Introduction
Non-small cell lung cancer (NSCLC) is a lethal and
aggressive malignancy that accounts for the majority of lung cancer
cases. It is known to have low sensitivity to chemotherapy and even
with optimal treatment, the mortality of NSCLC patients remains
unacceptably high (1); therefore, the
development of new treatment approaches for NSCLC is of great
importance.
NSCLC has been shown to be induced by interplay
between environmental factors and aberrant gene expression.
However, a considerable amount of work has been performed to
elucidate its genetic basis. The tyrosine-protein kinase receptor
(TYRO3), tyrosine-protein kinase receptor UFO (AXL) and MER
proto-oncogene tyrosine kinase (MER-TK) TAM receptor family, which
share a common ligand termed growth arrest-specific 6 (Gas6), are
characterized by a combination of 2 immunoglobulin-like domains,
dual fibronectin type III repeats in the extracellular region and a
cytoplasmic kinase domain. The activation of TAM signaling has been
reported to be involved in several biological processes, including
cell survival, proliferation, migration and adhesion (2). Belonging to the TAM subfamily, AXL, a
receptor tyrosine kinase that shares the same structure and
function with TYRO3 and MER, has been shown to be highly expressed
in cancers including blood, breast, ovarian and prostate cancers
(3–8).
However, to the best of our knowledge, its potential role has not
been fully addressed in NSCLC on a clinical level.
To fully understand its function in clinical
patients, the present study enrolled a cohort of patients with
NSCLC, of which the expression profile of AXL in cancer tissues and
normal tissues were compared. A significant differential expression
between tissues, and its association with clinical characteristics
is reported. In addition to the clinical study, the in vitro
experiments using AXL siRNA present consistency with the results of
the present study.
Materials and methods
Patients and specimens
All samples used for paraffin-embedded sections were
collected from the First Hospital of China Medical University
(Shenyang, China) between January 2003 and December 2004, and
consisted of a total number of 257 patients with surgically
resected NSCLC and lung tissue adjacent to carcinoma tissues. All
paracancerous lung tissues were at least 5 cm from the tumor edge.
Frozen tissue samples (35 pairs) were obtained between July and
December 2013 and kept in liquid nitrogen immediately following
surgical resection and stored in a −70°C refrigerator. None of the
patients received any preoperative anticancer treatment. Relevant
clinical data including gender, age, tumor size, location,
histological type, differentiation degree and lymph node metastasis
were collected. The sampling procedures in all cases were reviewed
and approved by the Ethics Committee of the Taizhou Hospital
(Taizhou, China). The pathological diagnosis was confirmed by ≥2
experienced pathologists.
Immunohistochemistry analysis
Paraffin-embedded tissue sections were
deparaffinized, rehydrated using xylene and a descending ethanol
series, and washed with PBS. Antigen retrieval was performed by
heating to 93°C for 15 min. Following 30 min of endogenous
peroxidase quenching and 30 min of blocking at 37°C (both
UltraSensitive™ SP kit; Maxim Biotech, Inc., Rockville, MD, USA),
samples were incubated with the primary anti-AXL rabbit polyclonal
antibody (1:100; cat. no. ab37861; Abcam, Cambridge, UK) overnight
at 4°C. Samples were subsequently washed with PBS and incubated
with a biotin-labeled secondary antibody for 30 min at 37°C, prior
to incubation with streptavidin-peroxidase (both UltraSensitive™ SP
kit) at 37°C for 30 min, according to the manufacturer's protocol.
3,3-Diaminobenzidine (DAB) reagent (Maxvision™ DAB kit; Maxim
Biotech, Inc.) was added for 45 sec to stain the samples. Images
were captured using an inverted microscope (IX53; Olympus
Corporation, Tokyo, Japan). The results were reported as the
product of staining density score and staining intensity score. To
determine the staining density score, which was defined as the
percentage of the positive staining area, samples with a staining
density <30% scored 1 point, samples with a staining density of
between 30 and 60% scored 2 points, and samples with a staining
density >60% scored 3 points. To determine the staining
intensity score, samples with no color or yellowish color scored 1
point, samples with brown staining scored 2 points, and samples
with dark brown staining scored 3 points. The results were
determined by 2 experienced pathologists, and the mean of the three
observations was taken to be the final score.
Cell culture
The 3 NSCLC cell lines used in the present study,
adenocarcinoma A549, adenocarcinoma H1299 and squamous cell
carcinomas SK-MES-1 were all obtained from the Cell Culture Center
of the Forth Hospital of China Medical University (Shenyang,
China). The H1299 cell line was cultured in RPMI-1640 medium
(Hyclone: GE Healthcare Life Sciences, Logan, UT, USA), and the
SK-MES-1 and A549 cell lines were cultured in Dulbecco's modified
Eagle's medium (Hyclone: GE Healthcare Life Sciences). The media
were supplied with 10% fetal bovine serum (Hyclone: GE Healthcare
Life Sciences) without antibiotics. Cells were cultured in a 37°C
incubator containing 5% CO2. The culture media were
changed every 2–3 days. Cells with 80% confluency were passaged.
Cells at 60–70% confluency were transfected with AXL-siRNA using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), Lipofectamine 2000 treatment alone was taken as the mock
control. The target sequences were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China) and are shown as follows:
AXL-siRNA sense, 5′-GGAGACCCGUUAUGGAGAATT-3′ and antisense,
5′-UUCUCCAUAACGGGUCUCCTT-3′; and negative control siRNA sense,
5′-GCGACGAUCUGCCUAAGAUdTdT-3′ and antisense
5′-AUCUUAGGCAGAUCGUCGCdTdT-3′.
