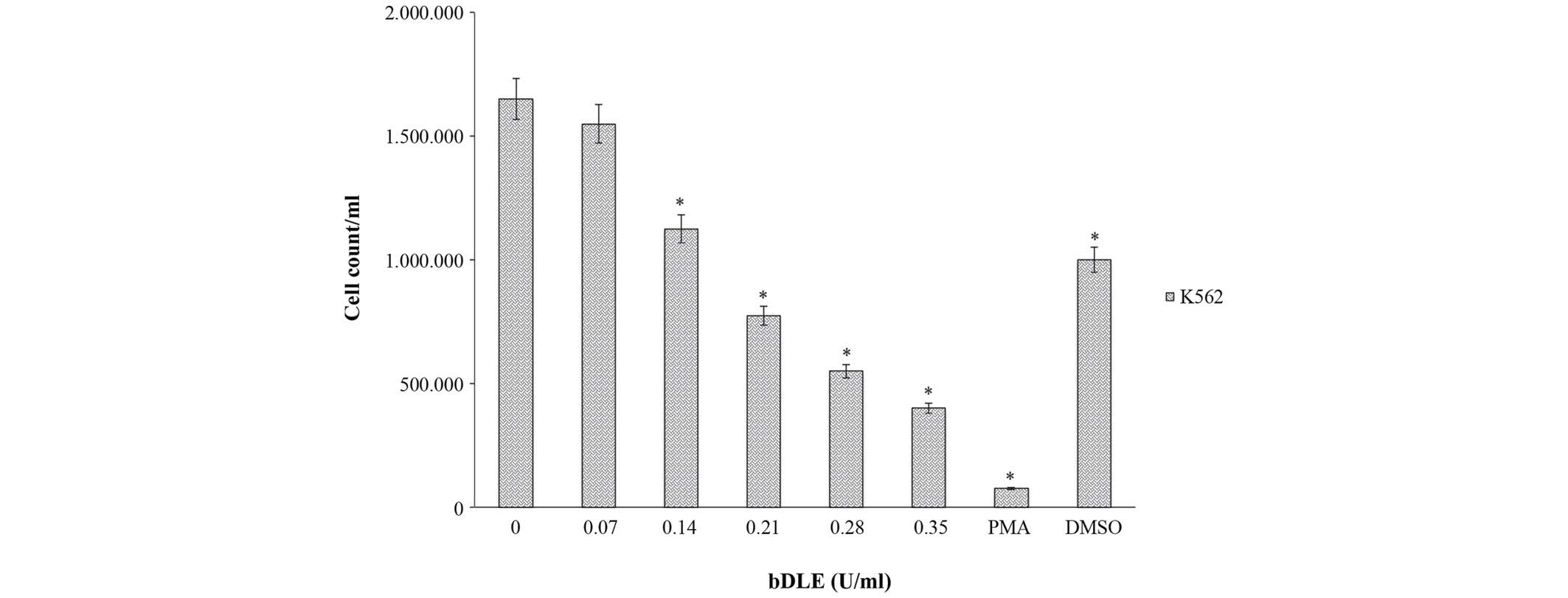
cells (44.43%). Furthermore, bDLE treatment for 96 h significantly
inhibited the K562 cell proliferation rate in a dose-dependent
manner (P=0.05) [0.07 U/ml (1,550,000 cells/ml), 0.14 U/ml
(1,125,000 cells/ml), 0.21 U/ml (775,000 cells/ml), 0.28 U/ml
(550,000 cells/ml) and 0.35 U/ml (400,000 cells/ml]. Treatment with
PMA (75,000 cells/ml) and DMSO (1,000,000 cells/ml) obtained
similar results when compared with untreated cells (1,650,000
cells/ml), in which the cellular proliferation was the highest
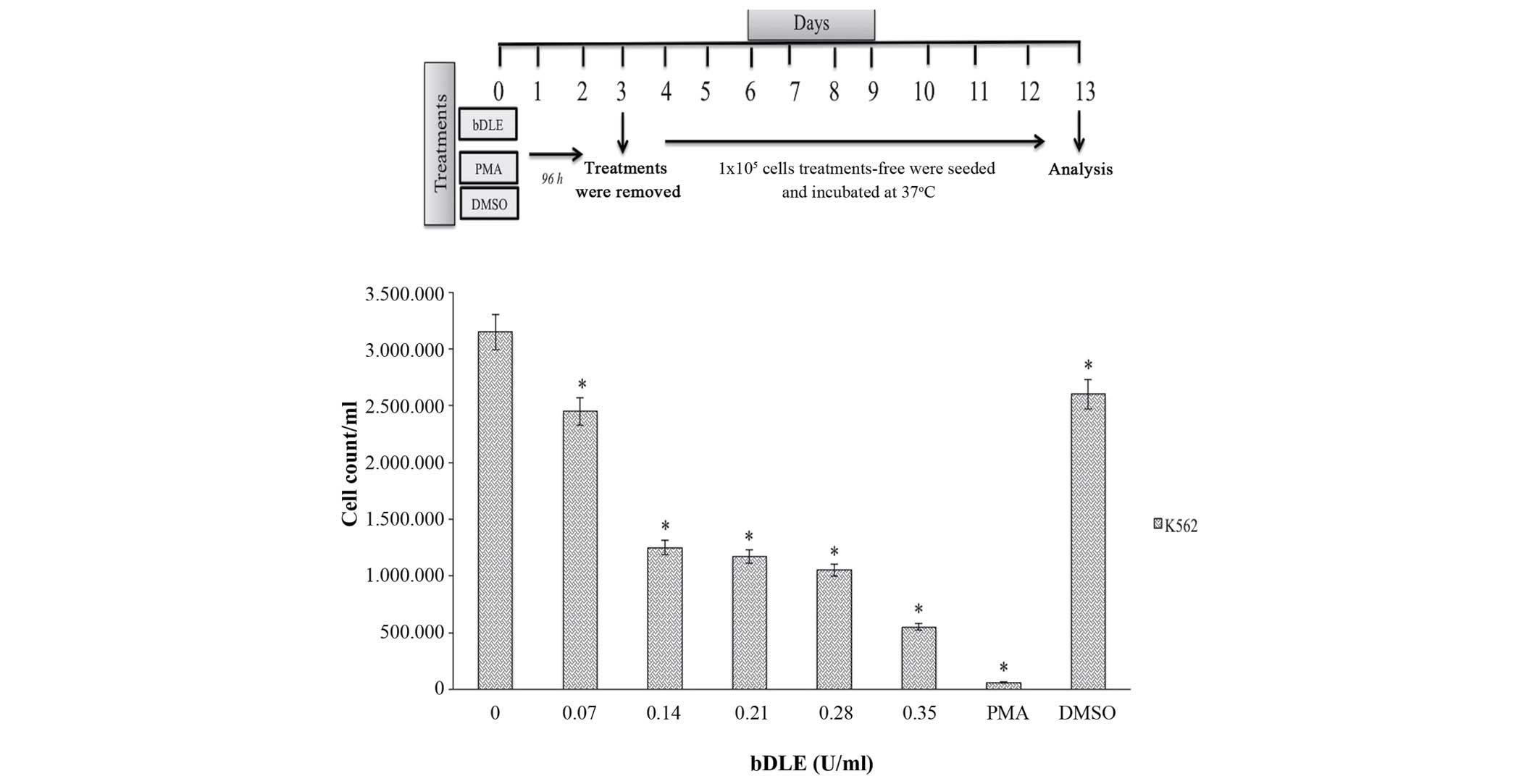
(P=0.05) (Fig. 5). Subsequently, the
K562 cells previously differentiated by treatments with bDLE, PMA
or DMSO for 96 h were incubated for 10 days, and it was observed
that the bDLE treatment decreased the rate of cell growth in a
dose-dependent manner (P=0.03) compared with the control. The
cellular growth was significantly decreased following PMA and DMSO
treatment (P=0.005) compared with the control (Fig. 6).
