Introduction
Colorectal cancer is the third and second most
common malignant tumor in males and females worldwide, respectively
(1). According to the International
Agency for Research on Cancer, 1.2 million novel cases of
colorectal cancer were diagnosed worldwide in 2008, accounting for
8% of all cancer-related mortalities (2,3). The
incidence of colorectal cancer is highest in developed countries
and regions: Due to economic development and rapid urbanization in
recent years in China, which has resulted in dietary and lifestyle
changes within the population, the colorectal cancer morbidity and
mortality rates in China are now higher than the average rates,
worldwide (4,5).
In 2013, the incidence of colorectal cancer was 45.1
per 100,000 individuals, with >5,000 deaths per year and an
average of 13.1 years of life lost (1). Colorectal cancer operation is an
important method for the treatment of colorectal cancer (5). The clinical manifestations of colorectal
cancer may appear in the following ways: Altered defecation habits,
stomach ache, abdominal masses, gastrointestinal hemorrhage,
jaundice and a change in character of the stool. All patients have
postoperative complications, with have a great impact on the
patient's quality of life, and even endanger the patient's life
(6). Therefore, it is important that
physicians investigate multiple potential treatments for colorectal
cancer, with the aim of preventing these treatment complications
(7).
Naphthazarin is a natural bioactive substance
present in numerous plants that has been demonstrated to exhibit
antitumor effects (8). Naphthazarin
is an important active ingredient, which exhibits extensive
pharmacological activities, including antitumor activity, and due
to its low toxicity, it has gained considerable attention (9,10).
However, to date the anticancer effects of naphthazarin on human
colorectal cancer cells have not been reported. In the present
study, the anticancer effects of naphthazarin, as well as its
effect on the B-cell lymphoma 2 (Bcl-2)/B-cell associated X protein
(Bax) signaling pathway were investigated in human SW480 colorectal
cancer cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
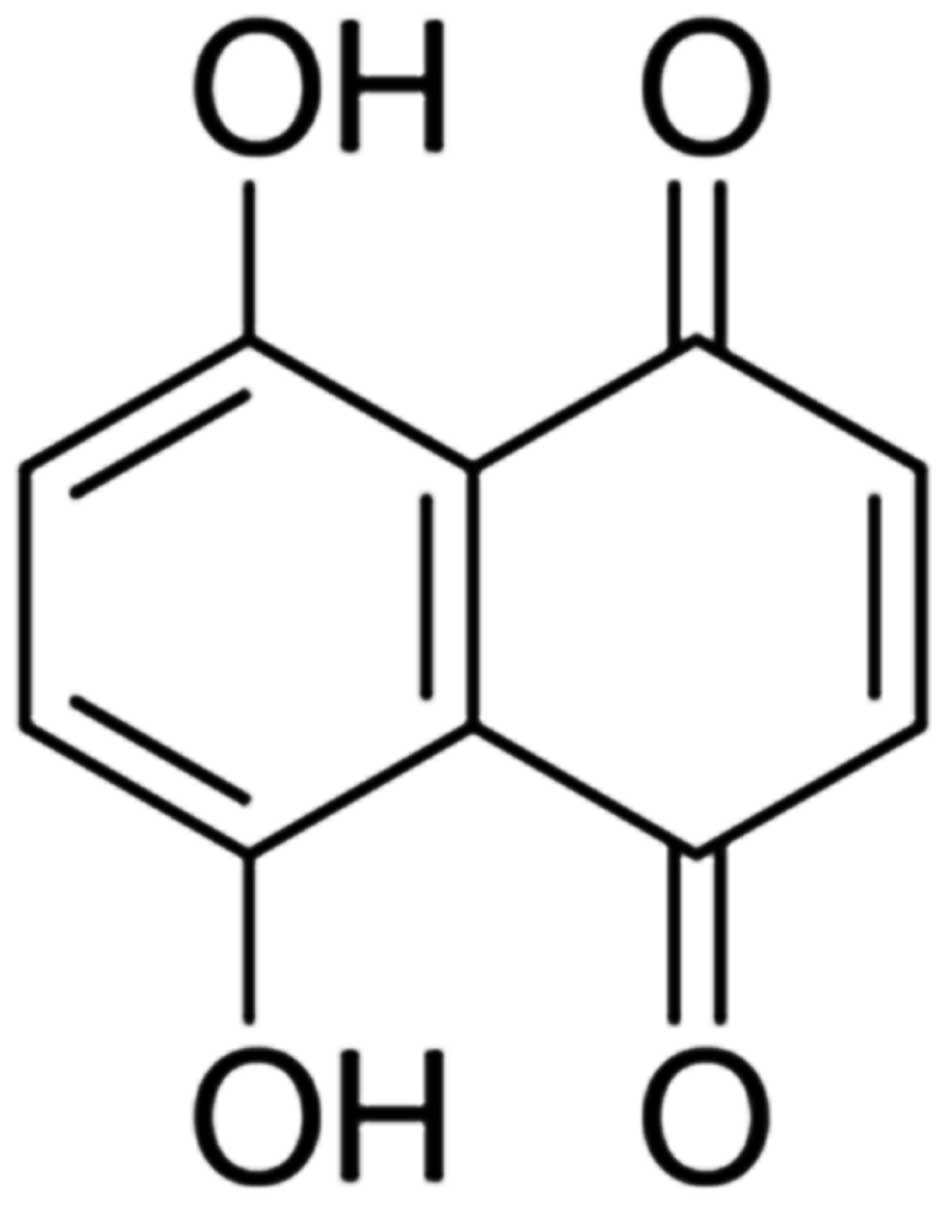
bovine serum (FBS), naphthazarin (Fig.
1) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) solution and a lactate dehydrogenase (LDH) assay were
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis and caspase-3 activation kits were purchased from KeyGen
Biotech Co., Ltd., (Nanjing, China).
Cell culture and cell viability
assay
The human colorectal cancer SW480 cell line was
obtained from the Cell Bank of Chinese Academy of Sciences
(Shanghai, China) and maintained in DMEM (Sigma-Aldrich; Merck
Millipore) supplemented with 10% FBS in a humidified atmosphere of
5% CO2 at 37°C. A total of 1×104 cells/well
were seeded in a 96-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. After incubation, SW480 cells
were treated with 0, 0.5, 1, 5, 10 and 20 µM naphthazarin for 24 h.
Subsequently, 20 µl MTT solution (Sigma-Aldrich; Merck Millipore)
was added to each well and incubated at 37°C in a 5% CO2
incubator for 4 h. Following incubation, the culture medium was
replaced and 200 µl DMSO was added to each well and agitated for 20
min at room temperature. Cell viability was analyzed at a
wavelength of 490 nm using an ELISA reader (Multiskan EX; Thermo
Labsystems, Helsinki, Finland). .
LDH assay
SW480 cells (1×104 cells/well) were
seeded in a 96-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. After incubation, SW480 cells
were treated with to 0, 0.5, 1, 5, 10 and 20 µM naphthazarin for 24
h. Subsequently, 100 µl LDH solution was added to each well and
cultured for 30 min. The absorbance was read at a wavelength of 490
nm using an ELISA reader (Multiskan EX; Thermo Labsystems).
Annexin V-FITC/PI apoptosis assay
SW480 cells (1×106 cells/well) were
seeded in a 6-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. After incubation, SW480 cells
were treated with 0, 0.5, 1 and 5 µM naphthazarin for 24 h. SW480
cells were trypsinized (Sangon Biotech Co., Ltd., Shanghai, China),
washed with phosphate-buffered saline (PBS) and fixed in precooling
75% ethanol at 4°C overnight. Next, Annexin-V FITC and PI were
added and incubated for 10 min at room temperature in the dark.
Flow cytometry analysis was performed on a FACScan flow cytometer
(BD Biosciences, San Diego, CA, USA) using emission filters of 525
and 575 nm. Data were analyzed using CellQuest Pro software version
5.1 (BD Biosciences).
4′,6-diamidino-2-phenylindole (DAPI)
staining assay
SW480 cells (1×106 cells/well) were
seeded in a 6-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. Following incubation, SW480
cells were treated with 0, 0.5, 1 and 5 µM naphthazarin for 24 h.
SW480 cells were then incubated with 0.1% sodium citrate containing
0.1% Triton X-100 (Beyotime Institute of Biotechnology, Haimen,
China) for 5 min at 4°C. Cell nuclei were observed using a
fluorescent microscope (Zeiss Axio Observer A1; Carl Zeiss, Inc.,
Oberkochen, Germany).
Western blot analysis
SW480 cells (1×106 cells/well) were
seeded in a 6-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. After incubation, SW480 cells
were treated with 0, 0.5, 1 and 5 µM naphthazarin for 24 h. SW480
cells were harvested with PBS and extracted in cold
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology). Protein concentrations were determined
using the by Pierce BCA protein assay kit (BD Biosciences). Equal
amounts of protein were resolved on 6–15% SDS-PAGE gel and
transferred to polyvinylidene fluoride membranes (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Membranes were then
incubated with antibodies against poly (ADP-ribose) polymerase
(PARP; 1:1,000; D161071; Santa Cruz Biotechnology, Inc.), Bax
(1:500; AF0057; Beyotime Institute of Biotechnology), Bcl-2 (1:500;
AF0060; Beyotime Institute of Biotechnology) and β-actin (1:500;
AA128; Beyotime Institute of Biotechnology) overnight at 4°C.
Membranes were next washed with TBS containing Tween 20 and
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (1:2,000; Sangon Biotech, Co., Ltd.) at 37°C for 2
h. The protein band was detected using ImageLab 3.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Caspase-3 activation assay
SW480 cells (1×106 cells/well) were
seeded in a 6-well plate and incubated at 37°C in a 5%
CO2 incubator for 24 h. After incubation, SW480 cells
were treated with 0, 0.5, 1 and 5 µM naphthazarin for 24 h. SW480
cells were harvested with PBS and extracted in cold RIPA lysis
buffer (Beyotime Institute of Biotechnology). Protein
concentrations were determined using the Pierce BCA protein assay
kit (BD Biosciences). Equal amounts of the total protein were mixed
with Ac-DEVD-pNA (Beyotime Institute of Biotechnology) for
caspase-3 expression and incubated at 37°C for 2 h in the dark.
