Introduction
Breast cancer is one of the most prevalent
carcinomas and is the second leading cause of mortality in women
worldwide, with more than one million cases occurring and more than
400,000 cases of associated mortality annually worldwide (1). Breast cancer is a heterogeneous disease,
which can be further divided into subtypes via their
histopatological and gene expression profiles. In particular,
triple-negative breast cancers (TNBCs) are among the most
aggressive and treatment-resistant breast subtypes. TNBC comprises
~15–20% of all breast cancer cases (2), and is defined as breast carcinoma that
does not express the estrogen receptor (ER), progesterone receptor
(PR) or human epidermal growth factor receptor type 2 (HER2). TNBC
is characterized by invasive potential, aggressive behaviors with a
high recurrence rate and poor prognosis. However, the driving
factors underlying TNBC invasion remain poorly defined and a better
understanding of TNBC invasion mechanisms is required for the
development of rational strategies for the prevention and treatment
of TNBC recurrence.
Cancerous inhibitor of protein phosphatase 2A
(CIP2A), a recently identified human oncoprotein that stabilizes
c-Myc by inhibiting protein phosphatase 2A-mediated
dephosphorylation of Myc at serine 62 (3). Previous studies have shown that CIP2A
serves a critical role in the progression of several cancer types,
including head and neck squamous cell carcinoma, oral squamous cell
carcinoma, oesophageal squamous cell carcinoma, colon, gastric,
breast, prostate, tongue, lung, cervical cancer and acute myeloid
leukaemia (3–9). In 2009, Côme et al (10) demonstrated that CIP2A is associated
with clinical aggressivity in human breast cancer and promotes the
malignant growth of breast cancer cells (10). Then in 2012, Tseng et al
(11) found that CIP2A is a target of
the proteasome inhibitor bortezomib in human TNSC cells. However,
the expression and the role that CIP2A serves in pathogenesis of
human TNBC requires further investigation.
In the present study, the expression and the
functional role of CIP2A in TNSC cells is examined. The results
show that CIP2A is overexpressed in TNBC cell lines. CIP2A
depletion led to proliferation and clonogenic activity inhibition
of TNBC cell lines MDA-MB-231 and MDA-MB-468. Interestingly, CIP2A
depletion in TNBC cells induced autophagy and apoptosis. In
addition, the invasive behavior of MDA-MB-231 cells was examined by
CIP2A small interfering (si)siRNA, and found that CIP2A depletion
inhibits the invasion and migration of MDA-MB-231. Previously,
CIP2A has been shown to be an oncoprotein capable of modulating
phosphorylated-Akt (pAkt) (9,12). Results of the present study
demonstrated that CIP2A depletion inhibits phosphorylation of Akt
and its downstream molecules, mechanistic target of rapamycin
(mTOR) and p70 ribosomal protein S6 kinase (P70S6K). The protein
kinase mTOR is an Akt signaling protein and a critical regulator of
cellular metabolism, growth and proliferation, with p70S6K1 and
4E-BP1 (eIF4E binding protein 1) as two important effectors
(13). These results suggest that
CIP2A promotes invasion and migration of TNSCs through
Akt/mTOR/P70S6K signaling pathways, therefore the function of CIP2A
in TNSC warrants further investigation.
Materials and methods
Cell culture
Human breast cancer cell lines MCF-7
(ER+/PR+/HER2-), MDA-MB-231 (ER-/PR-/HER2-), MDA-MB-468
(ER-/PR-/HER2-) and BT549 (ER-/PR-/HER2-) were obtained from
American Type Culture Collection (Manassas, VA, USA). MCF-7 and
BT549 cells were maintained in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Chalfont, UK) and antibiotics, and
incubated in a humidified atmosphere with 5% CO2 at
37°C. MDA-MB-231 and MDA-MB-468 cells were maintained in
Leibovitz's-15 (L-15) (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and antibiotics and incubated in a
humidified atmosphere without CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression of the CIP2A gene was examined by
quantitative polymerase chain reaction (PCR) normalized to the
expression of GAPDH. Total RNA was extracted from cell lines or
patients' cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed using SYBR Premix Ex Taq (Perfect Real Time;
Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol (14). RT-qPCR analysis of
CIP2A was performed with 2 µg of total RNA and ReverTra Ace
qPCR RT kit (Toyobo Co., Ltd., Osaka, Japan). The reverse
transcription conditions were as follows: 37°C for 15 min and 98°C
for 5 min, followed by storage at −20°C. For qPCR, the following
primers were used: CIP2A forward,
5′-5′-TGCGGCACTTGGAGGTAATTTC-3′ and reverse,
5′-AGCTCTACAAGGCAACTCAAGC-3′; and GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse 5′-CTCCACGACGTACTCAGCG-3′.
RT-qPCR was performed in an ABI StepOnePlus™ Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction
mix contained: 10 µl SYBR Green PCR Master mix, 200 nm forward and
reverse primers, 100 ng cDNA template and ddH2O up to 20
µl volume. The PCR cycling conditions consisted of the following:
94°C for 3 min for denaturation, 94°C for 30 sec for annealing and
58°C for 40 sec for extension, for a total of 40 cycles. The
threshold cycle for each sample was selected from the linear range
and converted to a starting quantity by interpolation from a
standard curve generated on the same plate for each set of primers.