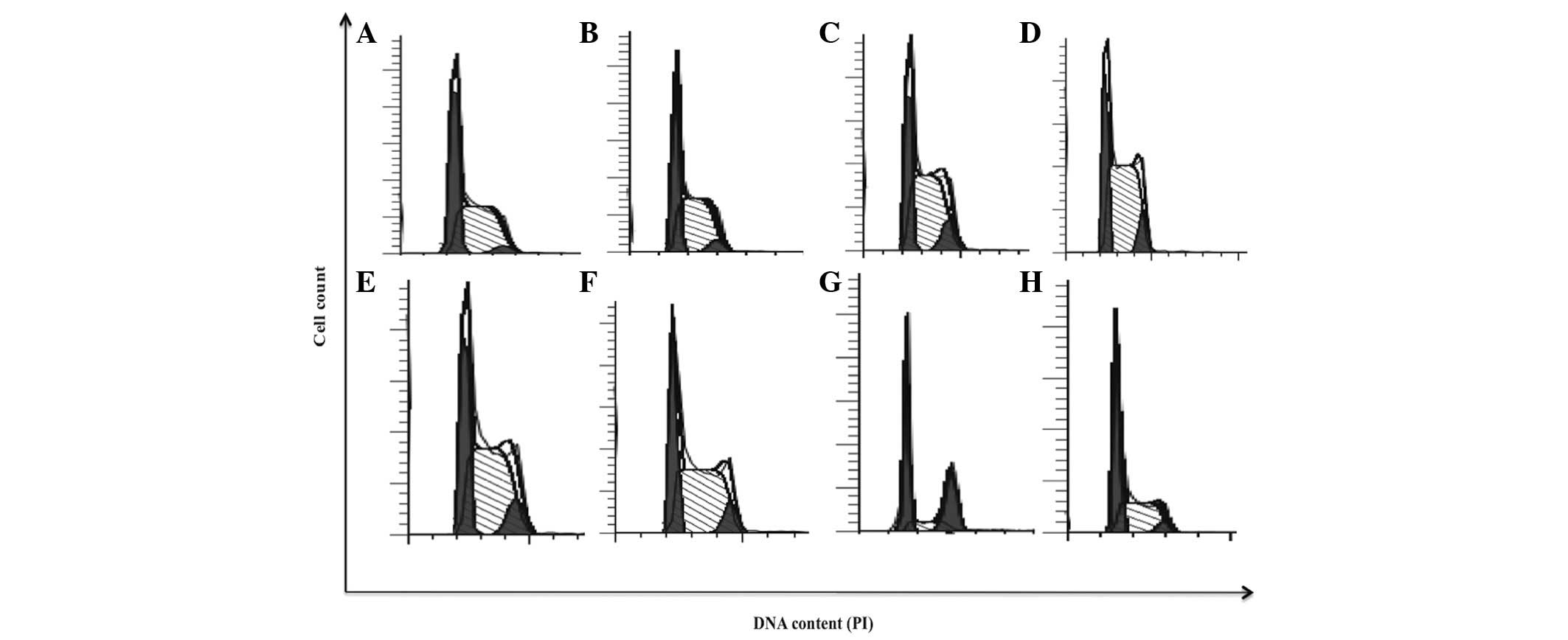 | Figure 4.bDLE induces arrest in S and
G2/M phases of the cell cycle in K562 cells. (A)
Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14 U/ml bDLE, (D) 0.21
U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml bDLE, (G) 10 ng/ml
phorbol myristate acetate or (H) dimethyl sulfoxide (1.5%, v:v)
were incubated for 96 h, and cell cycle progression analysis was
performed using the Cycle Test™ Plus DNA Reagent kit according to
the manufacturer's protocol and ModFit software. PI, propidium
iodide; bDLE, bovine dialyzable leukocyte extract. |
 | Table I.bDLE induces arrest in the S and
G2/M phases of the cell cycle in K562 cells. |
Table I.
bDLE induces arrest in the S and
G2/M phases of the cell cycle in K562 cells.
|
| Percentage of
cells, % |
|---|
|
|
|
|---|
| Treatment |
G0/G1 | S |
G2/M |
|---|
| Untreated
cells | 44.43 | 52.34 | 3.24 |
| bDLE 0.07 U/ml | 37.61 | 57.28 | 5.11 |
| bDLE 0.14 U/ml | 30.80 | 59.62 | 9.57 |
| bDLE 0.21 U/ml | 27.36 | 64.45 | 8.19 |
| bDLE 0.28 U/ml | 31.86 | 58.68 | 9.46 |
| bDLE 0.35 U/ml | 33.09 | 58.64 | 8.27 |
| PMA | 50.84 | 16.09 | 33.07 |
| DMSO | 49.42 | 45.77 | 4.81 |
bDLE induces monocytic/macrophage and
megakaryocytic differentiation in K562 cells
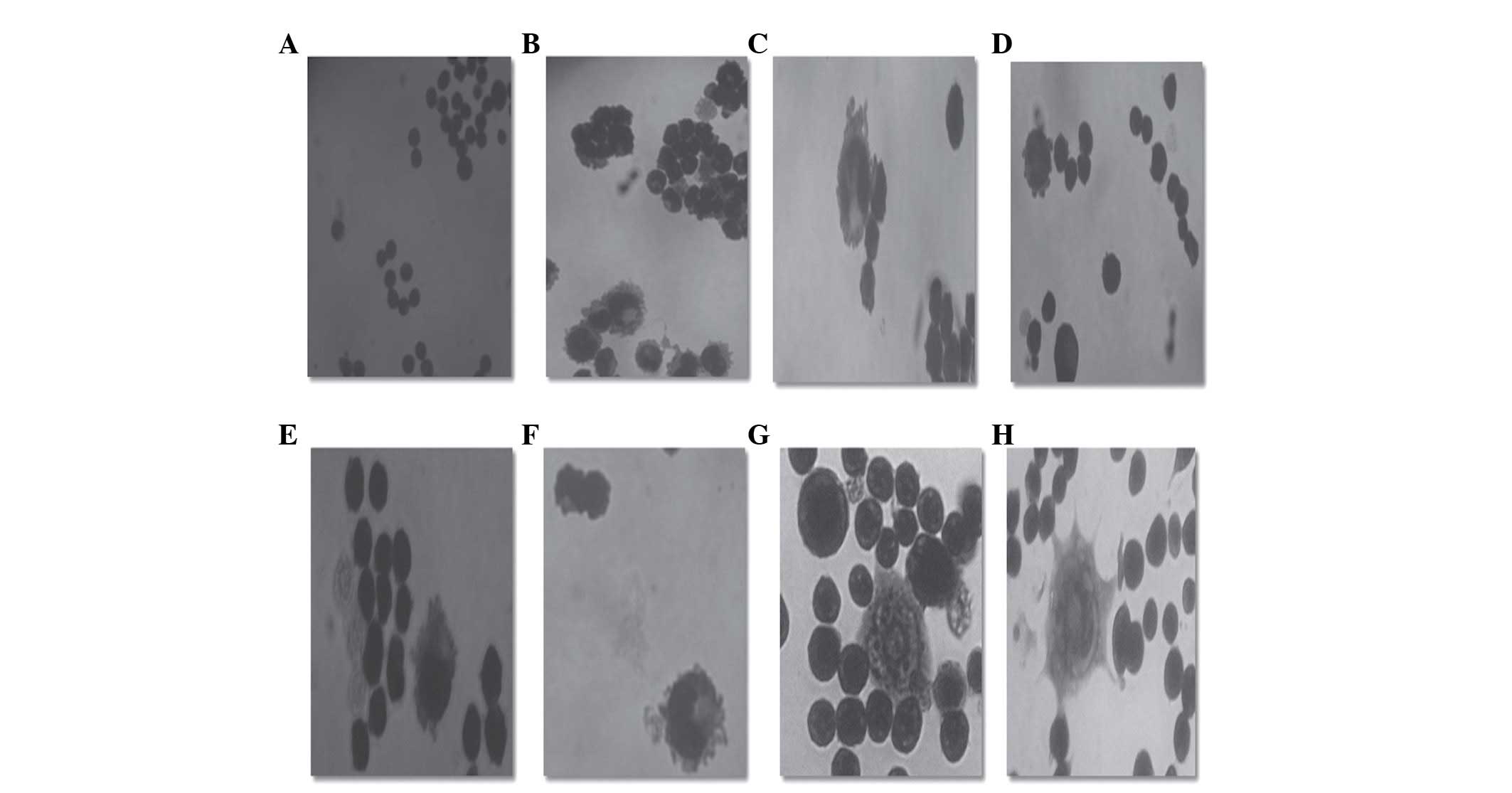
Microscopic examination by Romanowsky staining in
the K562 cells treated with bDLE (0.07–0.35 U/ml) for 96 h,
revealed morphological changes characteristic of
monocytic/macrophage differentiation occurring in a dose-dependent
manner. The cells developed pseudopodia extensions, increased cell
size and cytoplasm to nuclear ratio, as well as attachment to the
culture dishes, compared with untreated cells. These morphological
changes were similar to the effects obtained by PMA treatment in
terms of induced monocyte/macrophage differentiation in K562 cells
(Fig. 7). In order to confirm the
monocytic/macrophage differentiation induced by the bDLE and
inducer positive controls, the treated cells were monitored for
