Introduction
Familial adenomatous polyposis (FAP) is an inherited
disorder characterized by the development of hundreds to thousands
of adenomas in the rectum and colon during the second decade of
life (1–3). According to previous reports, including
the Chinese cancer registry annual report (4), FAP in China has an incidence at birth of
between 1 in 8,000 and 1 in 10,000 individuals; it manifests
equally in males and females, and accounts for 0.94% of colorectal
cancer (CRC) cases. Prevalence of FAP in China has been estimated
to be 1–1.5 in every 100,000 individuals (5–8).
Researchers in China have studied FAP for >30
years (5–10). Clinically, patients with FAP
presenting with numerous colorectal adenomas are easy to diagnose
(11); however, it is desirable to
screen out high-risk patients at earlier stages of development.
Currently, only certain individuals presenting with typical
extra-colonic manifestations, including congenital hypertrophy of
the retinal pigment epithelium (CHRPE), are recognized as high-risk
(7,8,11,12). In 1991, Groden et al (13) observed that FAP is caused by germline
mutations in the adenomatous polyposis coli (APC) gene, and
subsequent research confirmed that FAP is an autosomal dominant
disease (14–17). Members of families with a frequent
history of FAP may be at high risk of developing the disease
(15).
FAP is divided into three subtypes, including
classic FAP (CFAP), attenuated FAP (AFAP) and mutY DNA glycosylase
(MUTYH)-associated polyposis (MAP), each with unique genetics,
clinical features and prognoses (15,16).
Germline mutations and large rearrangements in the APC gene are the
primary causes of CFAP and AFAP (16–19), while
mutations in the MUTYH gene cause MAP (20–23).
Increasing numbers of pathogenic mutations have been reported to
predispose patients to FAP, and recent advances in the
understanding of FAP suggest that the genetics of each patient may
allow for early diagnosis and surveillance, and guide surgical and
chemopreventive management (24,25).
However, the genetics of FAP vary markedly between countries
(26–33). FAP in China has its own unique
characteristics, with the genotypes of patients with FAP varying
between regions and ethnicities (5,34–36).
As in Western countries, the primary priority for
patients with FAP in China is maintenance of a high quality of life
(5). In the present review, the
clinical manifestations and genetics of FAP in China are discussed,
as well as the surgical strategies, chemotherapeutics and
traditional Chinese medicines (TCM) used in its treatment.
Increased insight into the genetic and clinical features of FAP in
the Chinese population may aid in the prevention and management of
the disorder.
Data collection
PubMed (www.ncbi.nlm.nih.gov/sites/entrez?db=PubMed) and
Chinese search engines, including CNKI Data (www.cnki.net), Wanfang Data (www.wanfangdata.com.cn), SinoMed (www.sinomed.imicams.ac.cn) and Vip Information
(www.cqvip.com), were used to search the
literature for reference to FAP in China, using the key words:
‘familial adenomatous polyposis’, ‘APC’, ‘MYH’ and ‘Chinese’.
The majority of data on the genetic variations of
FAP in China is derived from published articles, databases and
unpublished experimental research, including the UMD APC mutations
database (www.umd.be/APC), APC-Database
(www.LOVD.nl/APC), Zhejiang University-Adinovo
Center APC Database (www.genomed.org/lovd2/home.php?select_db=APC)
(37) and the APC Mutation Database
(fap.taenzer.me). The MUTYH Mutation Database (www.LOVD.nl/MUTYH) was used to search for variation in
the MUTYH gene.
Clinical manifestations
Colonic manifestations
It is established that there are primarily three
subtypes of FAP. CFAP is the most common clinical phenotype and is
characterized by the presence of numerous colorectal adenomas of
differing sizes (Fig. 1), which if
left untreated progress into CRC. The majority of FAP cases in
China belong to this subtype (34–37). AFAP
is a less severe form of FAP, characterized by the presence of
<100 polyps and a later onset of CRC. AFAP in China has only
been diagnosed as an independent subclass to CFAP in the past ten
years (38–40). MAP is characterized by multiple
adenomatous polyps, with the majority of patients with MAP
presenting with fewer polyps compared with patients with CFAP. MAP
is reported to be the least common subtype, accounting for 1–5% of
FAP cases in China. This apparent decreased prevalence in MAP may
be due to poor recognition and detection of MAP (41–44).
Extra-colonic manifestations
Individuals with FAP are reported to develop a
variety of extra-colonic gastrointestinal manifestations (45,46).
However, fundic gland polyps in the stomach, adenomatous polyps in
the duodenum and periampullary region, and cancerization of upper
gastrointestinal adenomas is rare in Chinese patients with FAP,
particularly in those with CFAP (47). In 2015, Yan et al (48) reported a case of acute cholangitis due
to adenomas of the CBD in a patient with FAP, accompanied by
adenomatous changes in the stomach, duodenum and the ampulla of
Vater.
Extra-intestinal manifestations of FAP consist
mainly of cutaneous lesions, including fibromas, lipomas, and
sebaceous and epidermoid cysts (49,50). There
are specific FAP-associated syndromes reported in China, including
Gardner syndrome and Turcot syndrome (51). CHRPE is another characteristic
extra-intestinal manifestation in patients with FAP (52). In 1995, Li et al (53) initially reported CHRPE in 6 patients
with CFAP, and in 2010, Ding et al (54) detected CHRPE in 22 patients with FAP,
suggesting that CHRPE is an indicator of patients at a high risk of
FAP.