The CIP2A mRNA levels were normalized for each well to GAPDH mRNA
levels using the 2−ΔΔCq method (15). Each experiment was repeated three
times.
Western blot
Cell pellets were lysed in radioimmunoprecipitation
assay buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS,
0.5% deoxycholate, 1% NP-40, 1 mM DTT, 1 mM NaF, 1 mM sodium
vanadate, 1 mM PMSF (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) and 1% protease inhibitors cocktail (Merck Millipore).
Protein extracts were quantified and loaded (25 µg) on 8–12% sodium
dodecyl sulfate polyacrylamide gel, electrophoresed, and
transferred to a polyvinylidene difluoride membrane (Merck
Millipore). The membrane was blocked with 5% skimmed milk at room
temperature for 1 h. The membrane was incubated with primary
antibodies overnight at 4°C, followed by washing with TBST.
Subsequently, membranes were incubated with goat anti-rabbit or
anti-mouse horseradish peroxidase-conjugated secondary antibody
(1:10,000; cat. no., E030120-01 and E030110-01; EarthOx Life
Sciences, Millbrae, CA, USA) at room temperature for 1.5 h.
Detection was performed by using a SuperSignal® West
Pico Trial kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
(16). The defined sections of the
film were scanned for image capture and quantification using Adobe
Photoshop software (CS4; Adobe Systems, Inc., San Jose, CA, USA)
and ImageJ software (National Institutes of Health, Bethesda, MD,
USA). The primary antibodies used were anti-CIP2A (1:1,000; cat.
no. sc-80662; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-phospho-Akt (1:500; cat. no. sc-7985; Santa Cruz
Biotechnology, Inc.), anti-Akt (1:500; cat no. sc-8312; Santa Cruz
Biotechnology, Inc.), anti-c-Myc (1:500; cat. no. sc-788; Santa
Cruz Biotechnology, Inc.) anti-caspase-3 (1:1,000; cat. no. 9662;
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-poly ADP
ribose polymerase (PARP) (1:1,000; cat. no. 9542; Cell Signaling
Technology, Inc.), anti-LC-3 (1:1,000; cat no. 2775; Cell Signaling
Technology, Inc.), anti-phospho-P70S6K (1:1,000; cat. no. 2983;
Cell Signaling Technology, Inc.), anti-P70S6K (1:1,000; cat. no.
2708; Cell Signaling Technology, Inc.), anti-phospho-mTOR (1:1,000;
cat. no. 5536), anti-mTOR (1:1,000; cat. no. 2973; Cell Signaling
Technology, Inc.) and anti-GAPDH (1:5,000; cat. no. AB10016; Sangon
Biotech Co., Ltd., Shanghai, China).
Transfection of siRNA
Two siRNA targeting CIP2A were designed and
synthesized by Shanghai GenePharma Co. (Shanghai, China), referred
to as siRNA1 and siRNA2. The siRNA sequences were as follows:
5′-CUGUGGUUGUGUUUGCACUTT-3′ (CIP2A siRNA1),
5′-ACCAUUGAUAUCCUUAGAATT-3′ (CIP2A siRNA2) and
5′-UUCUCCGAACGUGUCACGUTT-3′ [negative control (NC) siRNA].
Using lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol,
MDA-MB-231 and MDA-MB-468 cells were transfected with 100 nM siRNA.
And 48 h transfection, the cells were harvested for western blot,
cell viability, soft-agar colony formation assay, invasion assay
and wound healing assay, as previously described (5,17).
Cell viability
Cell viability was estimated by trypan blue dye
exclusion, as previously described (18). Briefly, a 0.4% solution of trypan blue
was prepared in phosphate-buffered saline (PBS; pH 7.2–7.3). Then,
0.1 ml trypan blue stock solution was added to 1 ml cell
suspension. A hemacytometer was then loaded with the samples and
they were examined immediately under a microscope at low
magnification (IX70; Olympus Corporation, Tokyo, Japan). The number
of blue stained cells and the number of total cells were counted.
Cell viability should be ≥95% for healthy log-phase cultures.
Percentage viable cells = [1.00 - (Number blue cells / number total
cells)] × 100. To calculate the number of viable cells per ml of
culture, the following formula was used: Number of viable cells ×
(104 × 1.1) = cells/ml culture.
Soft agar colony formation assay
Cells were suspended in 1 ml L-15 containing 0.3%
low-melting-point agarose (Amresco Inc., Farmingham, MA, USA) and
10% FBS, and plated on a bottom layer containing 0.6% agarose and
10% FBS in 6-well plate in triplicate. After 2 weeks, plates were
stained with 0.2% gentian violet and the colonies were counted
under light microscope (5).
Invasion assay
An invasion assay was performed using a 24-well
plate (Corning). A polyvinyl-pyrrolidone-free polycarbonate filter
(8 µm pore size) (Corning, Inc., Corning, NY, USA) was coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). The lower
chamber was filled with medium containing 20% FBS as a
chemoattractant agent. The coated filter and upper chamber were
laid over the lower chamber. Cell suspension (1×104
cells/well) was seeded onto the upper chamber wells. After
incubation for 20 h at 37°C, the filter was fixed and stained with
2% ethanol containing 0.2% crystal violet (15 min). After drying,
the stained cells were enumerated under a light microscope at 10x
objective. For quantification, the invaded stained cells on the
other side of the membrane were extracted with 33% acetic acid. The
absorbance of the eluted stain was determined at 570 nm.