Western blot analysis
The protein from the fresh tissue was isolated and
quantified using radioimmunoprecipitation assay buffer and a BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China), respectively. The protein was denatured with loading buffer
at 100°C. The western blot analysis was conducted using a SDS-PAGE
Gel kit (cat. no. P0012A) and the DAB Horseradish Peroxidase Color
Development kit (cat. no. P0203) (both Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. The
anti-AXL antibody was used at a dilution of 1:300. The mouse
monoclonal antibodies against β-actin (cat. no. sc-47778; 1:500)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The results were analyzed using the Gel-Pro Analyzer software
(version 4.0; Media Cybernetics, Inc., Rockville, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Quantitative analysis of AXL mRNA expression was
achieved by RT-qPCR; the total RNA of the samples was isolated by
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China), the
first strand of cDNA was synthesized by PrimeScript® RT
Master Mix Perfect Real Time (Takara Biotechnology Co., Ltd.), and
the amplification was assessed using SYBR® Premix Ex
Taq™ (Takara Biotechnology Co., Ltd.). All were completed according
the manufacturer's protocols. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as internal control. The relative
expression was calculated by the 2−ΔΔCq
method. The primers were synthesized by Takara, and their sequences
were as follows: AXL, forward, 5′-GCAACCTTCACCTACCGAGTTC-3′ and
reverse, 5′-GGCCAACATGGTGAAACCCT-3′; GAPDH, forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′.
The PCR amplification and data analysis were assessed using a
ThermoCycler Dice Real Time System (Takara Biotechnology Co.,
Ltd.). The conditions were as follows: 1 cycle of 95°C for 30 sec;
40 cycles of 95°C for 5 sec and 60°C for 30 sec; and 1 cycle 95°C
for 30 sec (Table I). Experiments
were performed in triplicate and the mean ΔCq values were used for
subsequent analysis.
 | Table I.Reverse transcription quantitative PCR
reaction conditions. |
Table I.
Reverse transcription quantitative PCR
reaction conditions.
| Condition | Initial
denaturation | Amplification
stage | Melt curve |
|---|
| Cycle number | 1 | 40 | 1 |
|
|
|
|
|
| Temperature, °C | 95 | 95 | 60 | 95 |
| Time, sec | 30 | 5 | 30 | 30 |
Cell proliferation and invasion
assays
Cells were plated into 24-well plates at a density
of 3×104 cells/well 1 day prior to transfection. Cell
culture medium was replaced with fresh RPMI-1640 medium containing
10% fetal bovine serum without antibiotics one day following
AXL-siRNA transfection. Following another 24, 48 and 72 h of
culture, the MTT Cell Proliferation Assay kit (American Type
Culture Collection, Manassas, VA, USA) was used to perform the MTT
