Introduction
Ovarian cancer is the seventh most common cancer in
women, and affects 239,000 women worldwide each year (1); however, the natural history of these
cancers as well as their underlying tumor biology are poorly
understood (2). Carcinomas are the
most frequent type of ovarian cancer, which can be divided into
five main types: High-grade serous carcinoma (HGSC; accounting for
~70%), clear cell carcinoma (CCC; 10%), endometrioid carcinoma
(10%), mucinous carcinoma (3%) and low-grade serous carcinoma
(<5%) (3). At the time of surgery,
cancer involves both ovaries in 66% of patients with HGSC and in 8%
of patients with CCC (4). The cell
type(s) of origin in ovarian carcinogenesis is(are) unknown. It is
debated whether all HGSC arise in the fallopian tubes or if a
number of them stem from the surface epithelium of the ovaries
(2,5).
In CCC, possible origins are the ovarian surface epithelium or
cells from the endometrium, as the latter tumor type is associated
with endometriosis in ~1/3 of the patients (4). We hypothesize that the developmental
history of bilateral ovarian carcinomas likewise represents a
conundrum; in principle, it may represent two primary tumors, a
primary tumor and its metastasis, or two metastases.
Genomic studies of bilateral carcinomas have
revealed a clonal association between the two tumors in each
patient, including similarities in karyotype and copy number
aberrations (6–8). Gene expression analyses of ovarian
tumors and their omental metastases have also demonstrated
similarities in gene expression profiles among the lesions, with a
number of the differently expressed genes being linked to the
metastatic process (9–11). To the best of our knowledge, no
comparison at the transcriptome level to search specifically for
genes differently expressed between the two tumors in bilateral
ovarian carcinomas has been published thus far.
As genes differentially expressed within bilateral
pairs may indicate the developmental association between the two
tumors, a microarray analysis of four pairs of ovarian carcinomas
(three pairs of HGSC and one pair of CCC) was conducted. The
results were compared with previous genomic data on the same tumors
(7) with the aim of identifying
possible mechanisms behind the RNA level differences.
The present tumor samples were originally selected
because the karyotypes of one of the samples in each tumor pair
displayed structural rearrangements of chromosome 19; the karyotype
analyses of the four remaining tumors were either inconclusive or
culture failure. Chromosome 19 is commonly and non-randomly
rearranged in ovarian carcinogenesis (12–15). Since
structural rearrangements of chromosome 19 have not been reported
as the sole anomaly, these changes are probably linked to tumor
progression rather than initiation. Therefore, in addition to the
global gene expression data set, a separate analysis of genes
mapping on chromosome 19 was performed in the present study.
Materials and methods
Patients and tissue samples
Bilateral ovarian carcinomas from four patients were
included in the present study (three pairs of HGSC and one pair of
CCC). Tumor grade and histology were revised by a gynecological
pathologist (Table I). The same
material had previously been examined by karyotyping and
comparative genomic hybridization (CGH) (7). The tumors were surgically removed at The
Norwegian Radium Hospital (Oslo, Norway) between January 2001 and
December 2004. The samples were originally selected because
rearrangement(s) of chromosome 19 had been noticed in the karyotype
of one of the bilateral tumors. None of the patients had received
chemotherapy prior to surgery. The tumor biobank was registered
according to the Norwegian national legislation, and the study was
approved by the South East Regional Committee for Medical Research
Ethics (Oslo, Norway; project nos. S-07194a and 2.2007.425).
 | Table I.Clinical data. |
Table I.
Clinical data.
| Patient | Lab no. | Histology | FIGO stage | Agea |
|---|
| 1 A/B | 01-805/6 | HGSC | IIIC | 54 |
| 2 A/B | 01-837/8 | HGSC | IIIC | 61 |
| 3 A/B | 04-186/7 | HGSC | IIC | 64 |
| 4 A/B | 04-101/2 | CCCb | IIIB | 58 |
For comparison of technical variation and gene
expression levels, one healthy ovary sample (Human Ovary Total RNA;
catalogue no. HR-406; Zyagen, San Diego, CA, USA) was analyzed in
three separate replicates (N1-3).
Isolation of RNA
The tumor tissue adjacent to that used for
cytogenetic analysis and histological examination had been frozen
and stored at −80°C. Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
RNAeasy spin columns (RNeasy Mini kit; Qiagen GmbH, Hilden,
Germany). First, the tumor tissue was homogenized in TRIzol, and
the aqueous phase was then removed and used further with the
aforementioned kit, according to the manufacturer's protocol.
Quantification and quality control of the isolated total RNA were
performed using the NanoVue spectrophotometer (GE Healthcare Life
Sciences, Chalfont, UK) and the Experion automated electrophoresis
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total RNA
degradation was evaluated by reviewing the electropherograms and
the RNA quality indicator (RQI) numbers (RQI=7.5–9.0).