Role of mutations
Mutation analysis of the APC gene
The Leiden Open Variation Database reported >1600
different pathogenic APC mutations (www.chromium.lovd.nl/LOVD2/colon_cancer/home.php?select_db=APC).
A total of 275 of these mutations were reported in China, 194 of
which are unique to China (37). The
majority of mutations are nonsense mutations or small insertions
and deletions, which lead to a truncated APC protein. Mutations
causing FAP in China have been reported to occur mainly in three
regions of the APC gene: At the 5′ end, prior to codon 500; near
codon 1309 in the largest exon 16; or at the 3′ end, following
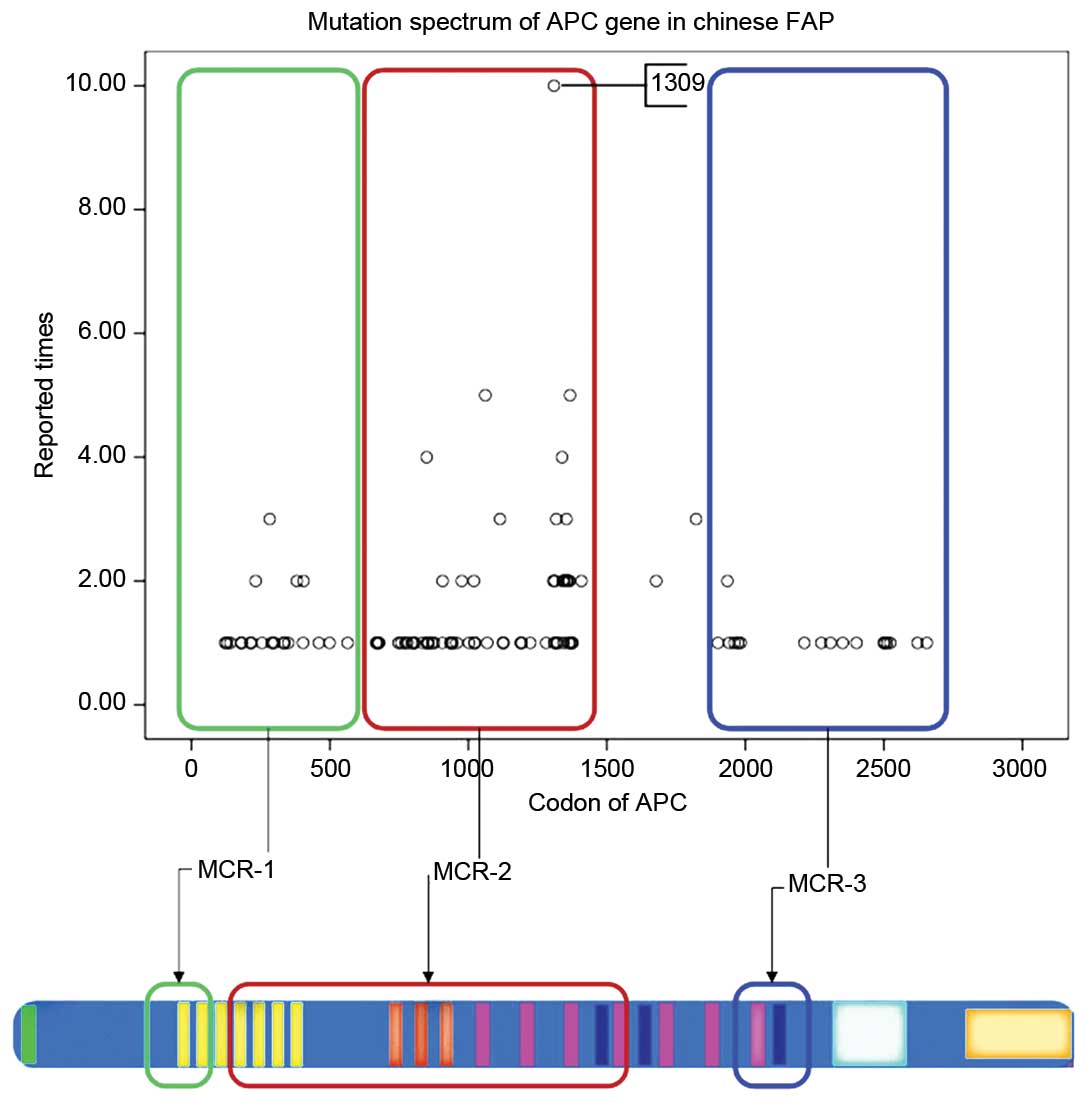
codon 1580 (37).
The mutation cluster region (MCR) in the APC gene in
Western countries has been established to be localized between
codons 1250 and 1464 (55,56). However, among the Chinese population,
the MCR in the APC gene is not consistent with Western countries,
instead localizing to exon 16, between codon 849 and 1376 (Fig. 1). According to the Chinese APC
database: The 5 bp deletion, c.3927_3931delAAAGA, at codon 1309 was
reported on eight occasions; the nonsense substitution,
c.3925G>T, at codon 1309 was reported on two occasions; the 2 bp
deletion, c.3182_3183delAA was reported once; the 5 bp deletion,
c.3181_3185delAAACA, at codon 1061 was reported once; and the 5 bp
deletion, c.3183_3187delACAAA, at codon 1061 was reported on three
occasions (37). The aforementioned
data indicate that the APC gene is frequently mutated between
codons 1309 and 1061 in Chinese families with FAP, which is
consistent with Western families (57). Furthermore, the substitution,
c.4479G>A, at codon 1493 was reported on five occasions; the 2
bp deletion, c.4393_4394delAG, at codon 1465 was reported on four
occasions; the nonsense substitution, c.4012C>T, at codon 1338
was reported on four occasions; and the nonsense substitution,
c.994C>T, at codon 332 was reported on four occasions. The data
suggest that the APC gene may frequently contain polymorphisms at
these points in Chinese patients with FAP (37).