Caspase-3 activation was analyzed at a wavelength of 405 nm using
an ELISA reader (Multiskan EX; Thermo Labsystems).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Differences between groups were
analyzed using the Student's t-test and the SPSS 17.0 program
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Naphthazarin decreases SW480 cell
viability
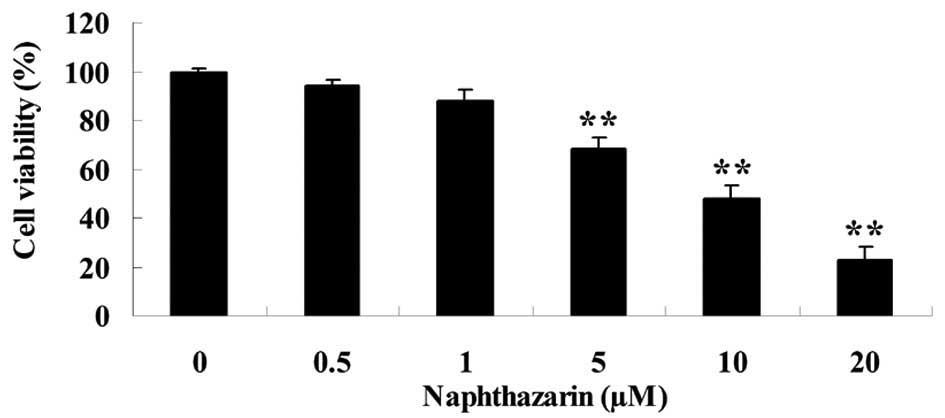
The effect of naphthazarin on cell viability was
investigated by MTT assay in SW480 cells. Following treatment with
5, 10 and 20 µM naphthazarin significantly decreased cell viability
of SW480 cells in a dose-dependent manner (P<0.01; Fig. 2). These results indicate that
naphthazarin exhibits an anticancer effect on human colorectal
cancer cells.
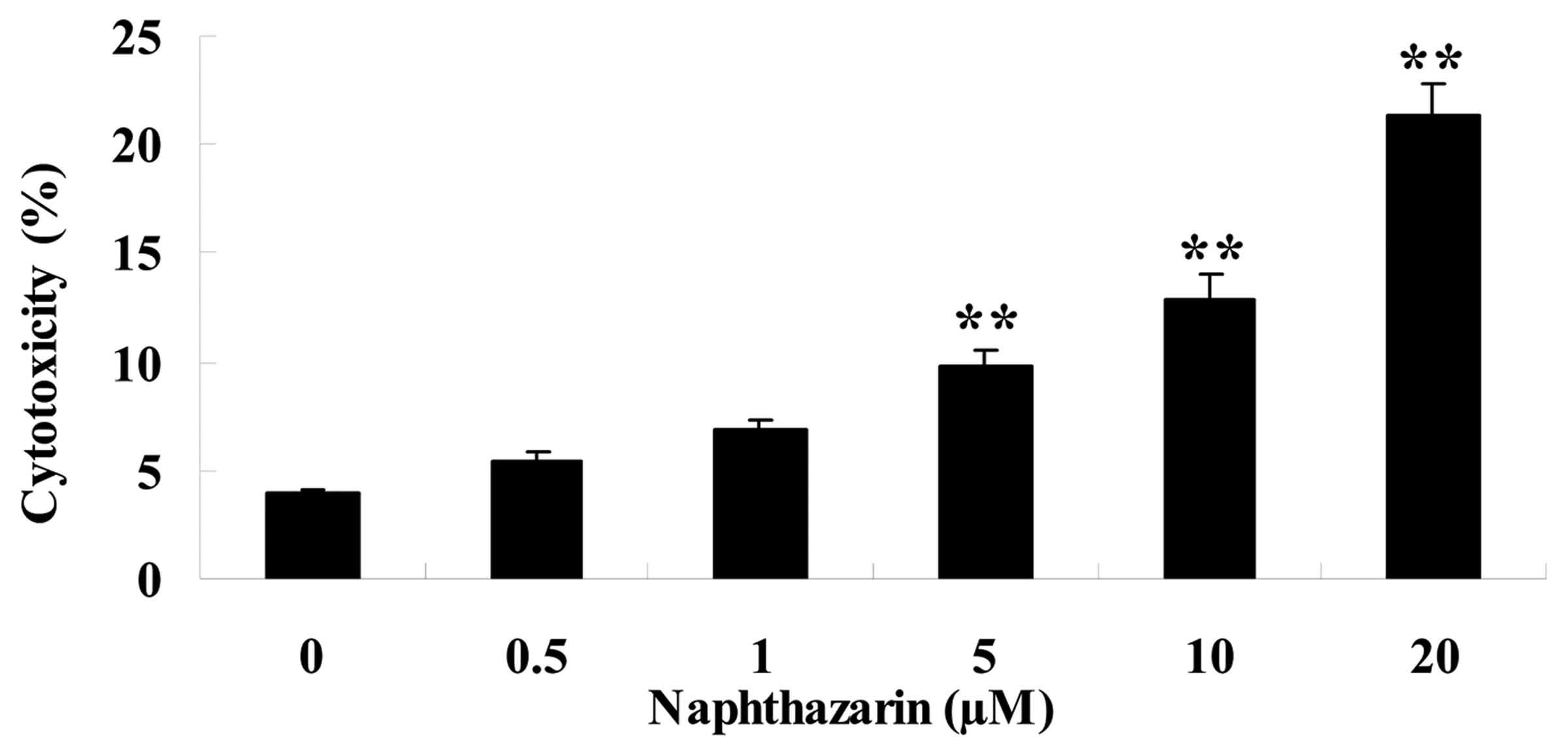
Naphthazarin increases cytotoxicity of
SW480 cells
A LDH assay was performed to investigate the effect
of naphthazarin on SW480 cell cyotoxicity. Naphthazarin
significantly increased cytotoxicity of SW480 cells in
dose-dependent manner following treatment with 5, 10 and 20 µM
naphthazarin for 24 h (Fig. 3).