expression of CD14+ monocytic marker. Flow cytometry
histograms revealed an increased expression of CD14+ on
K562 cells following treatment with bDLE in a dose-dependent
manner: 0.07 U/ml (14.9%), 0.14 U/ml (24.4%), 0.21 U/ml (31.0%),
0.28 U/ml (35.2%) and 0.35 U/ml (39.7%), compared with the
untreated cells that expressed 6.1%, DMSO (3.3%) or PMA (29.6%)
treatments (Fig. 8). When the
CD14+ cell population was gated the surface markers
CD68+ (M1-like phenotype) and CD163+ (M2-like
phenotype), characteristic of macrophage polarization, were
analyzed, it was determined that bDLE treatment at various doses
induced cells toward an M2-like phenotype, increasing the
CD163+ surface marker levels in a significant manner
(P=0.05) [0.07 U/ml (33.0%), 0.14 U/ml (47.6%), 0.21 U/ml (50.5%),
0.28 U/ml (51.7%), 0.35 U/ml (49.5%) compared with the untreated
cells (12.0%), PMA (26.6%) or DMSO (8.7%) treatments]. No
difference (P=0.15) was identified between treatments when the
CD68+ surface marker was evaluated. The double positive
population of CD68+/CD163+ increased slightly
with bDLE treatment at doses of 0.07 U/ml (4.2%), 0.14 U/ml (7.1%),
0.21 U/ml (4.8%), 0.28 U/ml (4.1%) and 0.35 U/ml (5.3%), with high
levels of expression following PMA treatment (27.5%), and no effect
on the percentage of marker-positive cells following DMSO treatment
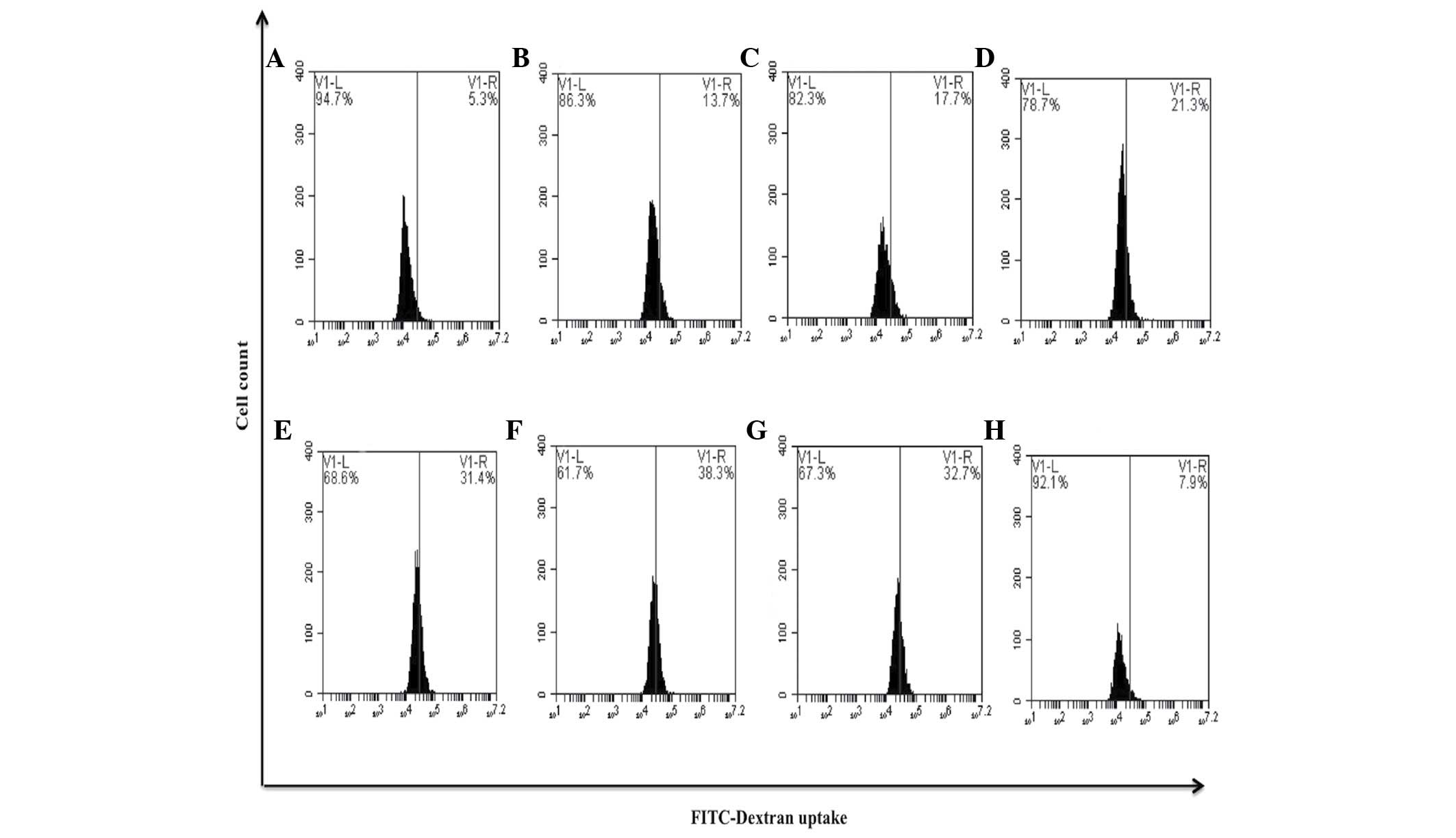
compared with untreated cells (0.3%) (P=0.35) (Fig. 9). It is well-known that differentiated
macrophages possess increased phagocytic capacity (11). The present study observed that the
bDLE treatments (0.07–0.35 U/ml) significantly increased (P=0.05)
in a dose-dependent manner the ability to uptake FITC-Dextran
reagent: 0.07 U/ml (13.7%), 0.14 U/ml (17.7%), 0.21 U/ml (21.3%),
0.28 U/ml (31.4%) and 0.35 U/ml (38.3%), similar to PMA treatment
(32.7%), when compared with untreated cells (5.3%). DMSO treatment
(7.9%) exerted a similar effect to that observed in the untreated
control cells (Fig. 10).