Certain rare mutations of the APC gene have been
reported in Chinese patients with FAP, including intron or promoter
point mutations. For example, the following mutations have been
detected in Chinese patients with FAP (58,59): The
nonsense substitution, c.220+40T>C, at intron 2+39; the nonsense
substitution, c.645+32C>T, at intron 5+32; the 1 bp deletion,
c.645+46delG, at intron 5+46. Furthermore, the nonsense
substitutions, c.1556C>G and c.1753G>A, have been reported at
the 3′ untranslated region of the APC gene. However, the
pathogenicity of these mutations remains unclear.
MUTYH mutation screening
There is no specialized website or database devoted
to mutations in the MUTYH gene in China, as the majority of MUTYH
gene screening is conducted sporadically in patients with CRC and
few of these screenings were performed in Chinese patients with
MAP. In 2008, Tong et al (43)
identified three single nucleotide polymorphisms (SNPs) of the
MUTYH gene, including IVS1-5A>C, IVS6+35A>G and c.G972C
(Q335H). The SNP IVS1-5A>C was confirmed to be significant in
the etiopathogenesis of CRC, and may be used in screening of
high-risk patients (43). Currently,
p.Y165C and p.G382D mutations in the MUTYH protein have only been
observed in patients from Western countries (60,61) and
there is no identified polymorphism in the MUTYH gene for MAP
patients in China.
Conventional screening techniques fail to identify
~30% of families with CFAP and ~90% of families with AFAP. A large
subset of families with history of FAP have undetectable pathogenic
changes defined as APC(−) and MUTYH(−) FAP (62,63). No
other specific genes predisposing an individual to FAP have been
identified in China (41).
Surgical strategies
Surgical intervention is the most effective therapy
for patients with FAP who present with colonic disorders, and
prophylactic removal of the tumorigenic colon is considered to be
the standard treatment for FAP (64,65). The
surgical strategies and optimal time of intervention vary between
each FAP subtype (64–66). In China, patients with CFAP and
advanced AFAP may be treated by total proctocolectomy with
ileostomy, subtotal colectomy with ileorectal anastomosis (IRA),
total colectomy or proctocolectomy with ileo-anal pouch anastomosis
(IPAA), total colectomy or proctocolectomy with IPAA, subtotal
colectomy plus rectal mucosectomy, IPAA through the muscular sheath
of the rectum, IPAA alone and subtotal proctocolectomy (66,67). IPAA
offers the best available prophylaxis in CFAP patients and remains
the primary alternative to IRA (66).
Furthermore, laparoscopic IPAA surgery is performed in Chinese
patients with FAP, and is considered to be safe, feasible and
effective (68).
Patients with early stage AFAP and MAP have
increased available treatment options compared with patients with
CFAP and advanced AFAP (69,70). Due to rapid progress in colonoscopy,
doctors in China are able to remove numerous adenomas using
endoscopic polyp electrocision and endoanal mucosal stripping
(69). In 2006, He et al
(71) reported the excision of 256
polyps in a single patient with FAP.
Chemoprevention
In order to delay the development of adenomas into
adenocarcinoma and to prevent the recurrence of adenomas in the
retained rectum of patients with FAP following surgical
intervention, multiple drugs and dietary supplements have been
identified as potential chemopreventatives (72,73). The
nonsteroidal anti-inflammatory drug (NSAID) sulindac (74,75) and
selective cyclooxygenase-2 (COX-2) inhibitor celecoxib (76,77) are
the typical drugs administered to control and reduce polyposis in
the retained rectum following surgery. However, gastrointestinal
toxicity has been observed following long-term treatment with
non-selective NSAIDs (78), leading
to an increase in the use of COX-2 inhibitors as the primary
chemopreventive agent for patients with FAP (79,80).
Treatment with celecoxib alone or combined with endoscopy has
proven to be effective in reducing the number of adenomas in
Chinese patients with FAP (81,82).
Roles of TCM
For Chinese patients with FAP who do not consent to
surgical intervention or chemotherapy, TCM is an available option.
In 1995, An et al (83)
reported that 15 patients diagnosed with CFAP receiving TCM through
oral administration and retention enema exhibited a reduction in
clinical CFAP symptoms, including diarrhea and hematochezia.
Furthermore, Huo et al (84)
reported the case of a patient with CFAP who rejected surgical
treatment and who, following 3 years of regular treatment with TCM,
had only a single polyp identified by endoscopic evaluation.
Conclusions
Previously, the majority of patients with FAP in
China were identified in the late stages of the disease, presenting
with bowel obstructions, rectal bleeding or adenocarcinomas
(5). Due to the poor prognosis and
genetic diversity of FAP, clinicians and geneticists in China have
studied the disease for the past three decades (5–10).