Microarray gene expression
analysis
Total RNA (100 ng) was used as input for global gene
expression analysis at the exon level using GeneChip®
Human Exon 1.0 ST Arrays (Affymetrix, Inc., Santa Clara, CA, USA).
Each microarray contained 1.4 million probe sets (the majority of
which were comprised of four probes), where each probe set
corresponded to ~1 known or computationally predicted exon. RNA
from each sample was individually amplified, reversely transcribed,
fragmented and labeled. Labeled sense strand complementary DNA was
hybridized onto the arrays, which were then washed, stained and
scanned as described previously (16). Cell intensity files from the eight
tumor samples were background corrected, inter-chip quantile
normalized and summarized at the gene level by the robust
multiarray average approach (17),
implemented in the Expression Console 1.1 software (Affymetrix,
Inc.). A total of 17,361 genes (transcript clusters annotated with
gene symbols) were identified using the HuEx-1_0-st-v2.r2 core
library files and the HuEx-1_0-st-v2.na33.hg19.transcript.csv
annotation file (Affymetrix, Inc.). In a second round of
normalization, which was performed as described above, the healthy
control was normalized together with the tumor samples. The gene
expression text file was imported into J-Express Pro 2012 (Molmine,
Bergen, Norway) for hierarchical clustering (Euclidean distance
measure and complete linkage) (18).
The expression of known ovarian cancer-related genes
was specifically evaluated. Genes were selected from the Cancer
Gene Census (http://cancer.sanger.ac.uk/census/, accessed on
October 12, 2015) by the search term ‘ovarian;’ and 19 genes were
identified, including AKT1, AKT2, AT-rich
interaction domain 1A, BRAF, breast cancer
(BRCA)1, BRCA2, cyclin E1, cyclin-dependent
kinase 12, catenin beta 1, Erb-B2 receptor yyrosine
kinase 2, forkhead box L2, MutL homolog 1,
MutS homolog (MSH)2, MSH6,
phosphatidylinositol 3-kinase regulatory subunit 1,
PMS1, PMS2, protein phosphatase 2, regulatory
subunit A, alpha and serine/threonine kinase
11.
Results
Gene expression
The gene expression analysis provided informative
results on 17,361 genes annotated with gene symbols. The
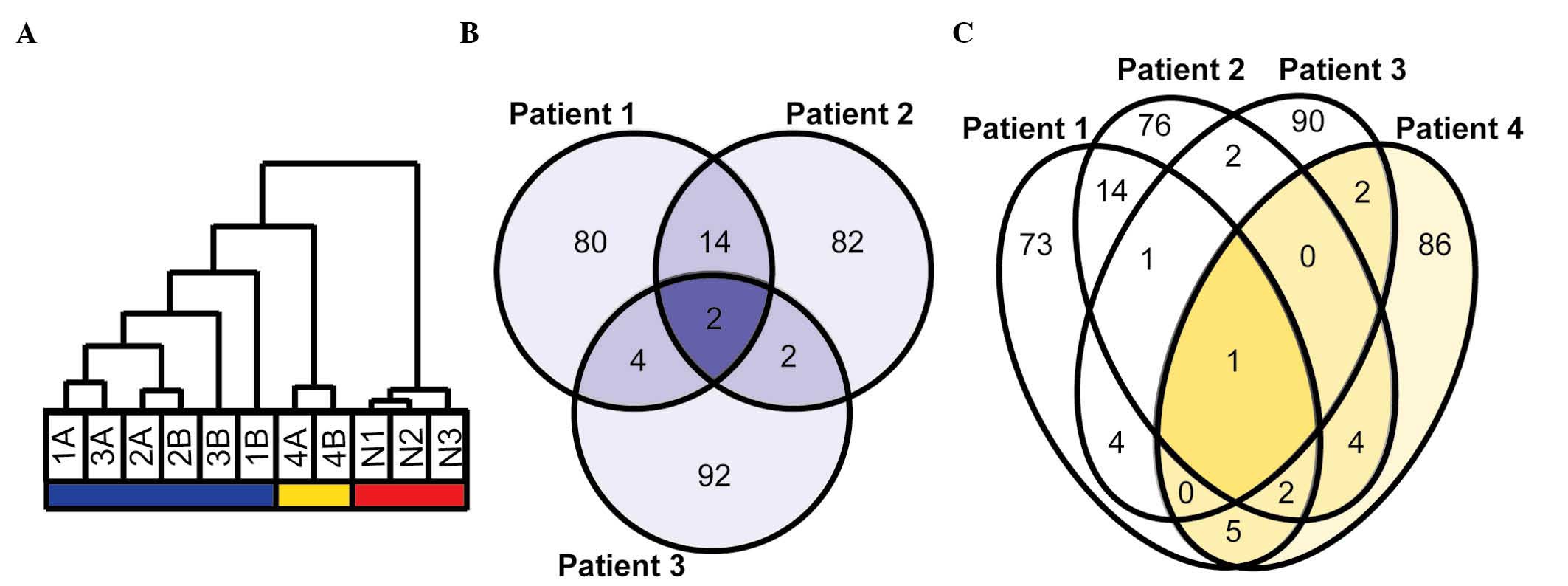
hierarchical clustering of all samples (six HGSC from three
patients, two CCC from one patient and three replicates of a
healthy whole ovarian tissue) based on all genes separated healthy
tissue from cancer, with the CCC samples clustering with the HGSC
samples. All bilateral tumors exhibited within-pair clustering.