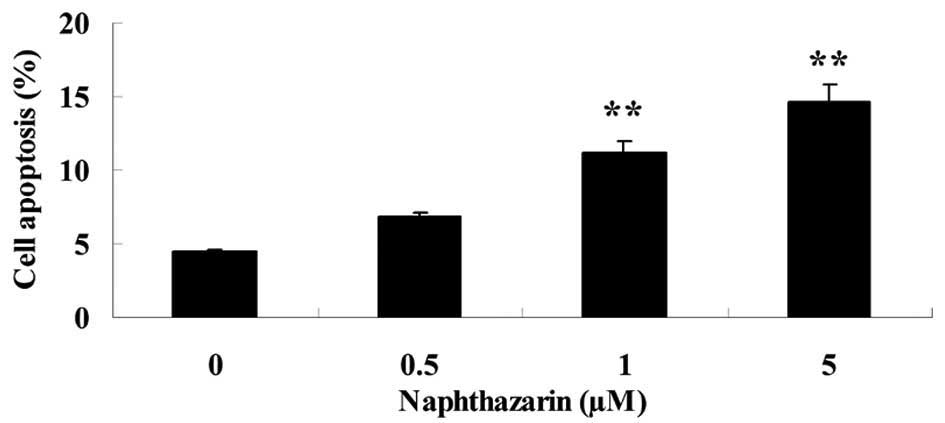
Naphthazarin induces apoptosis of
SW480 cells
The effect of naphthazarin on SW480 cell apoptosis
was investigated. Upon treatment with 1 and 5 µM naphthazarin for
24 h, the rate of cell apoptosis was significantly increased in
SW480 cells in a dose-dependent manner (Fig. 4).
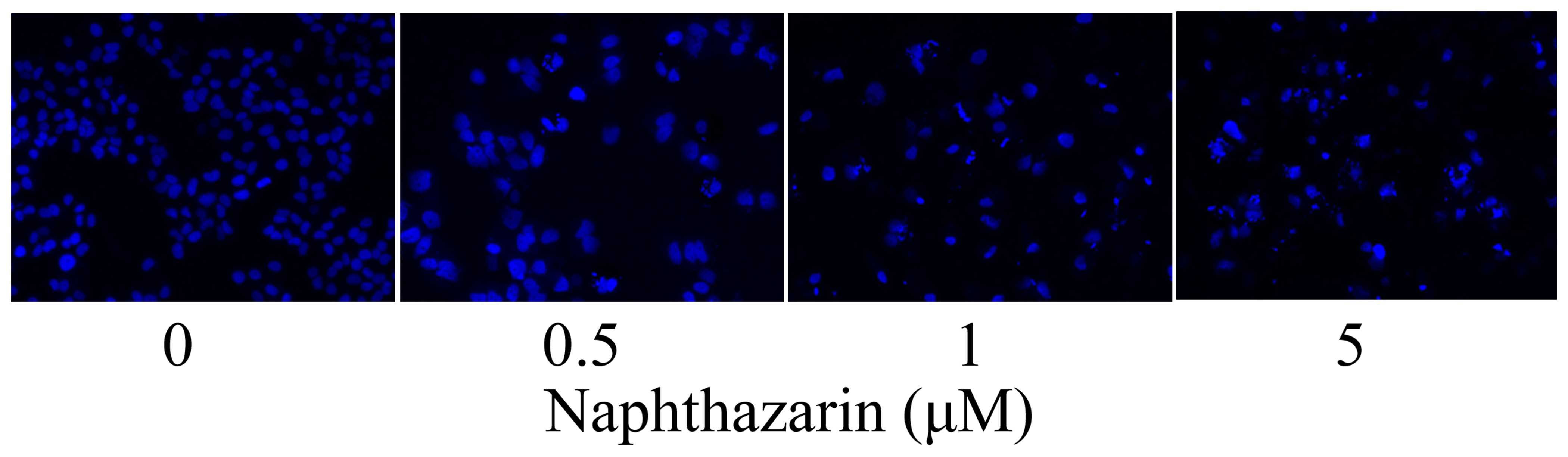
Naphthazarin induces nuclear apoptosis
in SW480 cells
SW480 cells were stained with DAPI and observed
under a fluorescent microscope to investigate the effect of
naphthazarinon nuclear apoptosis. Nuclear apoptosis was observed in
SW480 cells following treatment with 0.5, 1 and 5 µM naphthazarin
for 24 h (Fig. 5).