According to PubMed and multiple Chinese databases,
cases of FAP among the Chinese population are mainly of the subtype
CFAP. The colonic manifestations of these patients are typically
easy to diagnosis (11), and
extra-colonic manifestations, including CHRPE, are indicators of
CFAP (12). Patients presenting with
fewer adenomas (18,19) are now increasingly recognized as
having a separate subtype of FAP, known as AFAP, and are studied
separately. However, cases of MAP in China remain rare (44).
FAP is an autosomal dominant disease (1,13);
mutations of the APC gene are considered to be the main causes of
CFAP and AFAP (18,19), and mutations of the MUTYH gene are
associated with MAP (20–23). However, the FAP genotype varies
significantly between countries (26–33). FAP
in China has its own characteristics; the mutation spectrum of the
APC gene in China is not consistent with the mutation spectrum of
FAP in Western countries (5,34–36). The
MCR of APC in Chinese patients localizes to exon 16, between codons
849 and 1376, whereas the MCR of APC in Western patients is
reported to localize between codons 1250 and 1464 (50–51). In
addition, mutations of APC gene polymorphisms are frequently
located between codons 1309 and 1061 in Chinese patients with FAP,
which is consistent with polymorphisms identified in Western
countries (57). Other APC
polymorphisms frequently observed include, c.4393_4394delAG at
codon 1465, c.4012C>T at codon 1338 and c.994C>T at codon
332. As reports of MAP in the Chinese population are few in number,
the frequent MCR polymorphisms in the MUTYH gene remain unclear
(44). Additionally, a large subset
of Chinese patients with FAP have undetectable pathogenic
mutations. The rate of APC(−) and MUTYH(−) in China ranges between
30 and 60% depending on the province (62,63). There
are 56 ethnic groups in China, and living conditions vary between
provinces (85), which may affect the
genetic diversity of FAP in China.
Surgical intervention and chemotherapy has been
proven to benefit patients with FAP (64,65,72,73).
In China, laparoscopic IPAA surgery is considered the standard
treatment for patients diagnosed with CFAP and advanced AFAP
(66,67). Due to rapid progress in colonoscopy,
doctors in China are able to remove the numerous adenomas using
endoscopic polyp electrocision and endoanal mucosal stripping
(69,70). Celecoxib is the recommended FAP
chemotherapeutic used to delay the development of adenomas into
adenocarcinoma and prevent the recurrence of adenomas. Furthermore,
combined celecoxib treatment and endoscopy has been proven to
effectively reduce the number of adenomas in patients with FAP
(81,82).
For Chinese patients with FAP who do not consent to
surgical operation, TCM is another option and has been used in the
treatment of colonic disorders (86).
Having developed over thousands of years, TCM is considered to be a
complete system of healthcare and includes a complex herbal
therapeutic component (87).
Following treatment with TCM, patients with FAP have exhibited
long-term survival and reduced colorectal symptoms, which provides
evidence for the use of Chinese herbal medicine in the successful
prevention and treatment of FAP (83,84).
Researchers in China are attempting to study the active ingredients
of traditional herbal therapeutics and elucidate their mechanism of
action (86,88). However, further randomized controlled
trials on TCM are required.
In conclusion, as the understanding of
genotype-phenotype correlations between FAP subtypes increases,
patients diagnosed with FAP in China are gradually benefiting from
improved surgical intervention, colonoscopy, chemoprevention,
surveillance and TCM. However, multiple characteristics of FAP
remain unclear. There is a significant number of patients diagnosed
with FAP but with no identified genetic mutation, even following
sophisticated genetic testing (41,42,44); due
to this many high-risk individuals may be misdiagnosed. There is
clinical evidence to support the possibility of influencing the
manifestation of FAP through chemoprevention and lifestyle changes
(72,73). There are certain clinical cases
showing the efficacy of TCM in the treatment of FAP, however the
active ingredient remains to be elucidated. Therefore, the
mechanisms of FAP require further research and evaluation.
Acknowledgements
The present review was supported by the National
Natural Science Foundation of China (grant no., 81160245) and the
Science and Technology Planning Project of Yunnan Province, China
(grant no., 2011FB160).
References
|
1
|
Gardner EJ, Burt RW and Freston JW:
Gastrointestinal polyposis: Syndromes and genetic mechanisms. West
J Med. 132:488–499. 1980.PubMed/NCBI
|
|
2
|
Bianch KL, Buerke CA, Bennett AE, Lopez R,
Hasson H and Church JM: Fundic gland polyp dysplasia is common in
familial adenomatous polyposis. Clin Gastroenterol Heptatol.
6:180–185. 2008. View Article : Google Scholar
|
|
3
|
Nugent KP and Philips RK: Rectal cancer
risk in older patients with familial adenomatous polyposis and an
ileorectal anastomosis: A cause for concern. Brit J Surg.
79:1204–1206. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jie He and Wan-Qing Chen: Chinese cancer
registry annual report. 56–59. 2012.
|
|
5
|
Shu Z, Yanqin H and Ying Y: Hereditary
colorectal cancer in China. Hered Cancer Clin Pract. 3:155–164.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Parkin DM, Li LD, Chen YD and Bray
F: Estimation and projection of the national profile of cancer
mortality in China: 1991–2005. Br J Cancer. 90:2157–2166.