Wound healing assay
Cells (4×105 cells/2 ml) were seeded in a
6-well plate and incubated at 37°C until 90 to 100% confluent.
After the confluent cells were scratched with a 200 µl pipet tip,
followed by washing with PBS, they were then re-suspended in
complete medium. After 24 h incubation, the cells were fixed and
stained with 2% ethanol containing 0.2% crystal violet powder (15
min), and randomly chosen fields were photographed under a light
microscope at 4x objective. The number of cells migrated into the
scratched area was calculated.
Statistical analysis
All experiments were repeated at least three times
and the data are presented as the mean ± standard deviation unless
noted otherwise. Data were analyzed using SPSS version 17.0 for
Windows (SPSS, Inc., Chicago, IL, USA). All experiments were
repeated at least 3 times and the data are presented as the mean ±
standard deviation unless noted otherwise. Differences between data
groups were evaluated for significance using Student's t-test of
unpaired data of one way analysis of variance, followed by the
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CIP2A is overexpressed in TNSC cell
lines
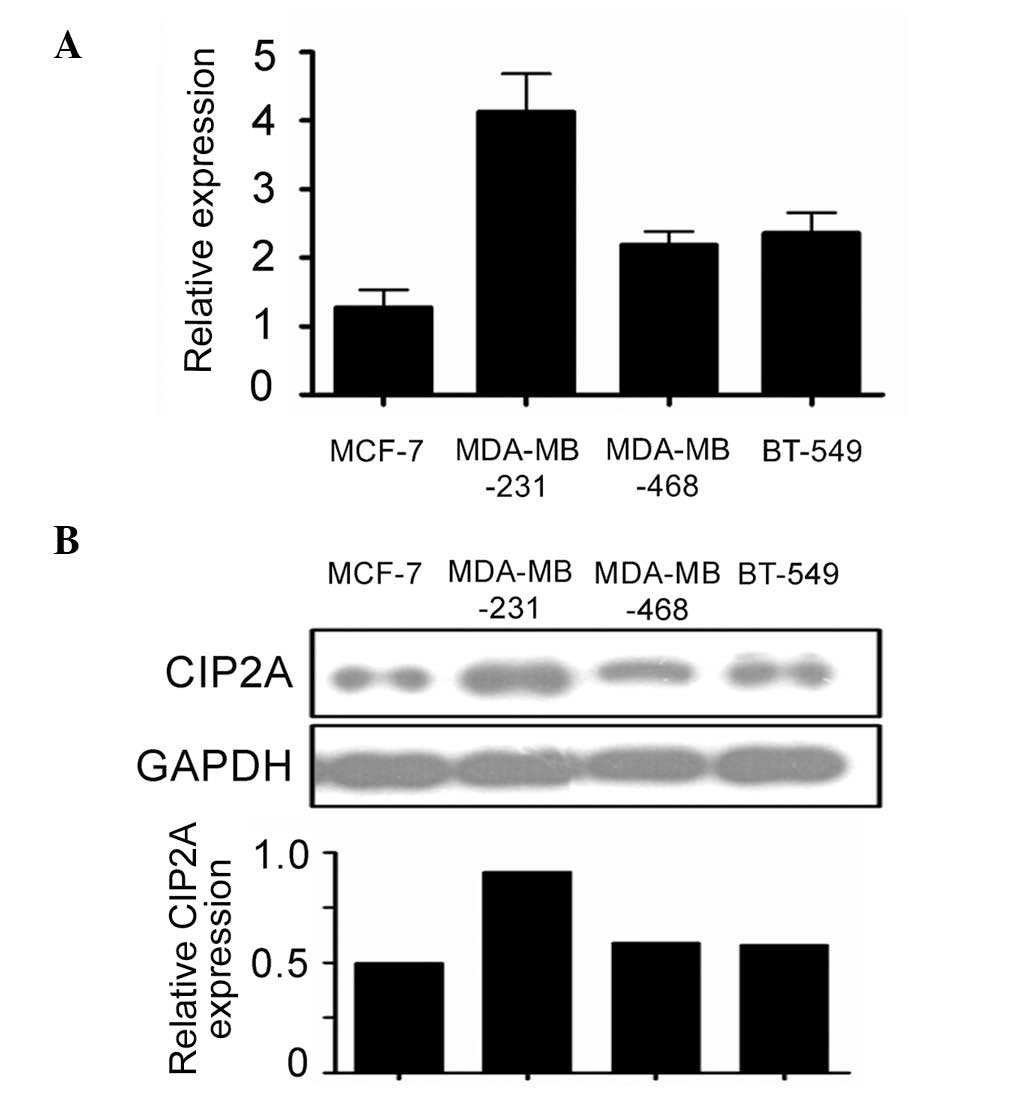
CIP2A expression was studied by RT-qPCR and western
blot analysis in TNSC cell lines MDA-MB-231, MDA-MB-468, and BT-549
cells. The poorly invasive ER+ breast cancer line MCF-7 was used as
a positive control. The results show that CIP2A is expressed in
BNSC cell lines at mRNA level. Interestingly, highly invasive TNSC
breast cancer cells have increased CIP2A expression compared with
poorly invasive ER+ MCF-7 cells (Fig.