assay, according to the manufacturer's protocol. Each group was
detected at a wavelength of 490 nm using a Model 680 Microplate
Reader (Bio-Rad, Hercules, CA, USA). Experiments were performed in
triplicate and the mean was used in the statistical analysis. The
Transwell invasion assay was performed using a 24-well, 8 µm pore
size Costar Transwell chamber system (Corning Life Sciences,
Lowell, MA, USA), according to the manufacturer's protocol. A total
of 1×105 cells/well were seeded in the upper chamber.
The number of cells in the lower chamber was counted in the lower
chamber following 36 h of culture for the transfection and control
groups. Experiments were performed in triplicate and the mean was
used in the statistical analysis.
Statistical analysis
The data were analyzed by SPSS 19.0 software (IBM
SPSS, Armonk, NY, USA); the means between two groups were compared
by paired t-test and Mann-Whitney U test, and the analysis
of the associations between AXL expression and clinical
characteristics was assessed by χ2 test or Fisher's
exact test. A two tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Differential expression of AXL in
NSCLC tissue and its clinical significance
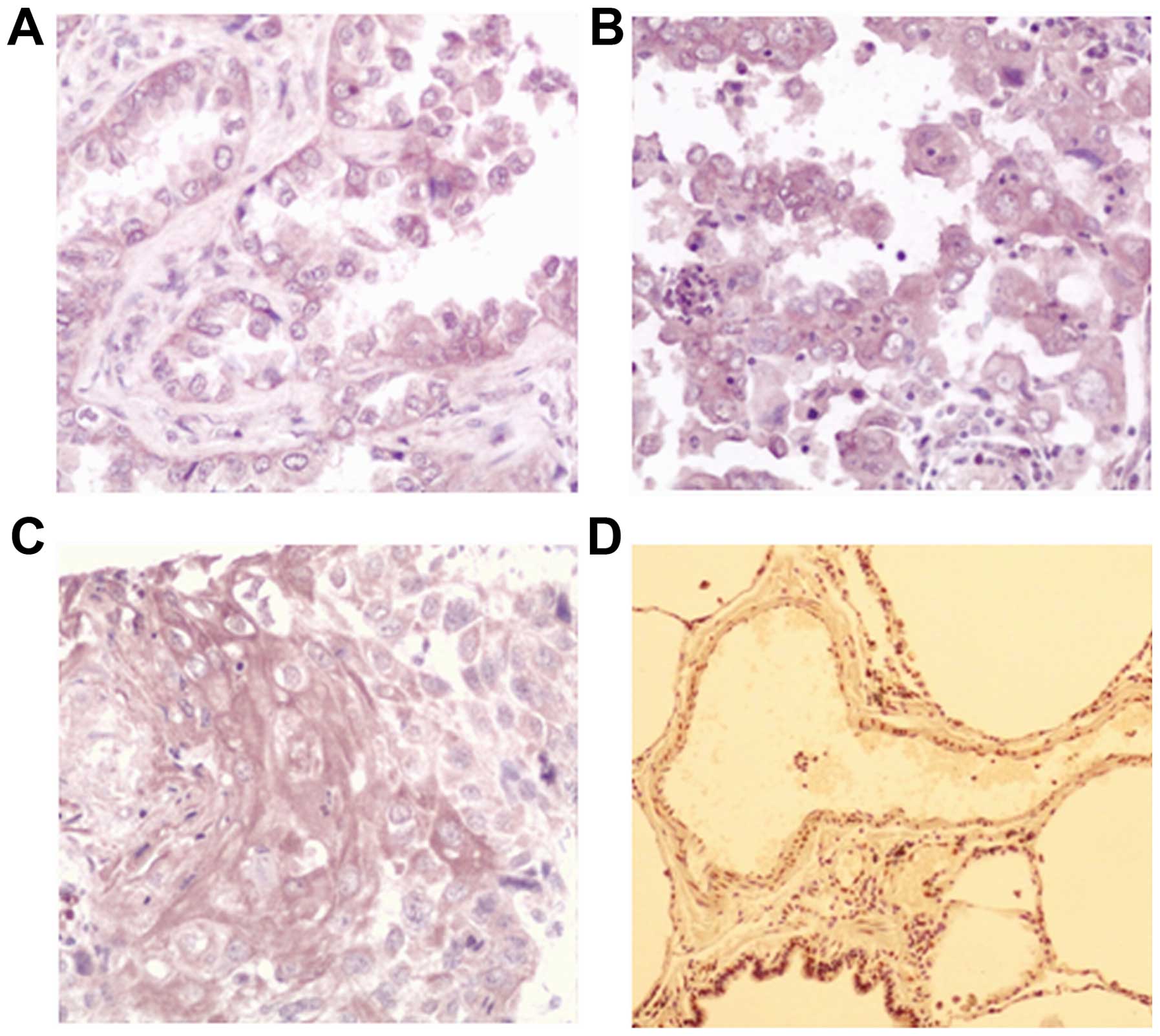
The results of the immunohistochemistry analysis are
shown in Fig. 1. The present study
found that the AXL staining was mainly located in the cytoplasm,
and a small portion of nuclear staining was also observed. Out of
the total 257 patients 147 showed positive staining for AXL, and
the average positive rate was 55.25%, which is significantly higher
than that of the adjacent lung tissue (26.85%; P=0.009; Table II).
 | Table II.Expression of AXL in tumor and
peritumoral tissues. |
Table II.
Expression of AXL in tumor and
peritumoral tissues.
|
|
| Expression of
AXL |
|
|
|---|
|
|
|
|
|
|
|---|
| Tissue | n | − | + | Positive rate | P-value |
|---|
| Tumor | 257 | 115 | 142 | 55.25% | 0.009a |
| Peritumoral | 257 | 188 | 69 | 26.85% |
|
The clinical characteristics and the statistical
analysis are shown in Table III.
The present study found that the expression level of AXL was
significantly associated with the degree of tumor differentiation.
The lower the differentiation of the tumor, the higher level of AXL
expressed (P=0.001). Patients with a stage higher than stage I
showed significantly higher expression level of AXL than those with
stage I (P=0.005). By contrast, no association between AXL
expression and age (P=0.722), gender (P=0.238), histological type
(P=1.000), lymph node metastasis (P=0.091) or tumor size (P=0.166)
was found.
 | Table III.Associations between the expression of
AXL and the clinical characteristics. |
Table III.
Associations between the expression of
AXL and the clinical characteristics.