Furthermore, megakaryocytic differentiation was assessed by the
expression of the CD42a+ marker, and it was observed
that PMA had the potential to induce the expression of this surface
marker in 24.8% of cells and bDLE treatment induced lower
expression levels in a dose-dependent manner (P=0.05) [(0.07 U/ml
(4.8%), 0.14 U/ml (7.6%), 0.21 U/ml (8.9%), 0.28 U/ml (13.1%) and
0.35 U/ml (13.9%)]. No difference (P=0.38) was observed between the
DMSO treated (0.2%) and untreated cells (0.9%). The results of the
present study demonstrated that bDLE had the potential to induce
monocytic/macrophage differentiation (Figs. 7–10),
and had a lower capacity to induce megakaryocytic differentiation
(Fig. 11).
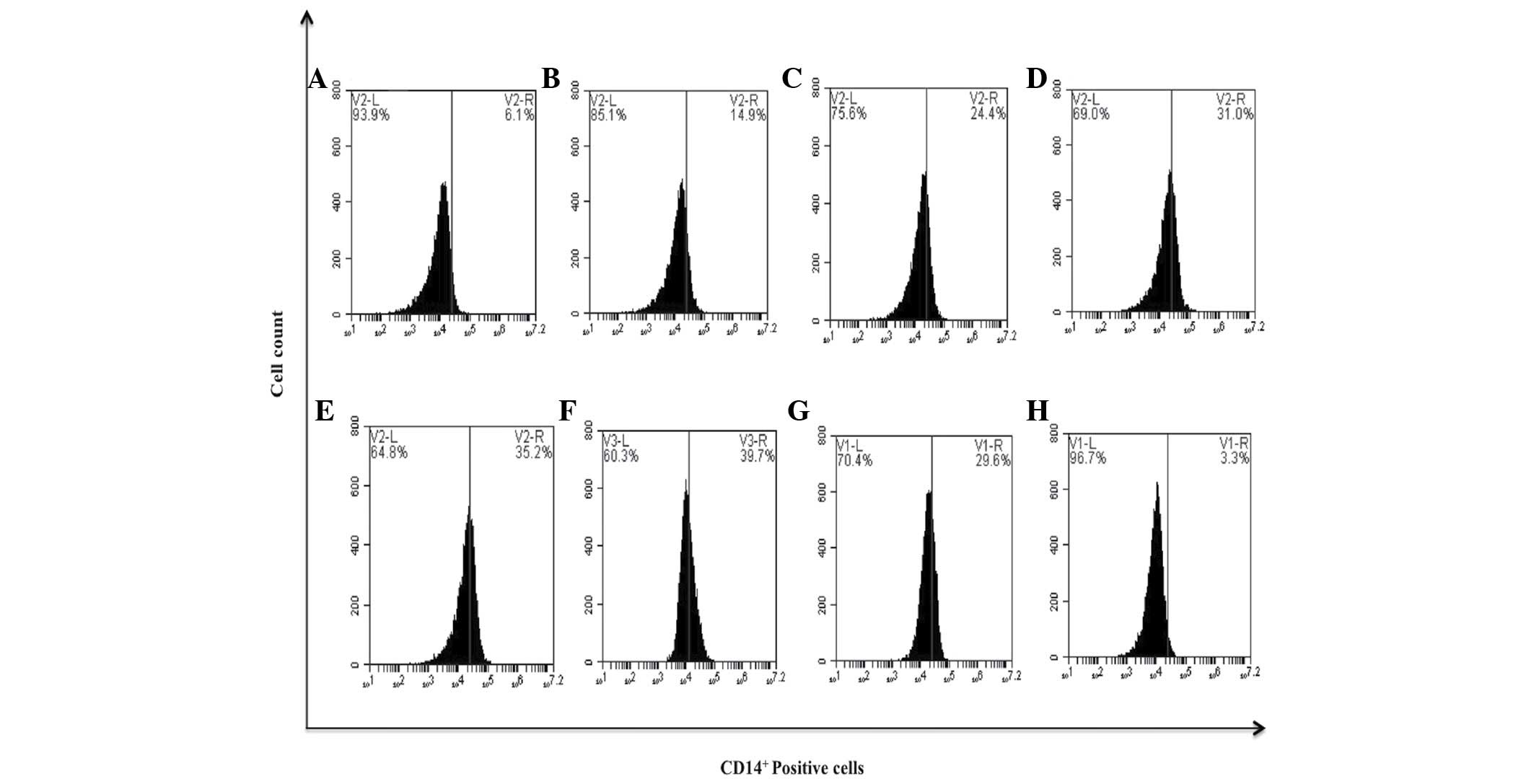 | Figure 8.bDLE induces monocytic differentiation
in K562 cells assessed by the expression of the CD14+
surface marker. (A) Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14
U/ml bDLE, (D) 0.21 U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml
bDLE, (G) 10 ng/ml phorbol myristate acetate or (H) dimethyl
sulfoxide (1.5%, v:v) were incubated for 96 h. Cells were harvested
and incubated with anti-CD14 phycoerythrin Texas red in PBS with 1%
fetal bovine serum and 0.1% sodium azide for 30 min at 4°C. Samples
were washed and resuspended in PBS, and 10,000 events were analyzed
by flow cytometry. Flow cytometry data shows representative results
from one of three independent experiments. CD, cluster of