2004.PubMed/NCBI
|
|
7
|
Zhang YZ, Sheng JQ, Li SR and Zhang H:
Clinical phenotype and prevalence of hereditary nonpolyposis
colorectal cancer syndrome in Chinese population. World J
Gastroenterol. 11:1481–1488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Hereditary Colorectal Cancer
Network, . Screening program of Chinese hereditary colorectal
cancer. Zhonghua Zhong Liu Za Zhi. 26:191–192. 2004.(In
Chinese).
|
|
9
|
Yutang G, Lu S and Yongbin X: Cancer
incidence trend in Shanghai 1972–1994. Tumor. 19:2551999.(In
Chinese).
|
|
10
|
Jiao Y, Gong E, Meng G, Liu S, Wen E and
Ding Y: Detection of APC gene germline mutations in familial
adenomatous polyposis patients. Beijing Yi Ke Da Xue Xue Bao.
28:267–269. 1997.(In Chinese).
|
|
11
|
Vasen HF: Clinical diagnosis and
management of hereditary colorectal cancer syndromes. J Clin Oncol.
18:(21 Suppl). 81S–92S. 2000.PubMed/NCBI
|
|
12
|
Romania A, Zakov ZN, McGannon E, Schroeder
T, Heyen F and Jagelman DG: Congenital hypertrophy of the retinal
pigment epithelium in familial adenomatous polyposis.
Ophthalmology. 96:879–984. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Groden J, Thliveris A, Samowitz W, Carlson
M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson
M, et al: Identification and characterization of the familial
adenomatous polyposis coli gene. Cell. 66:589–600. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinzler KW, Nilbert MC, Vogelstein B,
Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P,
Markham A, et al: Identification of a gene located at chromosome
5q21 that is mutated in colorectal cancers. Science. 251:1366–1370.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruhswurm I, Zehetmayer M, Dejaco C, Wolf B
and Karner-Hanusch J: Ophthalmic and genetic screening in pedigrees
with familial adenomatous polyposis. Am J Ophthalmol. 125:680–686.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Half E, Bercovich D and Rozen P: Familial
adenomatous polyposis. Orphanet J Rare Dis. 4:222009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powell SM, Petersen GM, Krush AJ, Booker
S, Jen J, Giardiello FM, Hamilton SR, Vogelstein B and Kinzler KW:
Molecular diagnosis of familial adenomatous polyposis. N Engl J
Med. 329:1982–1987. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sieber OM, Lipton L, Crabtree M, Heinimann
K, Fidalgo P, Phillips RK, Bisgaard ML, Orntoft TF, Aaltonen LA,
Hodgson SV, et al: Multiple colorectal adenomas, classic
adenomatous polyposis, and germ-line mutations in MYH. N Eng J Med.
348:791–799. 2003. View Article : Google Scholar
|
|
19
|
Quadri M, Vetro A, Gismondi V, Marabelli
M, Bertario L, Sala P, Varesco L, Zuffardi O and Ranzani GN: APC
rearrangements in familial adenomatous polyposis: Heterogeneity of
deletion lengths and breakpoint sequences underlies similar
phenotypes. Fam Cancer. 14:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramsoekh D, van Leerdam ME, Tops CM,
Dooijes D, Steyerberg EW, Kuipers EJ and Wagner A: The use of
genetic testing in hereditary colorectal cancer syndromes: Genetic
testing in HNPCC, (A)FAP and MAP. Clin Genet. 72:562–567. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poulsen ML and Bisgaard ML: MUTYH
associated polyposis (MAP). Curr Genomics. 9:420–435. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Venesio T, Balsamo A, D'Agostino VG and
Ranzani GN: MUTYH-associated polyposis (MAP), the syndrome
implicating base excision repair in inherited predisposition to
colorectal tumors. Front Oncol. 2:832012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sampson JR, Dolwani S, Jones S, Eccles D,
Ellis A, Evans DG, Frayling I, Jordan S, Maher ER, Mak T, et al:
Autosomal recessive colorectal adenomatous polyposis due to
inherited mutations of MYH. Lancet. 362:39–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kennedy RD, Potter DD, Moir CR and
El-Youssef M: The natural history of familial adenomatous polyposis
syndrome: A 24 year review of a single center experience in
screening, diagnosis, and outcomes. J Pediatr Surg. 49:82–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kennelly RP, Gryfe R and Winter DC:
Familial colorectal cancer: Patient assessment, surveillance and
surgical management. Eur J Surg Oncol. pii:S0748-S7983.30675-30678.
2016.
|
|
26
|
Torrezan GT, da Silva FC, Santos EM,
Krepischi AC, Achatz MI, Aguiar S Jr, Rossi BM and Carraro DM:
Mutational spectrum of the APC and MUTYH genes and
genotype-phenotype correlations in Brazilian FAP, AFAP, and MAP
patients. Orphanet J Rare Dis. 8:542013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vandrovcová J, Stekrová J, Kebrdlová V and
Kohoutová M: Molecular analysis of the APC and MYH genes in Czech
families affected by FAP or multiple adenomas: 13 novel mutations.