|
| Expression of AXL,
n |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | − | + | Positive rate, % | P-valuea |
|---|
| Total | 257 |
|
|
|
|
| Gender |
|
|
|
| 0.238 |
| Male | 190 | 81 | 109 | 57.37 |
|
|
Female | 67 | 36 | 31 | 46.27 |
|
| Age |
|
|
|
| 0.722 |
| ≤60
years | 111 | 48 | 63 | 56.76 |
|
| >60
years | 146 | 69 | 77 | 52.74 |
|
| Histological
type |
|
|
|
| 1.000 |
|
Adenocarcinoma | 143 | 66 | 77 | 53.85 |
|
| Squamous
cell carcinoma | 114 | 51 | 63 | 55.26 |
|
| Differentiation |
|
|
|
| 0.001 |
| Well | 44 | 33 | 11 | 25.00 |
|
|
Moderate | 140 | 71 | 69 | 49.29 |
|
| Poor | 73 | 13 | 60 | 82.19 |
|
| TNM stage |
|
|
|
| 0.005 |
| Stage
I | 47 | 35 | 12 | 25.53 |
|
| >Stage
I | 210 | 83 | 127 | 60.48 |
|
| Lymph node
metastasis |
|
|
|
| 0.091 |
|
Yes | 115 | 44 | 71 | 61.74 |
|
| No | 142 | 73 | 69 | 48.59 |
|
| Tumor size |
|
|
|
| 0.166 |
| ≤3
cm | 92 | 50 | 42 | 45.65 |
|
| >3
cm | 165 | 67 | 98 | 59.39 |
|
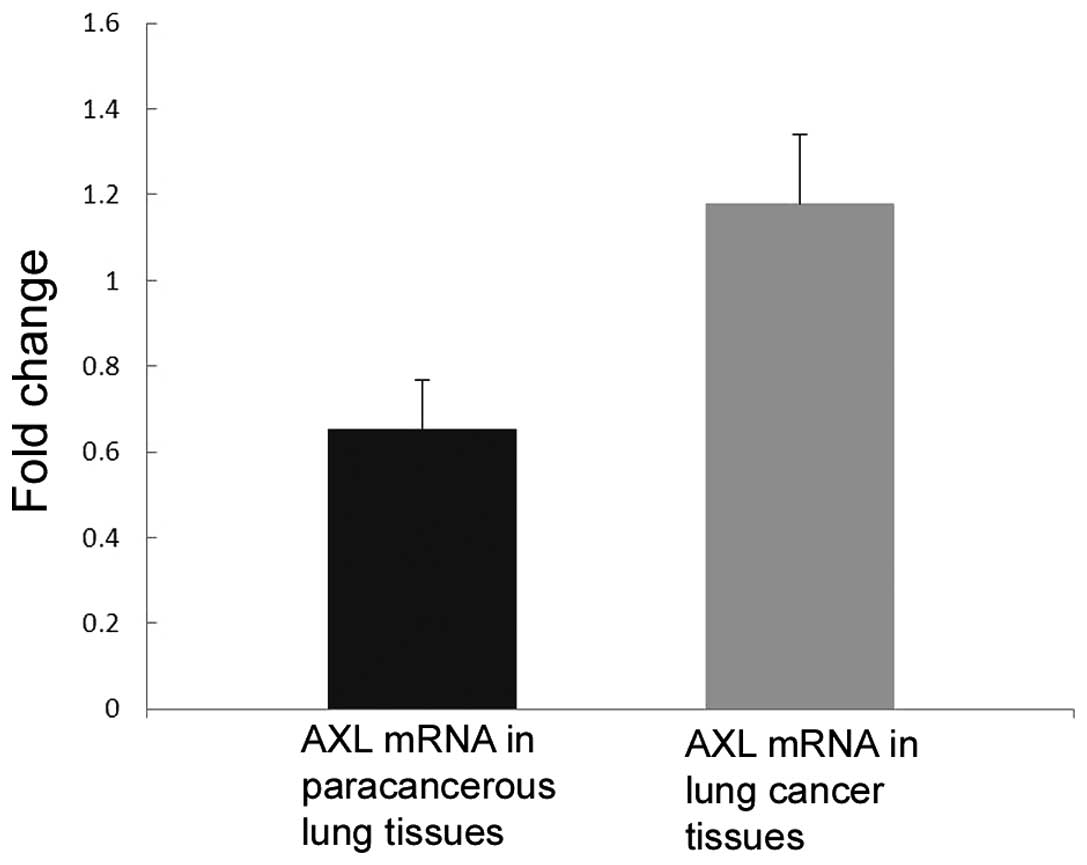
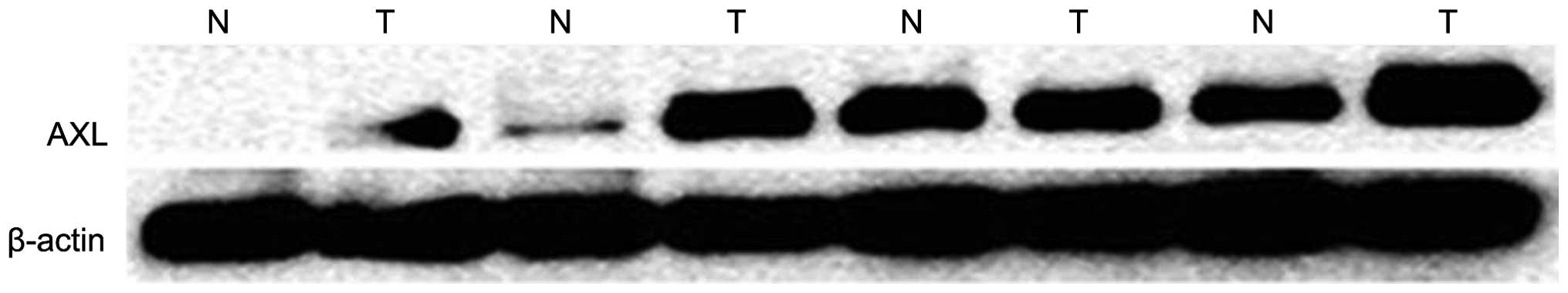
Next, the expression of AXL in fresh tissues was
detected by western blot analysis and qPCR. Consistent with the
immunohistochemistry results, the mRNA (P=0.037; Fig. 3) and protein (P=0.044; Fig. 2) levels of AXL were significantly
increased in NSCLC tissues compared with those in their adjacent
lung tissues.