1A). Consistent with mRNA expression, western blot analysis of
the protein level of CIP2A revealed that highly invasive TNSC cells
have increased CIP2A expression compared with poorly invasive ER+
MCF-7 cells (Fig. 1B). The results
show that CIP2A is overexpressed in TNSC cells, and that higher
expression of CIP2A in highly invasive TNSC cells may contribute
towards invasion.
CIP2A depletion in TNSC cell lines
inhibits cell proliferation and clonogenic activity
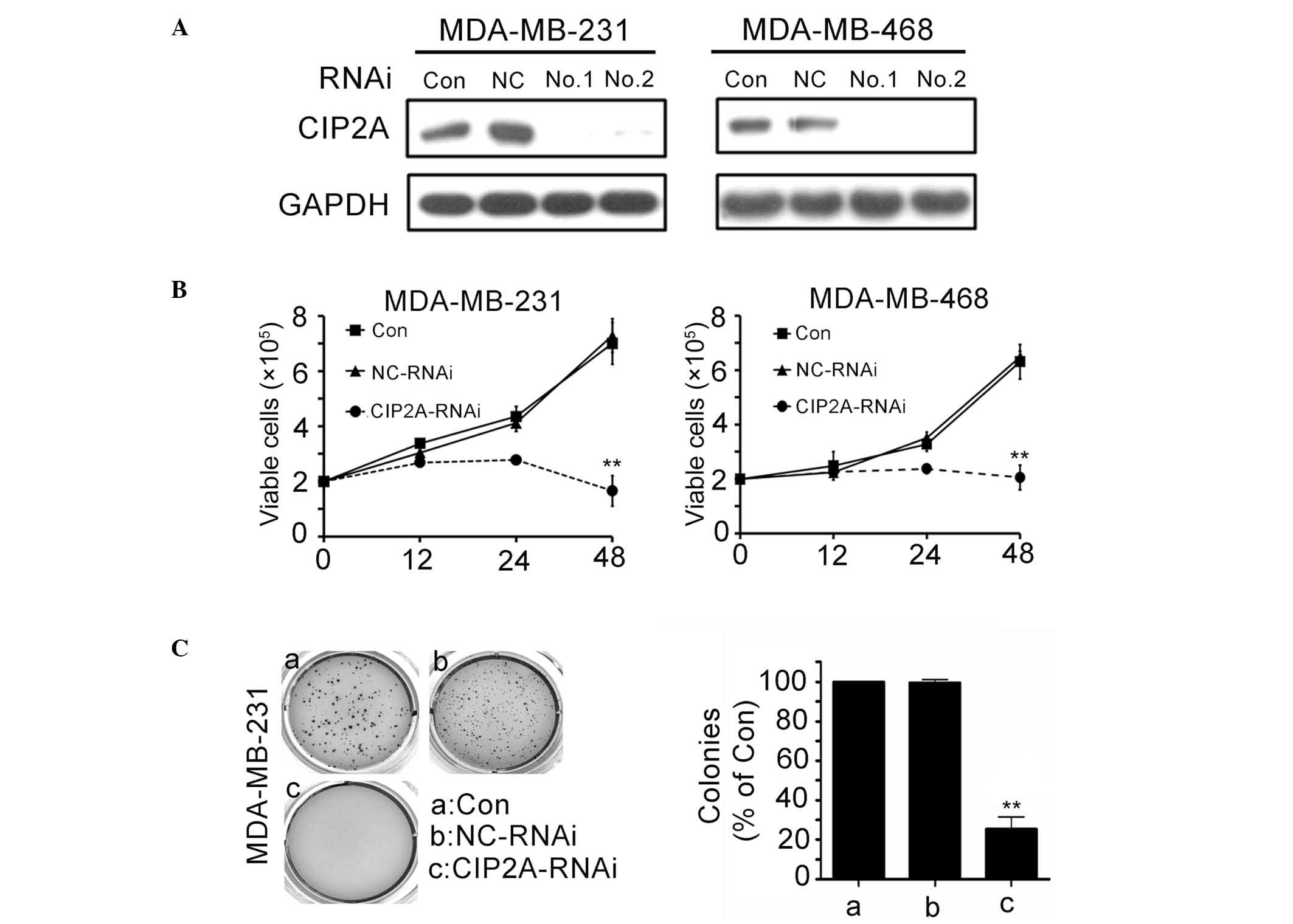
To evaluate the role that CIP2A overexpression
serves in TNSC cells, MDA-MB-231 and MDA-MB-468 cells were
transfected with siRNA1 or siRNA2 targeting CIP2A (Fig. 2A). CIP2A silencing significantly
inhibited proliferation of MDA-MB-231 and MDA-MB-468 cells
(Fig. 2B; P<0.01). The
proliferation rate was determined by trypan blue dye exclusion
assay. Consistent with the trypan blue dye exclusion results,
colony formation assay showed that CIP2A knockdown in MDA-MB-231
cells led to a significant decrease in focus numbers (Fig. 2C; P<0.01). These data demonstrate
that CIP2A serves a critical role in TNSC cell proliferation.