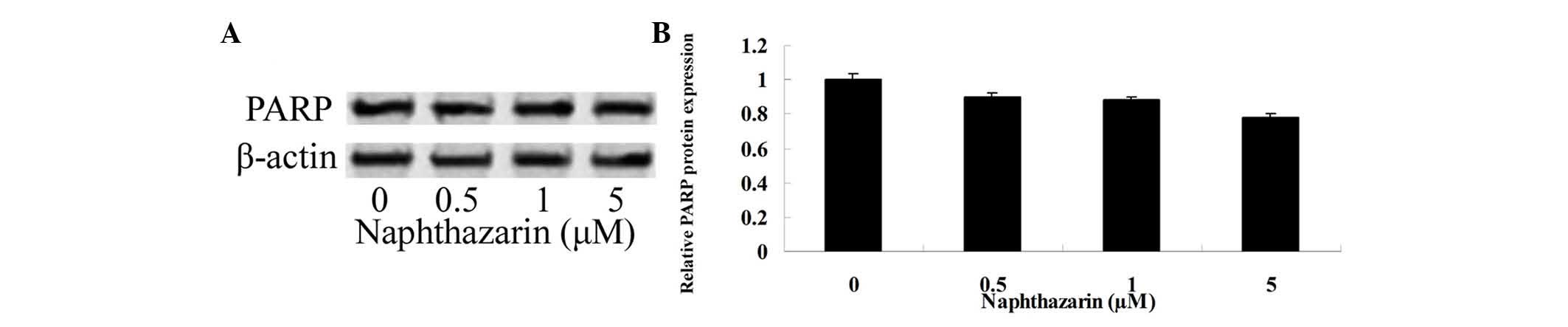
Effect of naphthazarin on PARP of
SW480 cells
To determine whether PARP is associated with the
effects of naphthazarin on cell viability and apoptosis in SW480
cells, PARP protein expression was evaluated by western blotting.
As shown in Fig. 6, treatment with
0.5, 1 and 5 µM naphthazarin for 24 h decreased PARP protein
expression, however, no significant differences were observed when
compared with the control group.
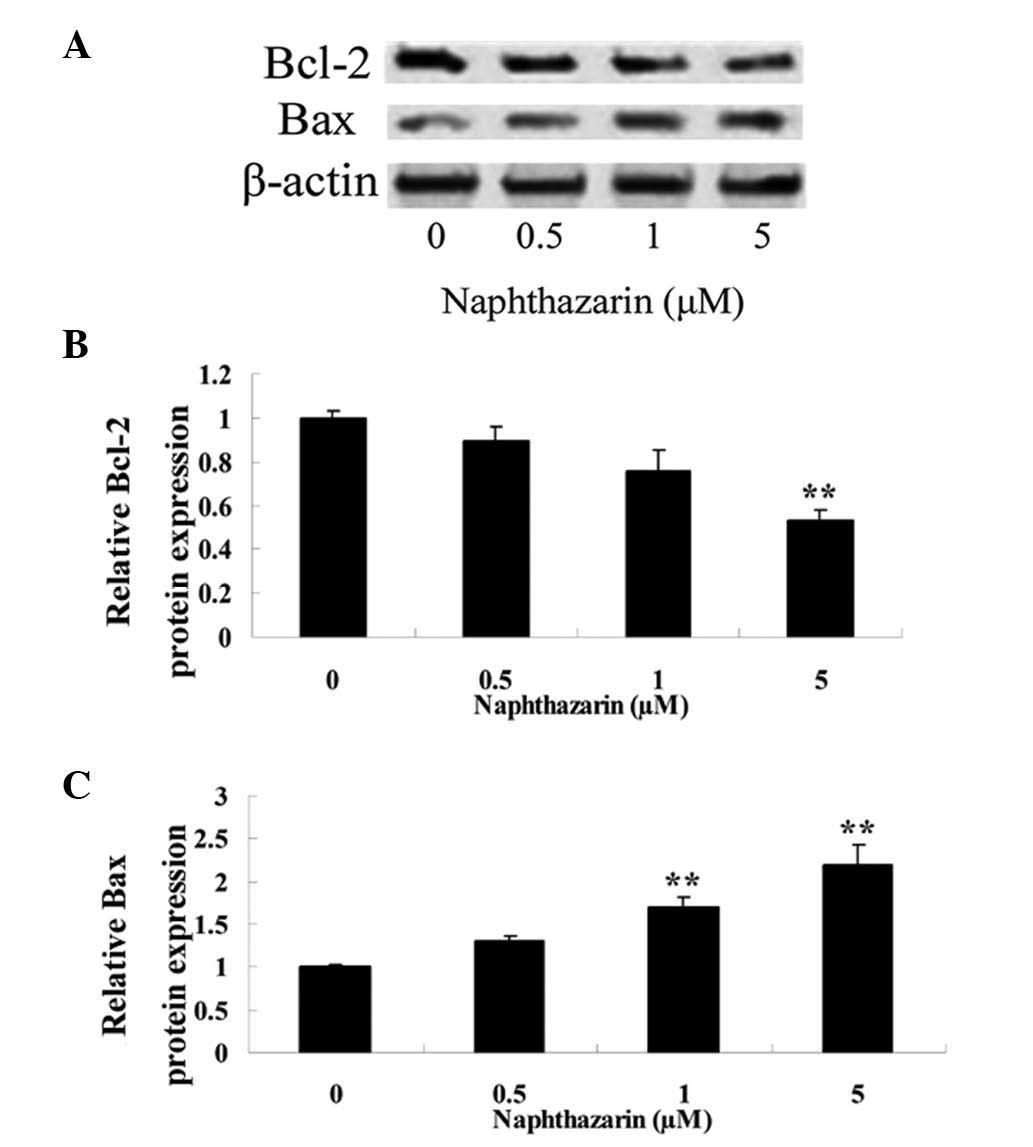
Naphthazarin decreases Bcl-2 and
increases Bax expression in SW480 cells
To further investigate the anticancer effect of
naphthazarin, the protein expression of Bcl-2 and Bax in human
colorectal SW480 cancer cells was analyzed by western blotting
following naphthazarin treatment (Fig.