Hum Mutat. 23:3972004. View Article : Google Scholar
|
|
28
|
Gómez-Fernández N, Castellví-Bel S,
Fernández-Rozadilla C, Balaguer F, Muñoz J, Madrigal I, Milà M,
Graña B, Vega A, Castells A, et al: Molecular analysis of the APC
and MUTYH genes in Galician and Catalonian FAP families: A
different spectrum of mutations? BMC Med Genet. 10:572009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivera B, Gonzalez S, Sánchez-Tomé E,
Blanco I, Mercadillo F, Letón R, Benítez J, Robledo M, Capellá G
and Urioste M: Clinical and genetic characterization of classical
forms of familial adenomatous polyposis: A Spanish population
study. Ann Oncol. 22:903–909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fostira F, Thodi G, Sandaltzopoulos R,
Fountzilas G and Yannoukakos D: Mutational spectrum of APC and
genotype-phenotype correlations in Greek FAP patients. BMC Cancer.
10:3892010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanter-Smoler G, Fritzell K, Rohlin A,
Engwall Y, Hallberg B, Bergman A, Meuller J, Grönberg H, Karlsson
P, Björk J and Nordling M: Clinical characterization and the
mutation spectrum in Swedish adenomatous polyposis families. BMC
Med. 6:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friedl W and Aretz S: Familial adenomatous
polyposis: Experience from a study of 1164 unrelated German
polyposis patients. Hered Cancer Clin Pract. 3:95–114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wachsmannova-Matelova L, Stevurkova V,
Adamcikova Z, Holec V and Zajac V: Polymorphisms in the adenomatous
polyposis coli gene in Slovak families suspected of FAP. Neuro
Endocrinol Lett. 30:(Suppl 1). S25–S28. 2009.
|
|
34
|
Liang J, Lin C, Hu F, Wang F, Zhu L, Yao
X, Wang Y and Zhao Y: APC polymorphisms and the risk of colorectal
neoplasia: A HuGE review and meta-analysis. Am J Epidemiol.
177:1169–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai Q, Sun M, Lu H, Zhang T, Mo S, Xu Y,
Cai S, Zhu X and Shi D: Clinicopathological and molecular genetic
analysis in Chinese typical hereditary nonpolyposis colorectal
cancer pedigrees. Zhonghua Bing Li Xue Za Zhi. 30:339–344. 2001.(In
Chinese). PubMed/NCBI
|
|
36
|
Sheng JQ, Cui WJ, Fu L, Jin P, Han Y, Li
SJ, Fan RY, Li AQ, Zhang MZ and Li SR: APC gene mutations in
Chinese familial adenomatous polyposis patients. World J
Gastroenterol. 16:1522–1526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan M, Cong P, Wang Y, Lin C, Yuan Y, Dong
J, Banerjee S, Zhang T, Chen Y, Zhang T, et al: Novel LOVD
databases for hereditary breast cancer and colorectal cancer genes
in the Chinese population. Hum Mutat. 32:1335–1340. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su F, Wang T, Zhang MF and Wang BM: The
significance of APC protein expression in the Dagnosis of AFAP.
Digestive Disease and Endoscopy. 2:26–30. 2008.(In Chinese).
|
|
39
|
Su F, Wang T and Wang BM: Attenuated
familial adenomatous. Zhong Guo Xiao Hua Nei Jing. 2:20–26.
2008.(In Chinese).
|
|
40
|
Lou Z, Yu ED and Meng RG: Attenuated
familial adenomatous polyposis. Zhong Hua Wei Chang Wai Ke Za Zhi
Bian Ji Bu. 9:81–83. 2006.(In Chinese).
|
|
41
|
Yang J, Liu WQ, Li WL, Chen C, Zhu Z, Wang
ZQ and Dong J: Detection of APC, MYH and AXIN2 mutations for
screening germline mutations predisposing to familial adenomatous
polyposis. Shijie Huaren Xiaohua Zazhi. 23:556–562. 2015.(In
Chinese).
|
|
42
|
Cheng J, Wang B and Wang T: Research
progress of MYH-associated polyposis. Guo Ji Xiao Hua Bing Za Zhi
She. 30:103–106. 2010.(In Chinese).
|
|
43
|
Jing Tong and Bing-Yuan Wang: Relationship
between MUTYH gene and occurrence of colorectal cancer in family
clan of familial adenomatous polyposis. Shijie Huaren Xiaohua
Zazhi. 16:3576–3581. 2008.(In Chinese).
|
|
44
|
Zhou HH: Inherited mutations of MUTYH and
colorectal cancer. Zhejiang Da Xue Xue Bao Yi Xue Ban. 36:406–411.
2007.(In Chinese). PubMed/NCBI
|
|
45
|
Griffioen G, Bus PJ, Vasen HF, Verspaget
HW and Lamers CB: Extracolonic manifestations of familial
adenomatous polyposis: Desmoid tumours, and upper gastrointestinal
adenomas and carcinomas. Scand J Gastroenterol Suppl. 225:85–91.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Konsker KA: Familial adenomatous
polyposis: Case report and review of extracolonic manifestations.
Mt Sinai J Med. 59:85–91. 1992.PubMed/NCBI
|
|
47
|
Bin Zhang: A case of samilial adenomatous
polyposis with fundic gland polyps. Clinical Misdiagnosis &
Mistherapy. 21:542008.(In Chinese).
|
|
48
|
Yan ML, Pan JY, Bai YN, Lai ZD, Chen Z and
Wang YD: Adenomas of the common bile duct in familial adenomatous
polyposis. World J Gastroenterol. 21:3150–3153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Hai-Zeng, Dong Shu-Xiao, Zhou
Zhi-Xiang and Shao Yong-FU: Familial adenomatous polyposis related
Desmoid tumors. The Practical Journal of Cancer. 21:189–194.