Differential expression of AXL in
NSCLC cell lines
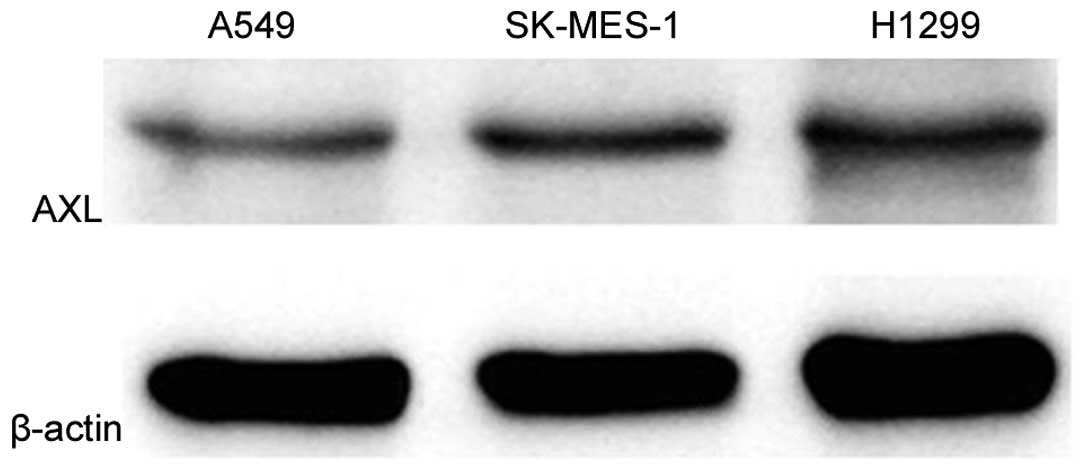
To investigate the function of AXL in vitro,
the current study first detected its expression in lung cancer cell
lines A549, H1299 and SK-MES-1 by western blot analysis and qPCR.
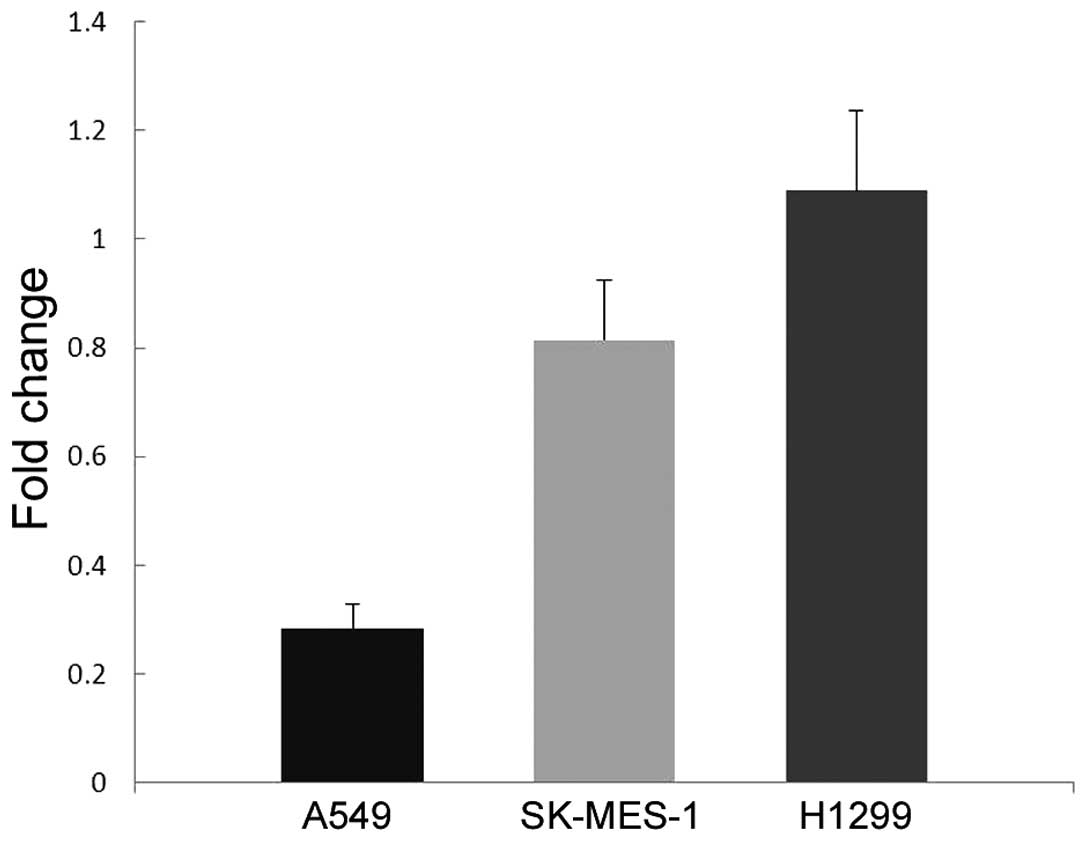
As shown in Fig. 4, H1299
demonstrated markedly higher level of AXL protein expression
compared with the other two cell lines. In addition, H1299 cells
demonstrated significantly increased AXL mRNA expression (P=0.003
vs. A549 cells; P=0.005 vs. SK-MES-1 cells; Fig. 5).
Effects of AXL-siRNA on the
proliferation and invasion activity of H1299 cells
Due to the relative high expression of H1299, a
methyl thiazolyl tetrazolium (MTT) assay was performed with a
Transwell migration assay to determine whether inhibition of AXL
expression could reduce its proliferation and invasion activity.