differentiation; bDLE, bovine dialyzable leukocyte extract. |
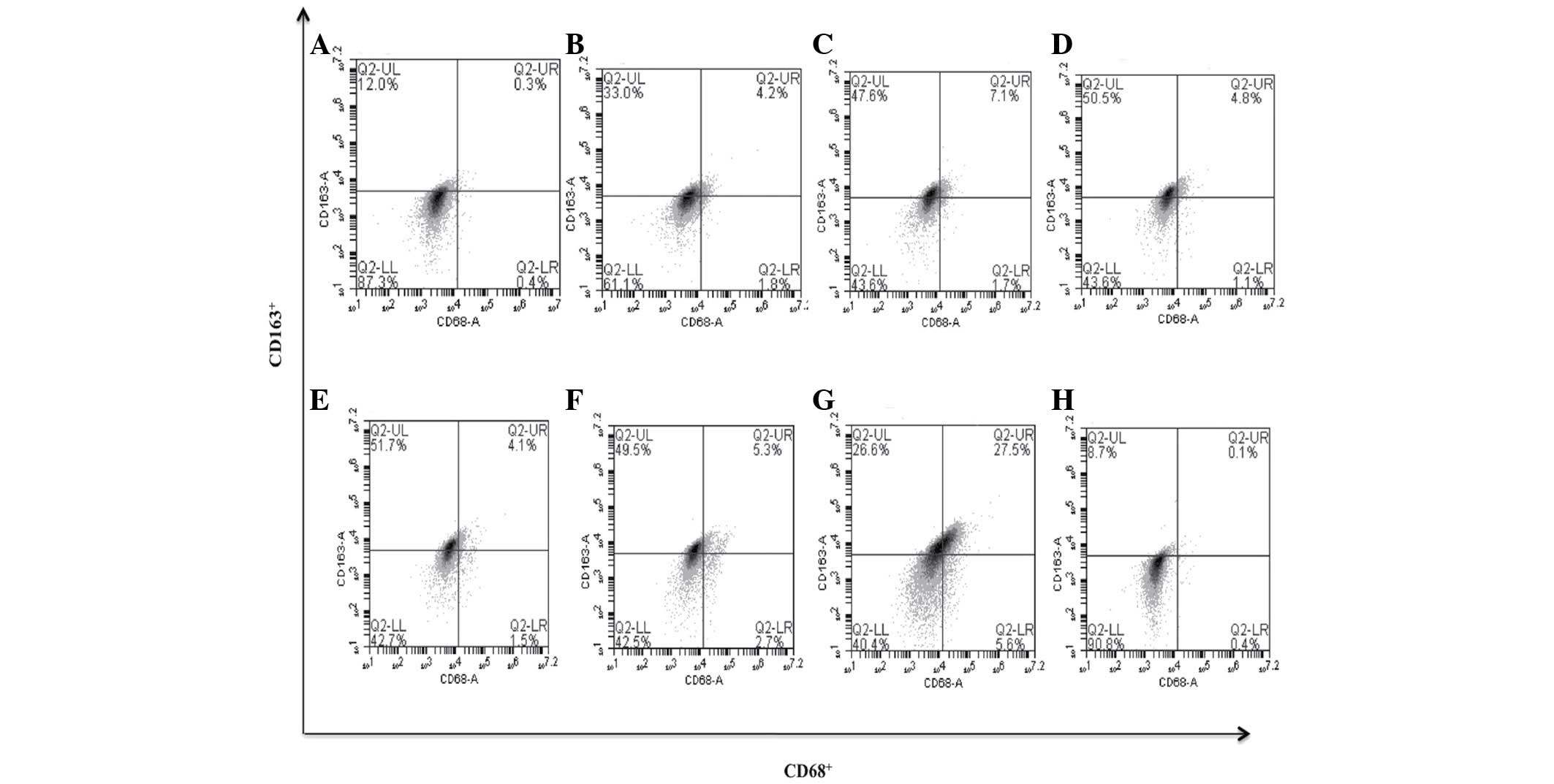 | Figure 9.bDLE induces macrophage polarization
to M2 assessed by expression of the CD163+ marker in
K562 cells. (A) Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14 U/ml
bDLE, (D) 0.21 U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml bDLE,
(G) 10 ng/ml phorbol myristate acetate or (H) dimethyl sulfoxide
(1.5%, v:v) were incubated for 96 h. Cells were harvested and
incubated with anti-CD68 and anti-CD163 in PBS with 1% fetal bovine
serum and 0.1% sodium azide for 30 min at 4°C. Samples were washed
and resuspended in PBS, and 10,000 events were analyzed by flow
cytometry. CD, cluster of differentiation; bDLE, bovine dialyzable
leukocyte extract. |
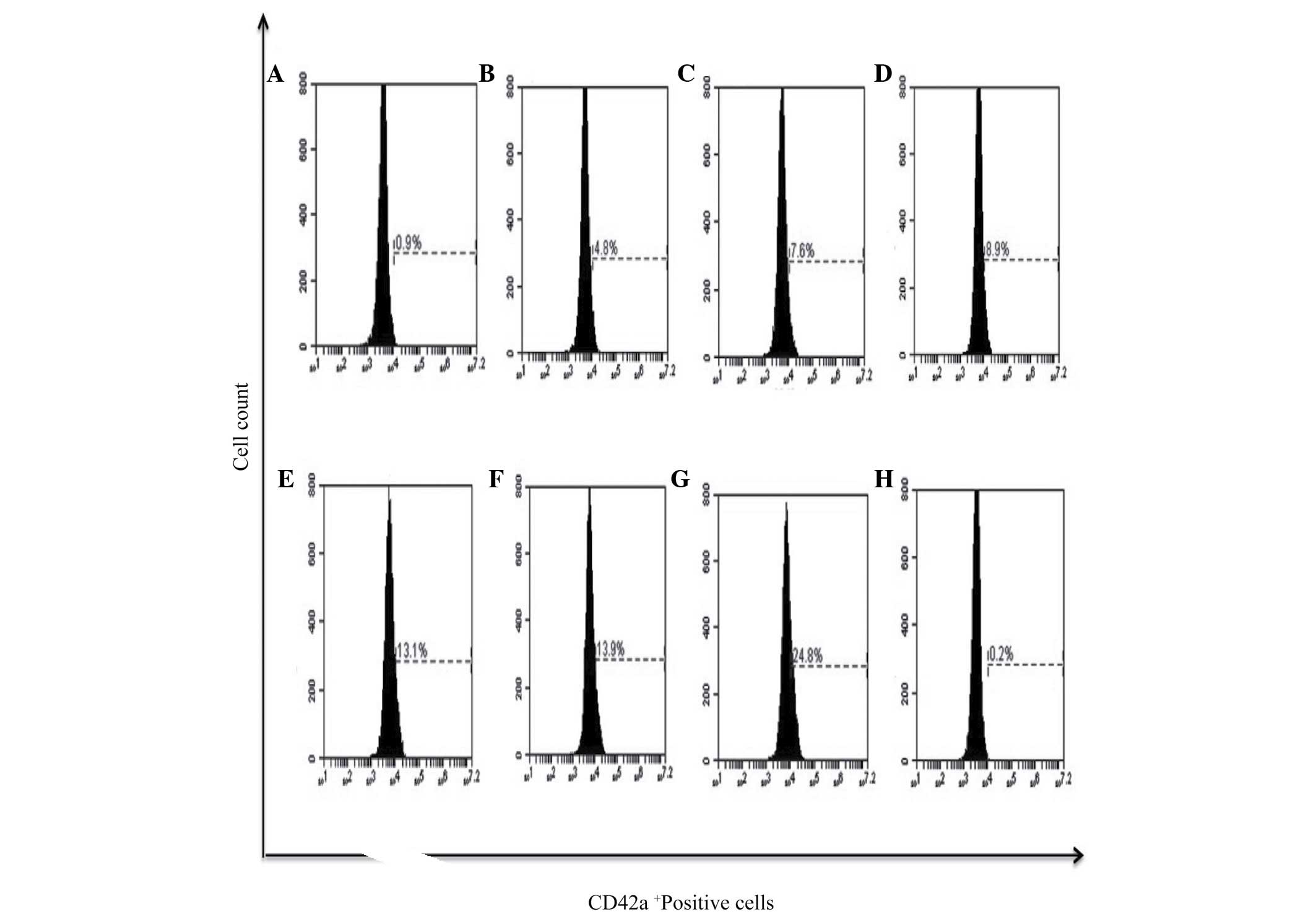 | Figure 11.bDLE increases the expression of
CD42a+ megakaryocytic marker differentiation in K562
cells. (A) Untreated cells, (B) 0.07 U/ml bDLE, (C) 0.14 U/ml bDLE,
(D) 0.21 U/ml bDLE, (E) 0.28 U/ml bDLE, (F) 0.35 U/ml bDLE, (G) 10
ng/ml phorbol myristate acetate or (H) dimethyl sulfoxide (1.5%,
v:v) were incubated for 96 h. Cells were harvested and incubated
with peridinin chlorophyll protein complex conjugated-anti-CD42a in
PBS with 1% fetal bovine serum and 0.1% sodium azide for 30 min at
4°C. Samples were washed and resuspended in PBS, and 10,000 events
were analyzed by flow cytometry. Flow cytometry data show
representative results from one of three independent experiments.