2006.(In Chinese).
|
|
50
|
Yu ED, Lou Z, Xu XD, Meng RG, Wang H, Jin
GX and Fu CG: Diagnosis and therapy of familial adenomatous
polyposis with desmoid tumour. Zhong Hua Pu Tong Wai Ke Za Zhi Bian
Ji Bu. 21:179–181. 2006.(In Chinese).
|
|
51
|
Cao HL, Wang BM and Cao XC: Clinical
features of Gardner syndrome and Turcot syndrome in Chinese
population: An analysis of 93 cases. Shijie Huaren Xiaohua Zazhi.
18:3922–3925. 2010.(In Chinese).
|
|
52
|
Nusliha A, Dalpatadu U, Amarasinghe B,
Chandrasinghe PC and Deen KI: Congenital hypertrophy of retinal
pigment epithelium (CHRPE) in patients with familial adenomatous
polyposis (FAP); a polyposis registry experience. BMC Res Notes.
7:7342014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Run Chun Li, Jin Wang and Jian Lin:
Screening value of congenital hypertrophy of the retinal pigment
epithelium for familial adenomatous polyposis. Chinese Journal of
Ocular Fundus Diseases. 11:1–3. 1995.(In Chinese).
|
|
54
|
Yan Ding, Yu Xu and Xiao-Dong Xu: Research
on the fundus fluorescein angiography in congenitaI hypertrophy of
the retinaI pigment epithelium in patients with familiaI
adenomatous polyposis. International Journal of Ophthalmology.
10:1157–1159. 2010.
|
|
55
|
Friedl W, Caspari R, Sengteller M, Uhlhaas
S, Lamberti C, Jungck M, Kadmon M, Wolf M, Fahnenstich J, Gebert J,
et al: Can APC mutation analysis contribute to therapeutic
decisions in familial adenomatous polyposis? Experience from 680
FAP families. Gut. 48:515–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bertario L, Russo A, Sala P, Varesco L,
Giarola M, Mondini P, Pierotti M, Spinelli P and Radice P:
Hereditary Colorectal Tumor Registry: Multiple approach to the
exploration of genotype-phenotype correlations in familial
adenomatous polyposis. J Clin Oncol. 21:1698–1707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Béroud C and Soussi T: APC gene: Database
of germline and somatic mutations in human tumors and cell lines.
Nucleic Acids Res. 24:121–124. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cai SR, Zhang SZ and Zheng S: Detection of
adenomatous polyposis coli gene mutations in 31 familial
adenomatous polyposis families by using denaturing high performance
liquid chromatography. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
25:164–167. 2008.(In Chinese). PubMed/NCBI
|
|
59
|
Chen J, Guo L, Peiffer DA, Zhou L, Chan
OT, Bibikova M, Wickham-Garcia E, Lu SH, Zhan Q, Wang-Rodriguez J,
et al: Genomic profiling of 766 cancer-related genes in archived
esophageal normal and carcinoma tissues. Int J Cancer.
122:2249–2254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gismondi V, Meta M, Bonelli L, Radice P,
Sala P, Bertario L, Viel A, Fornasarig M, Arrigoni A, Gentile M, et
al: Prevalence of the Y165C, G382D and 1395delGGA germline
mutations of the MYH gene in Italian patients with adenomatous
polyposis coli and colorectal adenomas. Int J Cancer. 109:680–684.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ashton KA, Meldrum CJ, McPhillips ML,
Kairupan CF and Scott RJ: Frequency of the Common MYH Mutations
(G382D and Y165C) in MMR Mutation Positive and Negative HNPCC
Patients. Hered Cancer Clin Pract. 3:65–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Castellsagué E, González S, Guinó E,
Stevens KN, Borràs E, Raymond VM, Lázaro C, Blanco I, Gruber SB and
Capellá G: Allele-specific expression of APC in adenomatous
polyposis families. Gastroenterology. 139:439–447.e1. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Renkonen ET, Nieminen P, Abdel-Rahman WM,
Moisio AL, Järvelä I, Arte S, Järvinen HJ and Peltomäki P:
Adenomatous polyposis families that screen APC mutation-negative by
conventional methods are genetically heterogeneous. J Clin Oncol.
23:5651–5659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Warrier SK and Kalady MF: Familial
adenomatous polyposis: Challenges and pitfalls of surgical
treatment. Clin Colon Rectal Surg. 25:83–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Leonard D, Wolthuis A, D'Hoore A, Bruyninx
L, Van De Stadt J, Van Cutsem E and Kartheuser A: Different
surgical strategies in the treatment of familial adenomatous
polyposis: What's the role of the ileal pouch-anal anastomosis?
Acta Gastroenterol Belg. 74:427–434. 2011.PubMed/NCBI
|
|
66
|
XIE Yu-Quan, Yuan Xing-Hua, Zheng Zhao-XU
and Fang YI: Meta analysis of surgical treatment of 231 cases of
familial adenomatous polyposis in China. Chinese Journal of Cancer
Prevention and Treatment. 15:537–540. 2008.(In Chinese).
|
|
67
|
Wen Y, Lu J, Zhu M, Wu Ch, Li L and Zhu W:
Surgical treatment of familial adenomatous polyposis: A report of
45 cases. Zhong Hua Pu Tong Wai Ke Za Zhi Bian Ji Bu. 13:673–675.