7A). The results revealed that treatment with 5 µM naphthazarin
for 24 h significantly decreased Bcl-2 expression in cells compared
with the control (P<0.01; Fig.
7B). Furthermore, treatment with 1 and 5 µM naphthazarin for 24
h significantly increased Bax protein expression in a
dose-dependent manner compared with the control (Fig. 7C).
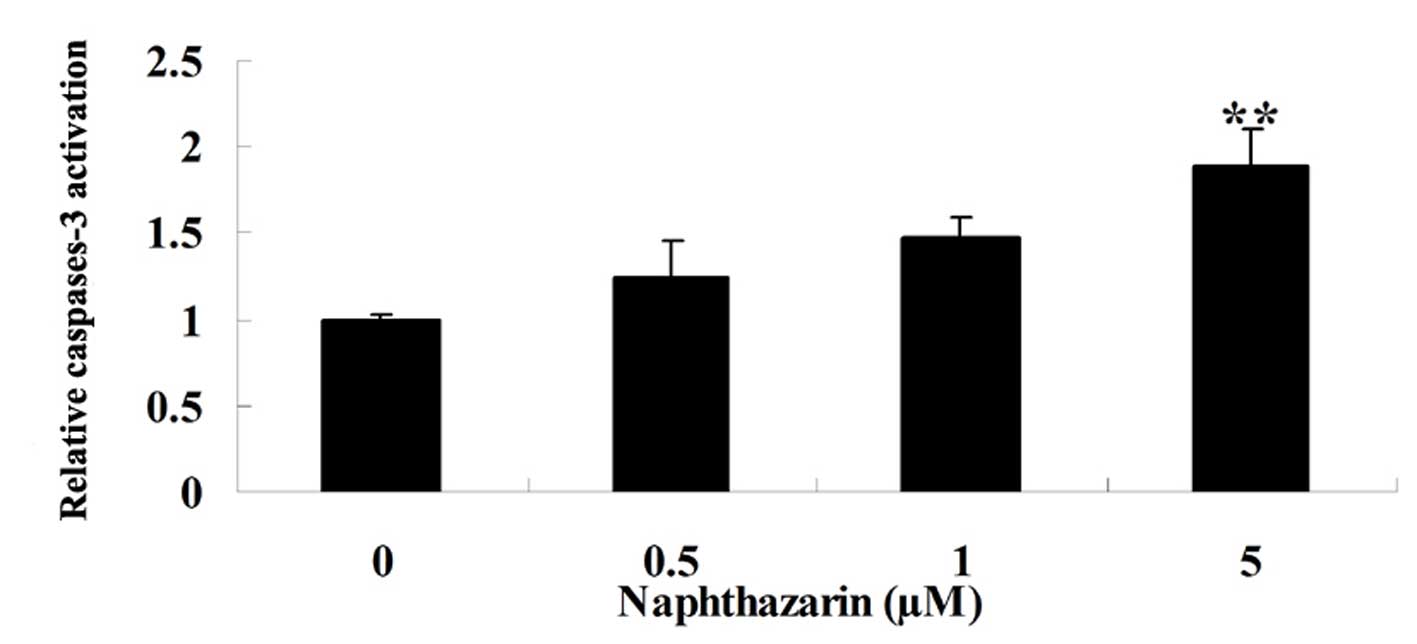
Effect of naphthazarin on caspase-3 of
SW480 cells
To confirm that potential mechanism of naphthazarin
on cell apoptosis of human colorectal cancer cell, we also examined
the activation of caspase-3 after naphthazarin treatment with for
24 h. Treatment with 5 µM naphthazarin resulted in significantly
increased levels of caspase-3 activation in SW480 cells compared
with the control (Fig. 8).