CIP2A depletion in TNBC cells induces
apoptosis and autophagy
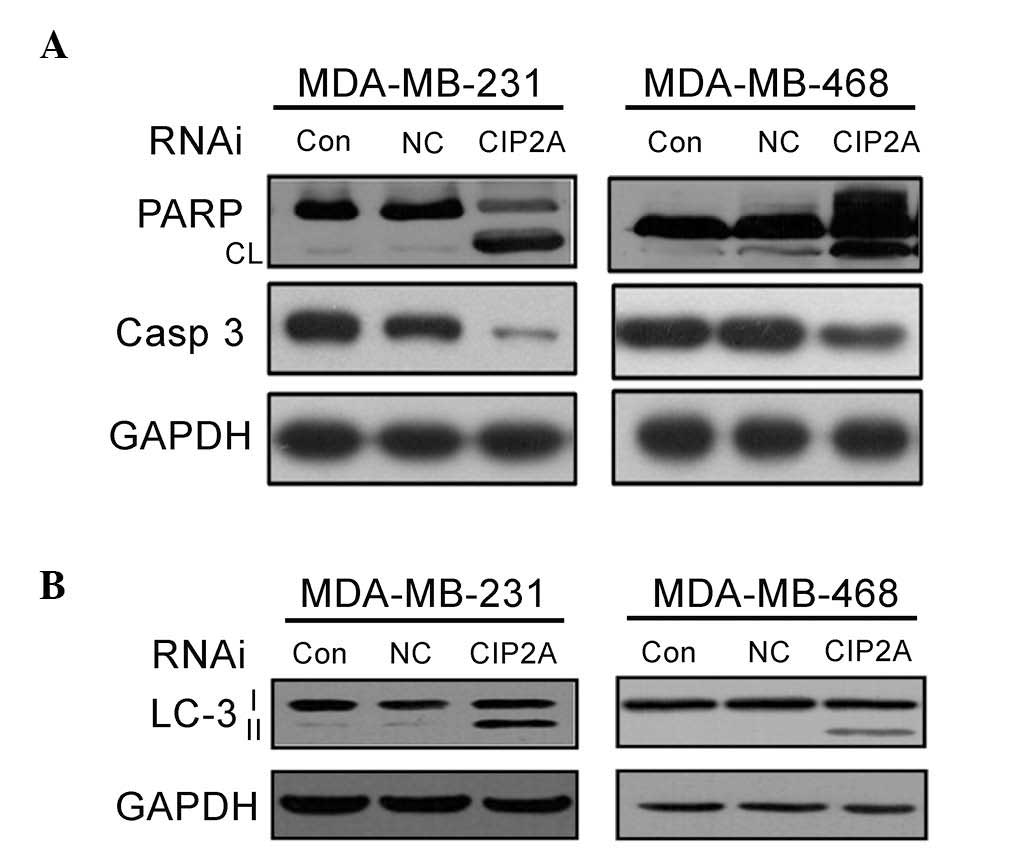
To determine which cell death pathway was induced by
CIP2A depletion, MDA-MB-231 and MDA-MB-468 cells were treated with
CIP2A siRNA for 48 h and apoptosis proteins were measured using
western blot analysis. As presented in Fig. 3A, the results showed that PARP
cleavage, as well as cleaved-caspases-3, were detected in CIP2A
depleted MDA-MB-231 and MDA-MB-468 cells. These results indicate
that the apoptosis pathway is primarily activated along with
caspase-dependent apoptosis in BNSC cells following CIP2A
depletion. To assess whether autophagy is also involved in CIP2A
siRNA-induced cell death, the expression level of LC-3 II in cells
treated with CIP2A siRNA was subsequently analyzed. Interestingly,
a marked increase in LC-3 II was observed following CIP2A siRNA
treatment for 48 h (Fig. 4B). These
findings suggest that CIP2A is associated with proliferation,
apoptosis and autophagy.
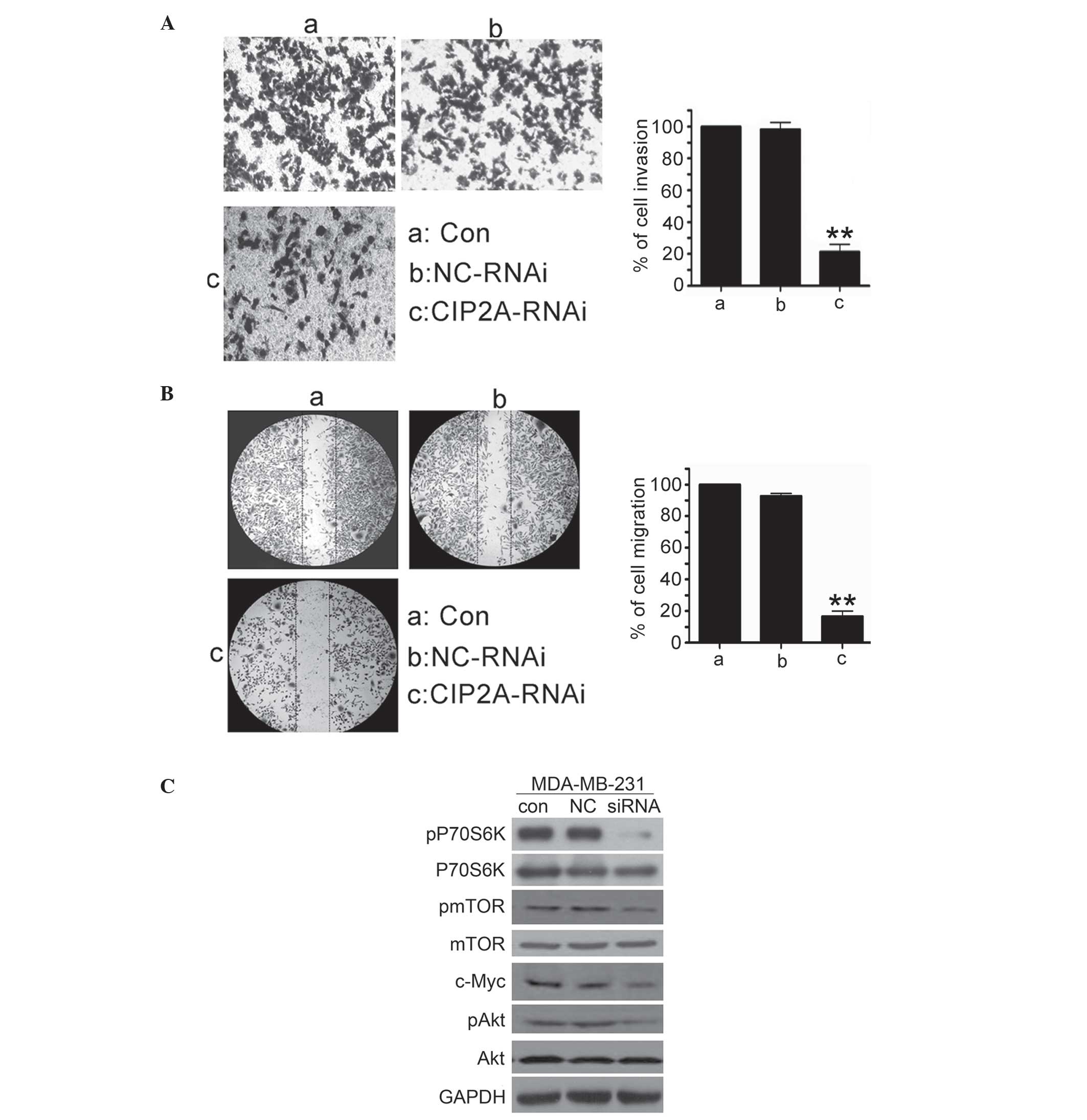 | Figure 4.CIP2A depletion in triple negative
breast cancer cells inhibits cell invasive behavior and
Akt/mTOR/P70S6K phosphorylation. (A) MDA-MB-231 cells were
transfected with 100 nM CIP2A-specific siRNA or NC siRNA for 48 h.
To evaluate cell invasion, the cells were analyzed for 20 h by
invasion assay. (B) MDA-MB-231 cells were transfected with 100 nM
CIP2A-specific siRNA or NC siRNA for 48 h. To evaluate cell
migration, the cells were analyzed for 24 h by wound healing assay.
(C) MDA-MB-231 cells were transfected with 100 nM CIP2A-specific
siRNA or NC siRNA for 48 h, then harvested for western blot
analysis. con, control; CIP2A, cancerous inhibitor of protein
phosphatase 2A; NC, negative control; RNAi, interfering RNA; siRNA,
small interfering RNA; P70S6K, p70 ribosomal protein S6 kinase;
pP70S6K, phosphorylated P70S6K; mTOR, mechanistic target of
rapamycin; pmTOR, phosphorylated mTOR; pAkt, phosphorylated
Akt. |
CIP2A depletion in TNSC cells inhibits