CD, cluster of differentiation; bDLE, bovine dialyzable leukocyte
extract. |
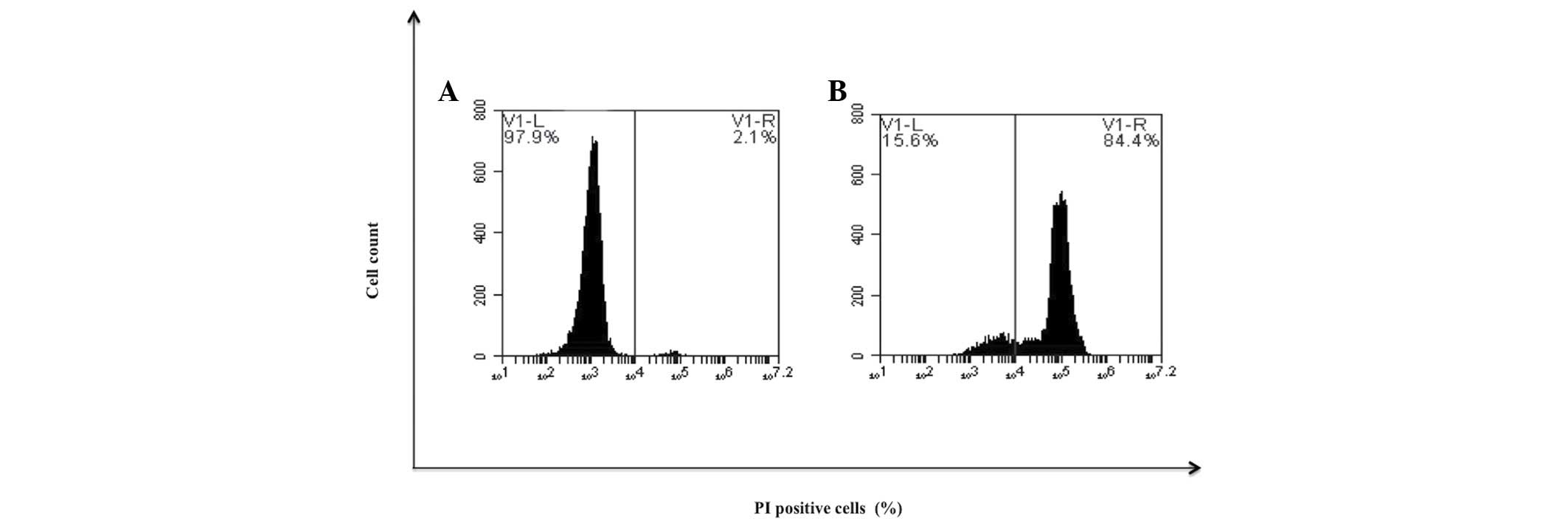
Determination of cellular
resistance
To determine if the previously differentiated cells
remained sensitive to treatment with bDLE, cells were treated with
cytotoxic doses. It was observed that treatment at 1 U/ml induced
cell death in 84.4% (Fig. 12).
Furthermore, murine peritoneal macrophages and primary human
monocytes treated with the an identical dose of bDLE demonstrated
17.4 and 27.9% cell death, respectively (Fig. 3).