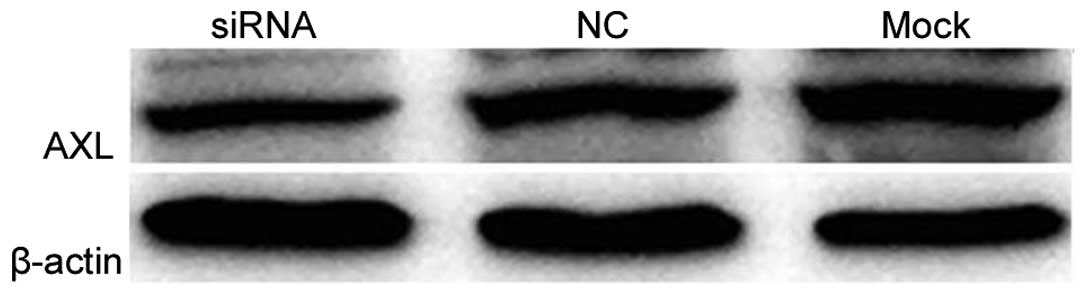
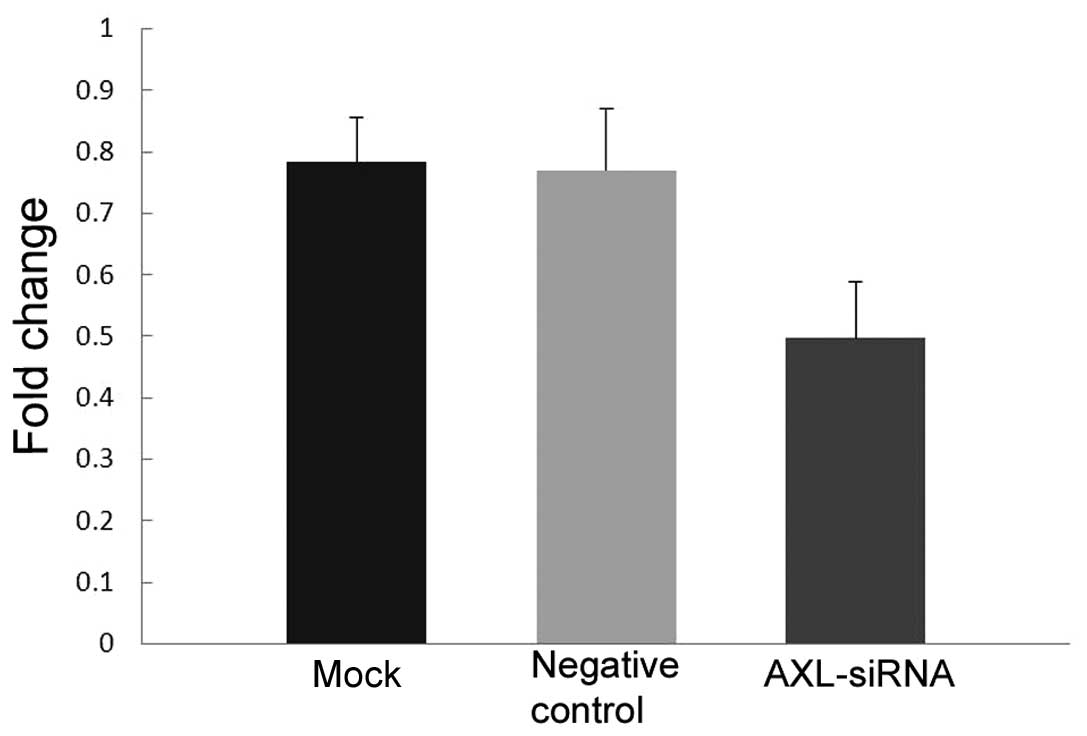
Western blotting and qPCR analysis confirmed the knockdown
efficiency of the AXL-siRNA. AXL protein expression was markedly
decreased (Fig. 6) and AXL mRNA
expression was significantly decreased (P=0.019 vs. the negative
control; P=0.013 vs. the mock transfection; Fig. 7) in H1299 cells following the
silencing of AXL by its specific siRNA.
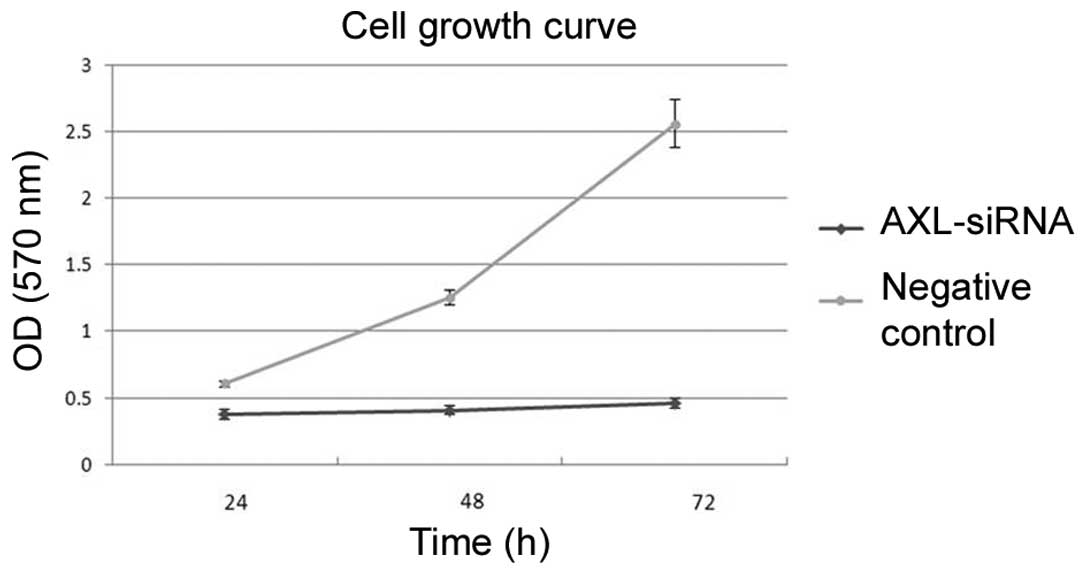
Subsequent to transfection, cells were incubated in
complete medium for another 24, 48 or 72 h. MTT results showed that
transfection with AXL-siRNA resulted in a significant decrease of
cell viability compared with the negative control at each time
point (P=0.007; Fig. 8). In addition,
the number of cells in the bottom layer of the culture was
significantly decreased following AXL-siRNA transfection compared
with the negative control (P=0.018). The number of cells that
migrated to the underside of the membranes was calculated by
counting 5 random separate fields, as shown by Transwell analysis
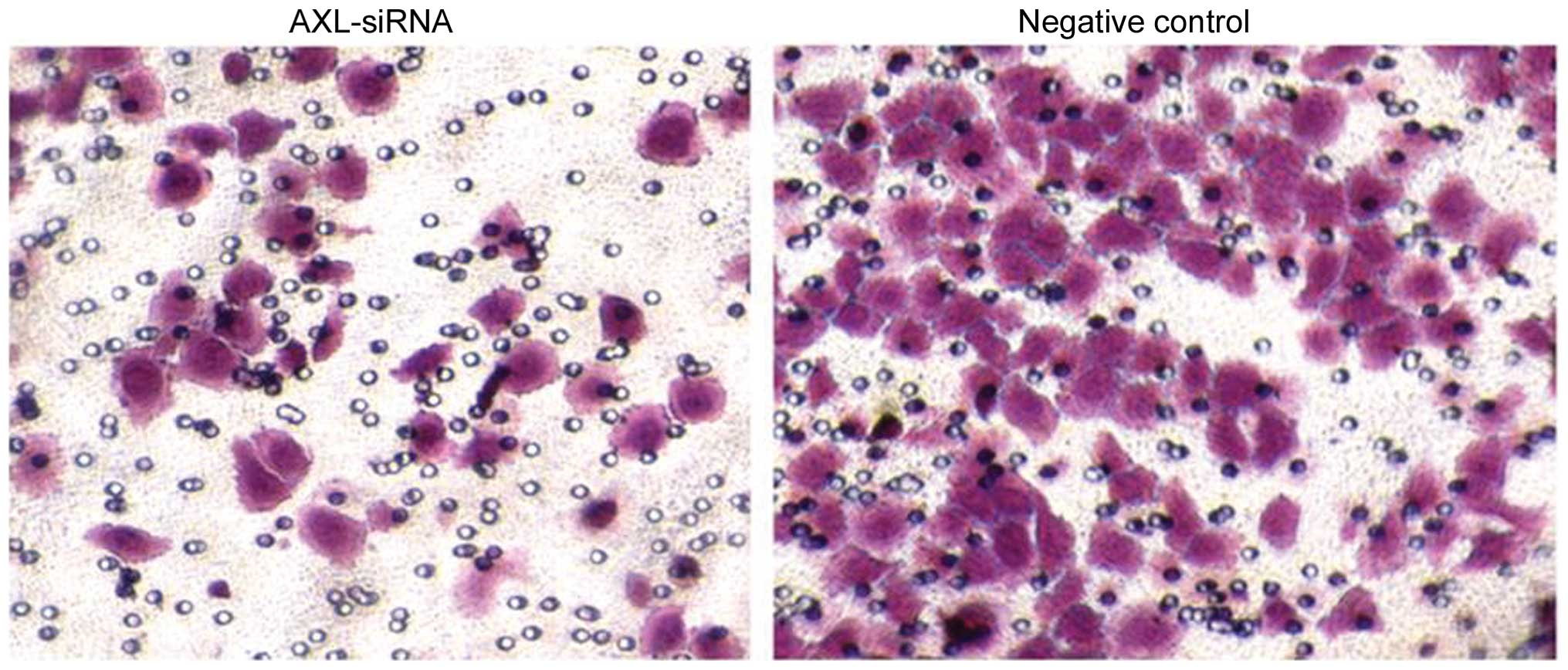
(Fig. 9), indicating the decreased
invasion activity subsequent to AXL inhibition.
Discussion
In the present study, the expression of AXL was
detected in the paraffin sections of NSCLC tissues. Consistent with
the feature of receptor tyrosine kinases, it was found that the
expression of AXL was largely localized in the cytoplasm, with the
exception of a small proportion of nuclear localization. More
importantly, the expression of AXL was found to be significantly
higher in tumor tissues compared with adjacent lung tissues in the
two paraffin sections and fresh tissues, which is in accordance
with the findings of a previous study (9). Subsequent to reviewing the clinical
characteristics statistically, the present study found a
significant association between high AXL expression and low
differentiation grade. As atypical histopathology forms an
important part of the low differentiation state, the current study
suggests that the higher AXL expression may be associated to an
atypical phenotype of NSCLC cells, thus represents a cancer type
with aggressive invasion and poor prognosis. Similarly, the present
data also revealed a strong association between high AXL expression
and tumor Union for International Cancer Control TNM Classification
of Malignant Tumors stage (10),
patients with a disease stage >I showed an overall higher AXL
expression compared with stage I patients. The current clinical
data therefore preliminarily demonstrated that AXL may be a risk
factor associated with the prognosis of NSCLC patients.