cell invasive behavior and Akt/mTOR/P70S6K phosphorylation
Considering that highly invasive TNSC cells have
higher CIP2A expression than poorly invasive MCF-7 cells, it was
determined whether CIP2A depletion inhibited the invasive behavior
of breast cancer cells. An invasion assay was performed in highly
invasive MDA-MB-231 cells using Matrigel-coated 24-well
microchemotaxis chambers. As shown in Fig. 3A, MDA-MB-231 cells were treated with
CIP2A siRNA (100 nM), and cell invasion was determined after 20 h.
CIP2A depletion markedly suppressed the invasion of MDA-MB-231
cells. In addition, the effect of CIP2A depletion on migration was
explored. MDA-MB-231 cells were treated with CIP2A siRNA (100 nM),
and cell migration was determined after 48 h. As shown in Fig. 3B, CIP2A depletion significantly
decreased MDA-MB-231 cell migration. To investigate the mechanism
underlying invasion and migration inhibition, the effect of CIP2A
knockdown on c-Myc, Akt, mTOR and P70S6K levels was explored. The
results indicated that depletion of CIP2A by siRNA resulted in
inhibition of c-Myc protein expression, Akt, mTOR and P70S6K
phosphorylation in MDA-MB-231 cells (Fig.
4C). These results indicated that CIP2A promotes TNSC cell
invasion through the c-Myc upregulatory effect on c-Myc expression,
and through the activation of the Akt/mTOR/P70S6K signaling
pathway.
Discussion
As one of the most common human malignancies, breast
cancer remains a challenging disease. TNBC are characterized by
occurrence in younger women, aggressive behaviors with a metastasis
potential, high recurrence rate and poor prognosis (11). Because of a lack of targeted therapies
(such as anti-HER2 therapy or hormone therapy), chemotherapy is
currently the primary treatment of TNBC. Therefore, the development
of novel diagnostic markers, understanding of the molecular
pathways implicated in TNBC pathogenesis, and targeted therapies,
remain an urgent and unmet need. One of the primary purposes of
this study was to address the above issues.