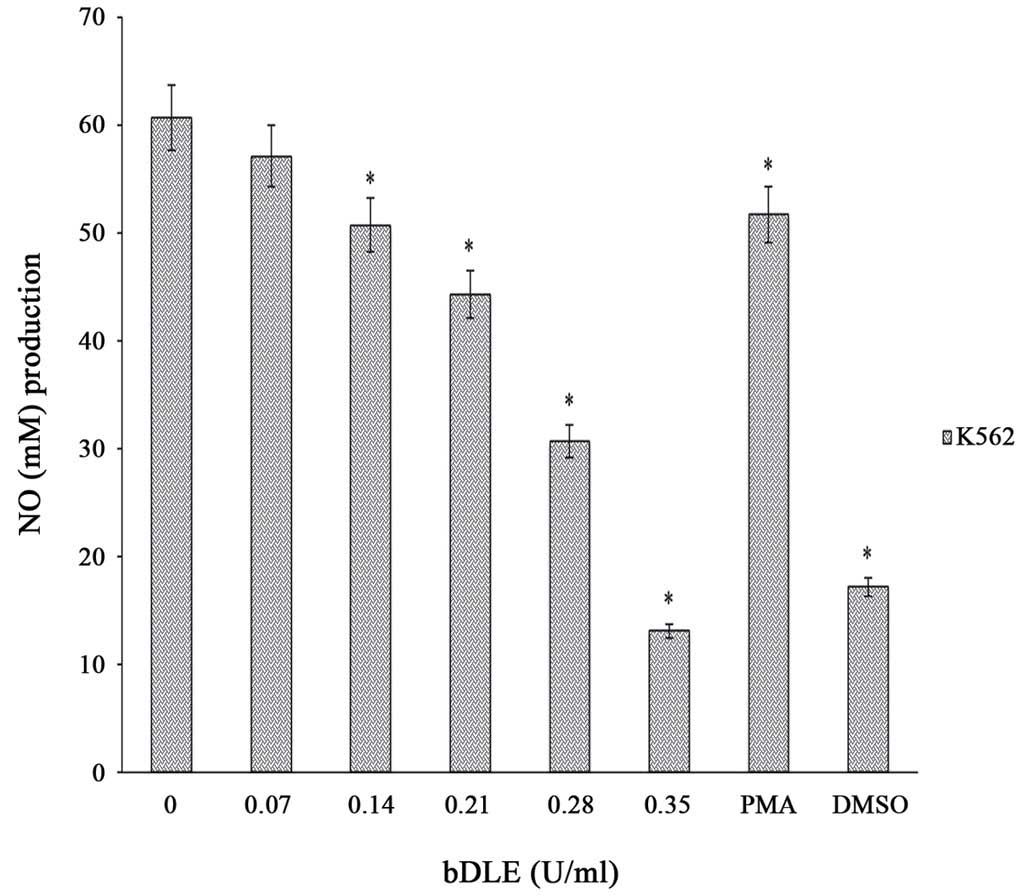
bDLE decreases NO production and
affects the cytokine and chemokine levels in K562 cells
The results of the present study demonstrated that
bDLE treatment significantly decreased (P=0.05) NO production in a
dose-dependent manner in K562 cells following 96 h of incubation
[0.07 U/ml (57.12 M), 0.14 U/ml (50.72 M), 0.21 U/ml (44.32 M),
0.28 U/ml (30.72 M), and 0.35 U/ml (13.12 M)] compared with
untreated cells (60.64 M) and PMA treated cells (51.68 M). DMSO
treatment significantly decreased NO production (17.2 M), similar
to the effects caused by 0.35 U/ml bDLE (Fig. 13). Furthermore, cytokine (IL-1b,
IL-6, IL-10, TNF-α and IL-12 p70) and chemokine production
(CCL2/MCP-1, CCL5/RANTES and CXCL8/IL-8) were evaluated in the K562
cell line treated with the inducers of differentiation. Untreated
cells demonstrated expression of IL-6, IL-8 and TNF-α, and IL-1b
and IL-12p70 were not expressed (Table
II); all chemokines evaluated in the present study were
observed to be expressed in untreated cells (Table III). PMA treatment induced
overexpression of the chemokines (Table
III) and cytokines (Table II)
evaluated, but IL-12p70 was not expressed. bDLE treatment at doses
of 0.07, 0.14, 0.21 and 0.28 U/ml increased the expression of all
cytokines evaluated, except IL-1b and IL-12 p70. bDLE at a dose of
0.28 U/ml decreased TNF-α expression. At doses of 0.35 U/ml the
expression levels of IL-6 and TNF-α were the lowest compared to the
control and PMA treatment groups, and IL-8 expression was elevated.
bDLE increased the expression of chemokines CCL2/MCP-1 in a
dose-dependent manner. At doses of 0.07 and 0.14 U/ml increased
expression of CCL5/RANTES was observed, and there was no difference
in expression between the doses evaluated compared to the control.
All the doses evaluated increased the expression of CXCL8/IL-8
(Table III). DMSO treatment reduced
the expression of all cytokines (Table
II) and chemokines evaluated (Table
III).
 | Table II.bDLE affects IL-6, IL-8 and TNF-α
production in K562 cells. |
Table II.
bDLE affects IL-6, IL-8 and TNF-α
production in K562 cells.
|
| Cytokine
production, pg/ml |
|
|
|---|
|
|
|
|
|
|---|
| Treatment | IL-1β | IL-6 | IL-8 | TNF-α |
|---|
| Untreated
cells | 0.0 | 126.03 | 18.84 | 830.47 |
| bDLE 0.07 U/ml | 0.0 | 307.83 | 56.22 | 1384.69 |
| bDLE 0.14 U/ml | 0.0 | 342.26 | 54.4 | 1882.90 |
| bDLE 0.21 U/ml | 0.0 | 293.94 | 23.22 | 950.22 |
| bDLE 0.28 U/ml | 0.0 | 213.17 | 27.06 | 431.83 |
| bDLE 0.35 U/ml | 0.0 | 115.18 | 34.95 | 310.54 |
| PMA | 7.17 | 991.06 | 17915.32 | 2347.26 |
| DMSO | 0 | 4.29 | 2.96 | 37.96 |
 | Table III.bDLE affects chemokine production in
K562 cells. |
Table III.
bDLE affects chemokine production in
K562 cells.
|
| Chemokine
production, pg/ml |
|---|
|
|
|
|---|
| Treatment | CCL2/MCP-1 | CCL5/RANTES | CXCL8/IL-8 |
|---|
| Untreated
cells | 25.07 | 0.7 | 17.84 |
| bDLE 0.07 U/ml | 70.42 | 4.17 | 47.35 |
| bDLE 0.14 U/ml | 66.07 | 2.76 | 46.29 |
| bDLE 0.21 U/ml | 74.02 | 0.7 | 19.44 |
| bDLE 0.28 U/ml | 115.81 | 0 | 20.05 |
| bDLE 0.35 U/ml | 164.15 | 0.31 | 28.23 |
| PMA | 9994.53 | 143.78 | 12105.23 |
| DMSO | 10.22 | 0 | 2.58 |
Discussion
The discovery of novel compounds with
differentiation-inducing activity in CML is required, as the degree
of sensitivity of cancer cells to ATRA is not universal (12). Previously, it has been reported that
bDLE induces cell death in several cancer cell lines (1). The present study demonstrated a novel
antileukemia activity of bDLE, as it exerted cytotoxic effects on
K562 and MOLT-3 leukemia cell lines, without affecting the cell
viability of monocytes and macrophages. Similar results are
observed when K562 cells are treated with Chemlali olive leaf
extract (7) or with Huangqi (Hex)
extract, which induce K562 and HEL cells to undergo cell
differentiation and death in a dose-dependent manner (13). Cell differentiation therapy is a novel
proposal that focuses on reducing the adverse effects of
chemotherapy and consists of forcing malignant cells to undergo
terminal differentiation. This treatment has attracted great
interest, particularly for treating leukemia (14). Numerous compounds have been reported
to induce differentiation of leukemia cells and some of these are
already approved for clinical use (7). The arrest of cell cycle progression
allows cells to undergo other processes, including apoptosis and
differentiation. By investigating the effect of bDLE on the cell
cycle distribution it was demonstrated that bDLE arrested at S and
G2/M phases cell cycle, similar results were obtained by
Imen et al (15) (2014) using
Chemlali olive leaf extract. The present study observed that bDLE
treatment for 96 h significantly inhibited the cell proliferation
rate in K562 cells, similar to the effects of PMA treatment. When
these treated cells were incubated by for 10 days with no
treatment, the percentage of cell proliferation was reduced
compared with untreated cells, suggesting that K562 cells may have
undergone a differentiation process. bDLE treatment induced K562
cell differentiation toward a monocyte/macrophage lineage. This was
determined by observation of morphological changes and increased
expression of the monocytic differentiation antigens,
CD14+ and CD163+. The CD163+
marker has been observed in acute myeloid leukemia with monocytic
differentiation (16). Furthermore,
the present study determined that K562 cells differentiated with
bDLE increased their phagocytic capacity, similar to cells treated
with PMA. It is known that K562 cells can be terminally
differentiated toward the macrophage lineage using PMA (17). The mechanism of action of bDLE in
decreasing NO production may be associated with antioxidant
properties (4). There is evidence of
certain antioxidants, including grape seed procyanidins, inducing
in vitro cell differentiation in leukemia cell lines
including K562 cells (8). bDLE also
induced the expression of the CD42a+ marker, which is
characteristic of megakaryocytic differentiation, and is considered
to be a classical inductor of megakaryocytic differentiation
(18). The macrophage adopts distinct
functional phenotypes in response to pathogenic and cytokine
signals, leading to the description of two divergent forms of
macrophage activation: M1 and M2 (19). In a tumor setting, M1-like macrophages
are thought to promote antitumor immunity, whereas M2-like
tumor-associated macrophages stimulate angiogenesis and tissue
repair (19). Enhanced cytokine and
chemokine production by flow cytometry was observed in bDLE and PMA
treatments, although this effect was more enhanced with PMA
treatment, suggesting that the production of these molecules is
associated with a process of cell differentiation. The fact that
bDLE at high doses affected the viability of K562 cells previously
differentiated with the identical treatment, and did not affect the
viability of murine peritoneal macrophage and human monocytes, may
suggest selectivity of action by receptors.