Demarchi et al demonstrated the
anti-apoptotic function of AXL by activating the nuclear factor κB
(NF-κB) pathway in NIH3T3 fibroblast cells (11). Shankar et al reported the
synergic effect of Gas6 on AXL in the regulation of apoptosis, and
found that Gas6 promoted cell survival and decreased apoptosis in
AXL-expressing oligodendrocytes in the presence of growth factor
deprivation and tumor necrosis factor-cytotoxicity (12). In addition to these findings, Gas6/AXL
activation has been shown to be involved in the processes of
proliferation and apoptosis of human pulmonary artery endothelial
cells, human umbilical vein smooth muscle cells and human umbilical
vein endothelial cells by several mechanisms including protein
kinase B activation, NF-κB phosphorylation, B-cell lymphoma 2
upregulation and caspase-3 suppression (13–15).
A number of studies have also highlighted the
significant role of AXL in lung cancer cell invasion and
metastasis. Tai et al (16)
suggested that NF-kB dependent matrix metalloproteinase-9
activation mediates the pro-metastatic effect of AXL on lung cancer
cells. Particularly, by co-immunoprecipitation and RNAi methods,
Vaughan et al (17)
demonstrated that mutated p53 is responsible for increased AXL
expression, and that increased expression may have accounted for
the enhancement of mitogenic activity. In the present study, since
the AXL expression was significantly increased in H1299 cells
compared with other NSCLC cell lines, the effect of AXL knockdown
on H1299 cell proliferation and migration was investigated. The
current data indicated the detrimental effect of AXL on cancer
cells, as exemplified by the decreased proliferation and migration
capacity subsequent to knockdown of AXL.
In summary, the present study reported aberrantly
higher expression of AXL in NSCLC tissues, and this expression
profile is prominently associated with the histological grade and
clinical stage, which offered insights into its potential
prognostic value. Previous studies have shed new light on AXL-based
targeted therapy; however, additional elucidations on the molecular
regulatory network of AXL in the development and progression of
lung cancer are required to facilitate its application in
translational medical research.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verma A, Warner SL, Vankayalapati H,
Bearss DJ and Sharma S: Targeting Axl and Mer kinases in cancer.
Mol Cancer Ther. 10:1763–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J,
Kallop D, Ludlam MJ and Pei L: Axl as a potential therapeutic
target in cancer: Role of Axl in tumor growth, metastasis and
angiogenesis. Oncogene. 28:3442–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong CC, Lay JD, Huang JS, Cheng AL, Tang
JL, Lin MT, Lai GM and Chuang SE: Receptor tyrosine kinase AXL is
induced by chemotherapy drugs and overexpression of AXL confers
drug resistance in acute myeloid leukemia. Cancer Lett.
268:314–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Greger J, Shi H, Liu Y, Greshock J,
Annan R, Halsey W, Sathe GM, Martin AM and Gilmer TM: Novel
mechanism of lapatinib resistance in HER2-positive breast tumor
cells: Activation of AXL. Cancer Res. 69:6871–6878. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunne PD, McArt DG, Blayney JK, Kalimutho
M, Greer S, Wang T, Srivastava S, Ong CW, Arthur K, Loughrey M, et
al: AXL is a key regulator of inherent and chemotherapy-induced
invasion and predicts a poor clinical outcome in early-stage colon
cancer. Clin Cancer Res. 20:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA and Giaccia AJ: AXL is
an essential factor and therapeutic target for metastatic ovarian
cancer. Cancer Res. 70:7570–7579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu
YW, Lee HS and Wu CW: Expression of axl in lung adenocarcinoma and
correlation with tumor progression. Neoplasia. 7:1058–1064. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
international union against cancer and the American Joint Committee
on cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demarchi F, Verardo R, Varnum B,
Brancolini C and Schneider C: Gas6 anti-apoptotic signaling
requires NF-kappa B activation. J Biol Chem. 276:31738–31744. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shankar SL, O'Guin K, Kim M, Varnum B,
Lemke G, Brosnan CF and Shafit-Zagardo B: Gas6/Axl signaling
activates the phosphatidylinositol 3-kinase/Akt1 survival pathway
to protect oligodendrocytes from tumor necrosis factor
alpha-induced apoptosis. J Neurosci. 26:5638–5648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melaragno MG, Cavet ME, Yan C, Tai LK, Jin
ZG, Haendeler J and Berk BC: Gas6 inhibits apoptosis in vascular
smooth muscle: Role of Axl kinase and Akt. J Mol Cell Cardiol.
37:881–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WP, Wen Y, Varnum B and Hung MC: Akt
is required for Axl-Gas6 signaling to protect cells from
E1A-mediated apoptosis. Oncogene. 21:329–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasanbasic I, Cuerquis J, Varnum B and
Blostein MD: Intracellular signaling pathways involved in
Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart
Circ Physiol. 287:H1207–H1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai KY, Shieh YS, Lee CS, Shiah SG and Wu
CW: Axl promotes cell invasion by inducing MMP-9 activity through
activation of NF-kappaB and Brg-1. Oncogene. 27:4044–4055. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaughan CA, Singh S, Windle B, Yeudall WA,
Frum R, Grossman SR, Deb SP and Deb S: Gain-of-function activity of
mutant p53 in lung cancer through Up-regulation of receptor protein
tyrosine kinase Axl. Genes Cancer. 3:491–502. 2012. View Article : Google Scholar : PubMed/NCBI
|