Discussion
Globally, colorectal cancer is the third most common
type of malignant tumor, after lung and breast cancer (11). The incidence of colorectal cancer
exhibits differences in regional distribution: Incidence is highest
in developed countries and regions, such as Australia, New Zealand,
Europe and North America, and lower in Asia and Africa (12–14). The
highest mortality rates occur in Central and Eastern European
countries, and the lowest mortality rates are observed in central
African regions (15). In China in
2009, colorectal cancer incidence and mortality rates were higher
than the world averages: Rates were than lower that observed in
Japan, Singapore and South Korea, but higher than that of countries
such as Iran, Laos and India (16,17). In
the present study, it was demonstrated that naphthazarin
significantly decreased cell viability, increased cytotoxicity and
induced cellular and nuclear apoptosis of SW480 cells in a
dose-dependent manner. Recent studies have demonstrated that
naphthazarin induces apoptosis of human breast cancer (18) and gastric cancer cells (19).
PARP is involved in DNA damage recognition and
signal transduction: PARP inhibitors can selectively prevent
defects in the DNA of tumor cells (20). A previous clinical trial revealed that
PARP inhibitors used for the treatment of ovarian cancer patients
harboring mutations in breast cancer susceptibility genes achieved
a good response rate, with few side effects (21). However, in the present study,
treatment with naphthazarin did not affect PARP protein expression
levels in SW480 cells. These results indicate that the PARP
signaling pathway may not be involved with the anticancer effects
of naphthazarin on human colorectal cancer cells.
Colorectal cancer is one of the most common
malignant tumors worldwide (22). It
has been demonstrated that the occurrence of tumor development
depends on the dynamic balance between cell proliferation and
apoptosis (23). Caspase-3 is a
important apoptotic protein for various cells, and caspase-3
activation can induce apoptosis in cancer cells (24). The Bcl-2 family of apoptosis-related
proteins includes important regulatory factors: Bcl-2 inhibits
apoptosis, whereas Bax and Bak promote apoptosis. Therefore,
changes in expression of these proteins affects the apoptosis of
both normal cells and tumor cells (25,26). The
results of the present study revealed that naphthazarin promoted
Bax expression and inhibited Bcl-2 protein expression, and
increased caspase-3 activation in SW480 cells. These results are in
accordance with those of Acharya et al (9) who reported that naphthazarin increases
the Bax/Bcl-2 protein ratio in A549 lung cancer cells.
In conclusion, the present study demonstrated that
naphthazarin suppressed cell proliferation and induced apoptosis in
human colorectal cancer cells via the Bcl-2/Bax signaling pathway.
Thus, we hypothesize that naphthazarin may present a potential
chemotherapeutic agent for colorectal cancer. However, further
studies are required to investigate the mechanisms underlying the
anticancer effects of naphthazarin on human colorectal cancers.
References
|
1
|
Sakurai J, Matsui Y, Hiraki T, Iguchi T,
Fujiwara H, Gobara H, Mitsuhashi T, Nagasaka T and Kanazawa S:
Single center prospective phase II trial of CT-guided
radiofrequency ablation for pulmonary metastases from colorectal
cancer (SCIRO-1401). Acta Med Okayama. 70:317–321. 2016.PubMed/NCBI
|
|
2
|
Huang L, Xu Y, Cai G, Guan Z and Cai S:
Downregulation of S100A4 expression by RNA interference suppresses
cell growth and invasion in human colorectal cancer cells. Oncol
Rep. 27:917–922. 2012.PubMed/NCBI
|
|
3
|
Kanefendt F, Lindauer A, Kinzig M,
Strumberg D, Scheulen ME, Mross K, Fischer R, Moritz B, Sörgel F
and Jaehde U: Biomarker response on exposure to sunitinib and its
primary metabolite (SU12662) in metastatic colorectal cancer
patients. Int J Clin Pharmacol Ther. 49:88–90. 2011.PubMed/NCBI
|
|
4
|
Xu F, Xu L, Wang M, An G and Feng G: The
accuracy of circulating microRNA-21 in the diagnosis of colorectal
cancer: A systematic review and meta-analysis. Colorectal Dis.
17:O100–O107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su B, Xu B and Wan J: Correlation between
long-term aspirin use and F-fluorodeoxyglucose uptake in colorectal
cancer measured by PET/CT. PLoS One. 9:e1094592014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa Willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Josa V, Krzystanek M, Eklund AC, Salamon
F, Zarand A, Szallasi Z and Baranyai Z: Relationship of
postoperative thrombocytosis and survival of patients with
colorectal cancer. Int J Surg. 18:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MY, Park SJ, Shim JW, Yang K, Kang HS
and Heo K: Naphthazarin enhances ionizing radiation-induced cell
cycle arrest and apoptosis in human breast cancer cells. Int J
Oncol. 46:1659–1666. 2015.PubMed/NCBI
|
|
9
|
Acharya BR, Bhattacharyya S, Choudhury D
and Chakrabarti G: The microtubule depolymerizing agent
naphthazarin induces both apoptosis and autophagy in A549 lung
cancer cells. Apoptosis. 16:924–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi SY, Son TG, Park HR, Jang YJ, Oh SB,
Jin B and Lee J: Naphthazarin has a protective effect on the
1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson's
disease model. J Neurosci Res. 90:1842–1849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012.PubMed/NCBI
|
|
12
|
Tatsumi S, Matsuoka H, Hashimoto Y, Hatta
K, Maeda K and Kamoshida S: Organic cation transporter 2 and tumor
budding as independent prognostic factors in metastatic colorectal
cancer patients treated with oxaliplatin-based chemotherapy. Int J
Clin Exp Pathol. 7:204–212. 2013.PubMed/NCBI
|
|
13
|
Liu Z, Huang Q, Liu G, Dang L, Chu D, Tao
K and Wang W: Presence of FOXP3(+)Treg cells is correlated with
colorectal cancer progression. Int J Clin Exp Med. 7:1781–1785.