CIP2A is a widespread oncogenic factor in human
neoplasms (4–9,3). Similar
to the Ras oncogene, CIP2A is required for anchorage-independent
cell growth and malignant transformation of human cells (4). More recently, CIP2A expression has been
found to be associated with the invasive function of
fibroblast-like synoviocytes and synovial hyperplasia in rheumatoid
arthritis (19). In addition, it has
been reported that CIP2A immunostaining level positively correlated
with metastasis in renal cell carcinomas (6). More recently, In addition, Zhai et
al (20) found that CIP2A was
overexpressed in osteosarcoma tissues, and that CIP2A depletion
attenuated cell proliferation and invasion. In addition, CIP2A
depletion inhibited matrix metalloproteinases-9 (MMP-9) mRNA
expression. MMP-9 is the enzyme most crucial to tumor invasion
owing to their ability to degrade extracellular matrix and basement
membrane. In conclusion, increasing evidence suggests that CIP2A is
an oncoprotein promoting proliferation and cell invasion. However,
the expression pattern of CIP2A in TNBC and its involvement in
aggressiveness of TNBC cells is less reported. The present study
provides evidence that CIP2A overexpression widely occurs in TNSC
cells and positively correlates with proliferation and
metastasis.
In the present study, it was identified that the
rate of CIP2A overexpression was high in TNBCs, which is known to
show relatively strong metastasis potential, aggressive behaviors
and thus poor prognosis compared with ER+ breast cancer cells of
MCF-7 (Fig. 1). To gain insight into
the potential mechanism of CIP2A overexpression in TNBCs, CIP2A
expression was knocked down in MDA-MB-231 and MDA-MB-468 cell
lines. Consistent with clinical findings by Côme (10), the experiments in the present study
demonstrated that CIP2A depletion significantly inhibits cell
proliferation and colony formation, suggesting that CIP2A depletion
inhibited the anchorage-dependent (cell proliferation) and
anchorage-independent (colony formation) growth of highly invasive
TNBCs.
Autophagy and apoptosis are two important
self-destructive processes that serve a pivotal role in the
maintenance of human health, as well as in the pathogenesis of
several tumors (21,22). In the present study, CIP2A depletion
significantly induced caspase-3 activation, followed by PARP
cleavage in two TNBC cell lines, suggesting that CIP2A depletion
induces caspase-dependent apoptosis in TNBC cells (Fig. 3A). Western blot analysis evaluated the
expression of LC-3 II, and it was identified that CIP2A depletion
induces autophagy of TNBC cells.
Migration and invasion are two important
prerequisites of TNBC cancer progression and metastasis. Therefore,
therapeutic strategies for preventing or suppressing cancer
invasion and metastasis can significantly improve the survival of
TNBC patients. The present data showed that CIP2A depletion
markedly inhibited the invasive and migratory abilities of
MDA-MB-231 cells. Recently, CIP2A expression has been found to be
associated with the invasive function of breast cancer (10). Based on the role of CIP2A in
stabilizing and upregulating the c-Myc oncoprotein, previous
reports found that c-Myc is involved in CIP2A-stimulated
invasiveness of tumor cells (3,9,10). The expression of c-Myc was analyzed by
western blot, and it was observed that c-Myc is downregulated by
CIP2A depletion. Myc oncoprotein confers a selective advantage on
cancer cells by promoting cell survival, proliferation,
differentiation blockade, angiogenesis and genetic instability, all
of which may contribute towards invasion and metastasis (23,24).
Recently, Myc has been reported to regulate the
epithelial-to-mesenchymal transition, a critical cellular programme
for migration and invasion (25,26). Above
reports of Myc may contribute to cancer cell invasion driven by
CIP2A, and further study is required to investigate the function of
Myc in CIP2A-mediated invasion and migration.
In conclusion, the present study reports that CIP2A
depletion induces autophagy of TNBC cancer cells. Studies show that
the CIP2A downstream molecule Akt serves an important role in
autophagy. The current study reports that while pAkt (Ser473) is
downregulated in cells upon CIP2A knockdown, p-mTOR (Ser2448) and
the mTOR effector protein P70S6K (Thr412/389) are decreased. A
previous study reported that CIP2A controls cell growth and
autophagy through mTORC1 activation in MCF-7 cells (22). The results of the present study
indicate that the downregulation of the Akt/mTOR/P70S6K signaling
pathway may contribute to autophagy induced by CIP2A depletion.
Collectively, these results indicate a critical role of CIP2A in
driving disease progression and the spread of TNSC cells.
Acknowledgements
The present study was supported by grants from the
National Natural Sciences Foundation of China (grant no.,
81502637), the Natural Science Foundation of Hubei Province of
China (grant no., 2016CFB528 and 2013CFC033), the Natural Science
Foundation of Hubei Provincial Department of Education (grant no.,
Q20152106 and B2014048), the Foundation of Hubei University of
Medicine (grant no., FDFR201605), the Faculty Development Grant
from Hubei University of Medicine (grant no., 2014QDJZR08), the Key
Discipline Project of Hubei University of Medicine and the National
Training Program of Innovation and Entrepreneurship for
undergraduates (grant no. 201610929001).
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yaman S, Gumuskaya B, Ozkan C, Aksoy S,
Guler G and Altundag K: Lymphatic and capillary invasion patterns
in triple negative breast cancer. Am Surg. 78:1238–1242.