The present study demonstrated for the first time
that bDLE exhibits an antileukemia effect on human CML cells. bDLE
was shown to inhibit the proliferation of K562 cells and induced
differentiation toward the monocyte/macrophage and megakaryocytic
lineages through increased expression of molecules associated with
differentiation. The present study provides an insight into the
underlying mechanism by which bDLE exhibits its antileukemic
activity.
Acknowledgements
The present study was supported by the Laboratory of
Immunology and Virology, Faculty of Biological Sciences, University
Autonomous of Nuevo León (UANL), Mexico, in collaboration with the
‘Network of Immunology in Cancer and Infectious Diseases’ CONACYT
(grant no. 253053).
Glossary
Abbreviations
Abbreviations:
|
CML
|
chronic myelogenous leukemia
|
|
DMSO
|
dimethyl sulfoxide
|
|
PMA
|
phorbol myristate acetate
|
|
bDLE
|
bovine dialyzable leukocyte
extract
|
References
|
1
|
Franco-Molina MA, Mendoza-Gamboa E,
Miranda-Hernández DF, Zapata-Benavides P, Castillo-León L,
Isaza-Brando C, Tamez-Guerra RS and Rodríguez-Padilla C: In vitro
effects of bovine dialyzable leukocyte extract (bDLE) in cancer
cells. Cytotherapy. 8:408–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franco-Molina MA, Mendoza-Gamboa E,
Zapata-Benavides P, Castillo-Tello P, Isaza-Brando C, Zamora-Ávila
D, Rivera-Morales LG, Miranda-Hernández DF, Sierra-Rivera CA,
Vera-García ME, et al: Antiangiogenic and antitumor effects of
IMMUNEPOTENT CRP in murine melanoma. Immunopharmacol Immunotoxicol.
32:637–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendoza-Gamboa E, Franco-Molina MA,
Zapata-Benavides P, Castillo-Tello P, Vera-García ME, Tamez-Guerra
RS and Rodríguez-Padilla C: Bovine dialyzable leukocyte extract
modulates AP-1 DNA-binding activity and nuclear transcription
factor expression in MCF-7 breast cancer cells. Cytotherapy.
10:212–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franco-Molina MA, Mendoza-Gamboa E,
Miranda-Hernández DF, Sierra-Rivera CA, Zapata-Benavides P,
Vera-García ME, Reyes S, Tamez-Guerra RS and Rodríguez-Padilla C:
Anti-inflammatory and antioxidant effects of IMMUNEPOTENT CRP in
Lipopolysaccharide (LPS)-stimulated human macrophages. Afr J
Microbiol Res. 5:3726–3736. 2011.
|
|
5
|
Wang XJ and Li YH: Inhibition of human
chronic myelogenous leukemia K562 cell growth following combination
treatment with resveratrol and imatinib mesylate. Genet Mol Res.
14:6413–6418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landau DA, Carter SL, Getz G and Wu CJ:
Clonal evolution in hematological malignancies and therapeutic
implications. Leukemia. 28:34–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samet I, Han J, Jlaiel L, Sayadi S and
Isoda H: Olive (Olea europaea) leaf extract induces apoptosis and
monocyte/macrophage differentiation in human chronic myelogenous
leukemia K562 cells: Insight into the underlying mechanism. Oxid
Med Cell Longev. 2014:1–16. 2014. View Article : Google Scholar
|
|
8
|
Wang M, Wang L, Pan XJ and Zhang H:
Monocytic differentiation of K562 cells induced by
proanthocyanidins from grape seeds. Arch Pharm Res. 35:129–135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gocek E and Marcinokowska E:
Differentiation therapy of acute myeloid leukemia. Cancers.
3:2402–2420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badisa RB, Darling-Reed SF, Joseph P,
Cooperwood JS, Latinwo LM and Goodman CB: Selective cytotoxic
activities of two novel synthetic drugs on human breast carcinoma
MCF-7 Cells. Anticancer Res. 29:2993–2996. 2009.PubMed/NCBI
|
|
11
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5:4912014.PubMed/NCBI
|
|
12
|
Aoki S, Kong D, Matsui K and Kobayashi M:
Erythroid differentiation in K562 chronic myelogenous cells induced
by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer
Res. 24:2325–2330. 2004.PubMed/NCBI
|
|
13
|
Cheng XD, Hou CH, Zhang XJ, Xie HY, Zhou
WY, Yang L, Zhang SB and Qian RL: Effects of Huangqi (Hex) on
inducing cell differentiation and cell death in K562 and HEL cells.
Acta Biochim Biophys Sin (Shanghai). 36:211–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spira Al and Carducci MA: Differentiation
therapy. Curr Opin Pharmacol. 3:338–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imen S, Junkyu H, Lobna J, Sami S and
Hiroko I: Olive (Olea europaea) leaf extract induces apoptosis and
monocyte/macrophage differentiation in human chronic myelogenous
leukemia K562 cells: Insight into the underlying mechanism. Oxid
Med Cell Longev. 2014:9276192014.PubMed/NCBI
|
|
16
|
Nguyen TT, Schwartz EJ, West RB, Warnke
RA, Arber DA and Natkunam Y: Expression of CD163 (hemoglobin
scavenger receptor) in normal tissues, lymphomas, carcinomas and
sarcomas is largely restricted to the monocyte/macrophage lineage.
Am J Surg Pathol. 29:617–624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutherland JA, Turner AR, Mannoni P,
McGann LE and Turc JM: Differentiation of K562 leukemia cells along
erythroid, macrophage, and megakaryocyte lineages. J Biol Response
Mod. 5:250–262. 1986.PubMed/NCBI
|
|
18
|
Bütler TM, Ziemiecki A and Friis RR:
Megakaryocytic differentiation of K562 cells is associated with
changes in the cytoskeletal organization and the pattern of
chromatographically distinct forms of phosphotyrosyl-specific
protein phosphatases. Cancer Res. 50:6323–6329. 1990.PubMed/NCBI
|
|
19
|
Liddiard K and Taylor PR: Understanding
local macrophage phenotypes in disease: Shape-shifting macrophages.
Nat Med. 21:119–120. 2015. View
Article : Google Scholar : PubMed/NCBI
|