2014.PubMed/NCBI
|
|
14
|
Shimizu D, Ishikawa T, Ichikawa Y, Togo S,
Hayasizaki Y, Okazaki Y, Danenberg PV and Shimada H: Prediction of
chemosensitivity of colorectal cancer to 5-fluorouracil by gene
expression profiling with cDNA microarrays. Int J Oncol.
27:371–376. 2005.PubMed/NCBI
|
|
15
|
Krützfeldt J, Rösch N, Hausser J,
Manoharan M, Zavolan M and Stoffel M: MicroRNA-194 is a target of
transcription factor 1 (Tcf1, HNF1α) in adult liver and controls
expression of frizzled-6. Hepatology. 55:98–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dimou A, Syrigos KN and Saif MW:
Disparities in colorectal cancer in African-Americans vs Whites:
Before and after diagnosis. World J Gastroenterol. 15:3734–3743.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehrabani D, Shamsdin SA, Dehghan A and
Safarpour A: Clinical significance of serum vascular endothelial
growth factor and complement 3a levels in patients with colorectal
cancer in southern Iran. Asian Pac J Cancer Prev. 15:9713–9717.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MY, Park SJ, Shim JW, Yang K, Kang HS
and Heo K: Naphthazarin enhances ionizing radiation-induced cell
cycle arrest and apoptosis in human breast cancer cells. Int J
Oncol. 46:1659–1666. 2015.PubMed/NCBI
|
|
19
|
Kim JA, Lee EK, Park SJ, Kim ND, Hyun DH,
Lee CG, Lee JH, Yang KM, Heo K and Son TG: Novel anti-cancer role
of naphthazarin in human gastric cancer cells. Int J Oncol.
40:157–162. 2012.PubMed/NCBI
|
|
20
|
Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y,
Kim M, Yoon JH, Kim CM, Chung HY and Kim ND: Corosolic acid induces
apoptotic cell death in HCT116 human colon cancer cells through a
caspase-dependent pathway. Int J Mol Med. 33:943–949.
2014.PubMed/NCBI
|
|
21
|
Hochster H, Wadler S, Runowicz C, Liebes
L, Cohen H, Wallach R, Sorich J, Taubes B and Speyer J: Activity
and pharmacodynamics of 21-Day topotecan infusion in patients with
ovarian cancer previously treated with platinum-based chemotherapy.
New York Gynecologic Oncology Group. J Clin Oncol. 17:2553–2561.
1999.PubMed/NCBI
|
|
22
|
Eng C, Bessudo A, Hart LL, Severtsev A,
Gladkov O, Müller L, Kopp MV, Vladimirov V, Langdon R, Kotiv B, et
al: A randomized, placebo-controlled, phase 1/2 study of tivantinib
(ARQ 197) in combination with irinotecan and cetuximab in patients
with metastatic colorectal cancer with wild-type KRAS who have
received first-line systemic therapy. Int J Cancer. 139:177–186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad S, Yadav VR, Sung B, Reuter S,
Kannappan R, Deorukhkar A, Diagaradjane P, Wei C,
Baladandayuthapani V, Krishnan S, et al: Ursolic acid inhibits
growth and metastasis of human colorectal cancer in an orthotopic
nude mouse model by targeting multiple cell signaling pathways:
Chemosensitization with capecitabine. Clin Cancer Res.
18:4942–4953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dastjerdi MN, Rarani MZ, Valiani A and
Mahmoudieh M: The effect of adenosine A1 receptor agonist and
antagonist on p53 and caspase 3, 8, and 9 expression and apoptosis
rate in MCF-7 breast cancer cell line. Res Pharm Sci. 11:303–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song J, Peng XL, Ji MY, Ai MH, Zhang JX
and Dong WG: Hugl-1 induces apoptosis in esophageal carcinoma cells
both in vitro and in vivo. World J Gastroenterol. 19:4127–4136.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chudecka-Głaz AM, Cymbaluk-Płoska AA,
Menkiszak JL, Sompolska-Rzechuła AM, Toloczko-Grabarek AI and
Rzepka-Górska IA: Serum HE4, CA125, YKL-40, bcl-2, cathepsin-L and
prediction optimal debulking surgery, response to chemotherapy in
ovarian cancer. J Ovarian Res. 7:622014. View Article : Google Scholar : PubMed/NCBI
|