2012.PubMed/NCBI
|
|
3
|
Junttila MR, Puustinen P, Niemelä M, Ahola
R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Ge Z, Liu C, Björkholm M, Jia J and
Xu D: CIP2A is overexpressed in gastric cancer and its depletion
leads to impaired clonogenicity, senescence, or differentiation of
tumor cells. Clin Cancer Res. 14:3722–3728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma L, Wen ZS, Liu Z, Hu Z, Ma J, Chen XQ,
Liu YQ, Pu JX, Xiao WL, Sun HD and Zhou GB: Overexpression and
small molecule-triggered downregulation of CIP2A in lung cancer.
PLoS One. 6:e201592011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren J, Li W, Yan L, Jiao W, Tian S, Li D,
Tang Y, Gu G, Liu H and Xu Z: Expression of CIP2A in renal cell
carcinomas correlates with tumour invasion, metastasis and
patients' survival. Br J Cancer. 105:1905–1911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Li W, Li L, Yu X, Jia J and Chen
C: CIP2A is over-expressed in acute myeloid leukaemia and
associated with HL60 cells proliferation and differentiation. Int J
Lab Hematol. 33:290–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucas CM, Harris RJ, Giannoudis A, Copland
M, Slupsky JR and Clark RE: Cancerous inhibitor of PP2A (CIP2A) at
diagnosis of chronic myeloid leukemia is a critical determinant of
disease progression. Blood. 117:6660–6668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Ma L, Wen ZS, Hu Z, Wu FQ, Li W,
Liu J and Zhou GB: Cancerous inhibitor of PP2A is targeted by
natural compound celastrol for degradation in non-small-cell lung
cancer. Carcinogenesis. 35:905–914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Côme C, Laine A, Chanrion M, Edgren H,
Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, et al:
CIP2A is associated with human breast cancer aggressivity. Clin
Cancer Res. 15:5092–5100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tseng LM, Liu CY, Chang KC, Chu PY, Shiau
CW and Chen KF: CIP2A is a target of bortezomib in human triple
negative breast cancer cells. Breast Cancer Res. 14:R682012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen KF, Liu CY, Lin YC, Yu HC, Liu TH,
Hou DR, Chen PJ and Cheng AL: CIP2A mediates effects of bortezomib
on phospho-Akt and apoptosis in hepatocellular carcinoma cells.
Oncogene. 29:6257–6266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Memmott RM and Dennis PA: Akt-dependent
and -independent mechanisms of mTOR regulation in cancer. Cell
Signal. 21:656–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu
YP, Yu XJ, Zhang XD, Ming PH, Zhou GB and Huang L: The natural
compound magnolol inhibits invasion and exhibits potential in human
breast cancer therapy. Sci Rep. 3:30982013.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Han M, Sun RH, Wang JJ, Zhang YP,
Zhang DQ and Wen JK: ABL-N-induced apoptosis in human breast cancer
cells is partially mediated by c-Jun NH2-terminal kinase
activation. Breast Cancer Research. 12:R92010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Chen XQ, Liang HX, Zhang FX, Zhang
B, Jin J, Chen YL, Cheng YX and Zhou GB: Small compound
6-O-angeloylplenolin induces mitotic arrest and exhibits
therapeutic potentials in multiple myeloma. PLoS One. 6:e219302011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Jiao J, Liu Y, Guo LX, Zhou B, Li
GQ, Yao ZJ and Zhou GB: Gefitinib analogue V1801 induces apoptosis
of T790M egfr-harboring lung cancer cells by up-regulation of the
BH-3 only protein noxa. PLoS One. 7:e487482012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J, Park EJ, Hwang JW, Oh JM, Kim H,
Bae EK, Choi YL, Han J, Ahn JK, Cha HS and Koh EM: CIP2A expression
is associated with synovial hyperplasia and invasive function of
fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatol
Int. 32:2023–2030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai M, Cong L, Han Y and Tu G: CIP2A is
overexpressed in osteosarcoma and regulates cell proliferation and
invasion. Tumour Biol. 35:1123–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha K, Fiskus W, Choi DS, Bhaskara S,
Cerchietti L, Devaraj SG, Shah B, Sharma S, Chang JC, Melnick AM,
et al: Histone deacetylase inhibitor treatment induces 'BRCAness'
and synergistic lethality with PARP inhibitor and cisplatin against
human triple negative breast cancer cells. Oncotarget. 5:5637–5650.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puustinen P, Rytter A, Mortensen M,
Moreira JM and Jäättelä M: CIP2A oncoprotein controls cell growth
and autophagy through mTORC1 activation. J Cell Biol. 204:713–727.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guffei A, Lichtensztejn Z, Gonçalves Dos
Santos SA, Louis SF, Caporali A and Mai S: c-Myc-dependent
formation of Robertsonian translocation chromosomes in mouse cells.
Neoplasia. 9:578–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaqué JP, Fernéndez-García B, García-Sanz
P, Ferrandiz N, Bretones G, Calvo F, Crespo P, Marín MC and León J:
c-Myc inhibits Ras-mediated differentiation of pheochromocytoma
cells by blocking c-Jun up-regulation. Mol Cancer Res. 6:325–339.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Helland A, Anglesio MS, George J, Cowin
PA, Johnstone CN, House CM, Sheppard KE, Etemadmoghadam D, Melnyk
N, Rustgi AK, et al: Deregulation of MYCN, LIN28B and LET7 in a
molecular subtype of aggressive high-grade serous ovarian cancers.
PLoS One. 6:e180642011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|