2004.(In Chinese).
|
|
68
|
Zhen Si-Hu, Hou Hui-Chi, Wang Shao-Wen, LI
Wei and Liu Wei: Laparoscopic total colectomy for familial
adenomatous polyposis with a report of 9 cases. Journal of Regional
Anatomy and Operative Surgery. 22:629–630. 2013.(In Chinese).
|
|
69
|
Moussata D, Napoleon B, Lepilliez V, Klich
A, Ecochard R, Lapalus MG, Nancey S, Cenni JC, Ponchon T,
Chayvialle JA and Saurin JC: Endoscopic treatment of severe
duodenal polyposis as an alternative to surgery for patients with
familial adenomatous polyposis. Gastrointest Endosc. 80:817–825.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nie Y, Zhang R, Fan K and Liang H:
Familial adenomatous polyposis: A report of 10 cases in 3
generations of a family and literature review. Zhonghua Nei Ke Za
Zhi. 53:290–292. 2014.(In Chinese). PubMed/NCBI
|
|
71
|
He JT, Li XL, Jiang XY, Li J, Gong LS,
Zhang YD and Lu Jand Liao CX: Clinical study on familial
adenomatous polyposis treated by high frequency electric cutting
under endoscope. China Journal of Modern Medicine. 16:568–570.
2006.(In Chinese).
|
|
72
|
Cooper K, Squires H, Carroll C,
Papaioannou D, Booth A, Logan RF, Maguire C, Hind D and Tappenden
P: Chemoprevention of colorectal cancer: Systematic review and
economic evaluation. Health Technol Assess. 14:1–206. 2010.
View Article : Google Scholar
|
|
73
|
Lang M and Gasche C: Chemoprevention of
colorectal cancer. Dig Dis. 33:58–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Waddell WR and Loughry RW: Sulindac for
polyposis of the colon. J Surg Oncol. 24:83–87. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li J, Lu Y, Gu F, An Y and Qian Y: The
long-term effects of sulindac on familial adenomatous polyposis.
Zhong Hua Ziao Hua Za Zhi Bian Ji Bu. 25:153–156. 2005.(In
Chinese).
|
|
76
|
Huang K, Gutierrez LP, Bülow S, Gallinger
S, Castells A, Eagle CJ and Church JM: Clinical characteristics and
outcomes in familial adenomatous polyposis patients with a
long-term treatment of celecoxib: A matched cohort study. Fam
Cancer. 10:303–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang J and Luo M: Treatment of upper
gastrointestinal polyps in familial adenomatous polyposis with
celexib. Chin J Surg. 43:1962003.(In Chinese).
|
|
78
|
Schnitzer TJ, Burmester GR, Mysler E,
Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B,
Matchaba P, et al: Comparison of lumiracoxib with naproxen and
ibuprofen in the Therapeutic Arthritis Research and
Gastrointestinal Event Trial (TARGET), reduction in ulcer
complications: Randomised controlled trial. Lancet. 364:665–674.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lynch PM, Ayers GD, Hawk E, Richmond E,
Eagle C, Woloj M, Church J, Hasson H, Patterson S, Half E and Burke
CA: The safety and efficacy of celecoxib in children with familial
adenomatous polyposis. Am J Gastroenterol. 105:1437–1443. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lynch PM, Burke CA, Phillips R, Morris JS,
Slack R, Wang X, Liu J, Patterson S, Sinicrope FA, Rodriguez-Bigas
MA, et al: An international randomised trial of celecoxib versus
celecoxib plus difluoromethylornithine in patients with familial
adenomatous polyposis. Gut. 65:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cao Yong and Zheng Chang-Qing: Effects of
celecoxib combined with high frequency electric cutting under
endoscopy on familial adenomatous polyposis. Practical Pharmacy And
Clinical Remedies. 14:209–211. 2011.(In Chinese).
|
|
82
|
Li SB, Tong Q, Gao YJ, Wang Q, Zhang WG
and Wang XH: A clinical study of celecoxib plus RFA/high frequency
electrocoagulation and electrocision in treatment of familial
adenomatous polyposis. Wei Chang Bing Xue Bian Ji Bu. 14:615–617.
2009.(In Chinese).
|
|
83
|
An A-Yue, Zheng Li-Hua and Huang Yue:
Treatment of 15 FAP patients by oral administration and retention
enema of TCM. Zhong Ji Yi Kan. 30:44–45. 1995.(In Chinese).
|
|
84
|
Huo Qing-Ping and Kong Lin: Treatment
experience of a familial adenomatous polyposis case. SH J TCM.
42:12–13. 2008.(In Chinese).
|
|
85
|
Jiao Kai-Shan: Analysis of spatial
statistics about Chinese minority population distribution and its
changes. Journal of Southwest University for Nationalities
(Humanities and Social Science). 10:26–32. 2014.(In Chinese).
|
|
86
|
Piao M, Cao H, He N, Yang B, Dong W, Xu M,
Yan F, Zhou B and Wang B: Berberine inhibits intestinal polyps
growth in Apc (min/+) mice via regulation of macrophage
polarization. Evid Based Complement Alternat Med. 2016:51375052016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu J and Yang Y: Traditional Chinese
medicine in the Chinese health care system. Health Policy.
90:133–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ping B: The effects of Chinese drugs for
supporting healthy energy and removing blood stasis on
postoperative metastasis of gastric carcinoma and ornithine
decarboxylase. J Tradit Chin Med. 18:3–6. 1998.PubMed/NCBI
|