When the hierarchical clustering analysis was based on the 1,000
genes with the greatest range in gene expression, cancer samples
were separated from healthy controls and HGSC were separated from
CCC (Fig. 1A). Since the aim of the
study was to identify genes with different expression within pairs,
no further studies on the similarities within tumor pairs were
performed.
Comparison of bilateral HGSC
The proportion of genes differently expressed within
the three pairs (≥2-fold-change between normalized expression
values) was 4.0, 2.6 and 4.8%, respectively. For each pair, the top
100 genes differing the most were selected for further analysis.
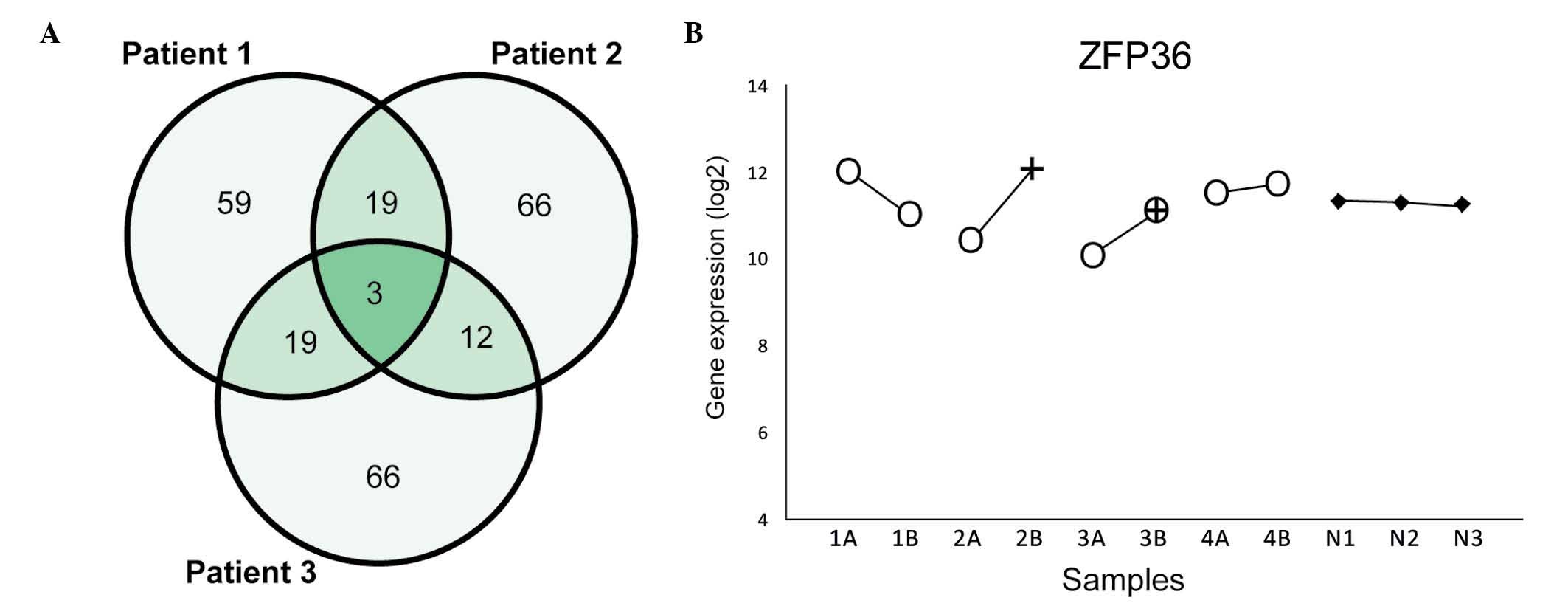
The comparison of these lists identified 276 unique genes (Fig. 1B), including 22 genes among the most
differently expressed genes in ≥2 patients (Table II). Two genes were among the most
differently expressed within all three pairs: Immunoglobulin
J (IGJ, Fig. 2A) and
serpin peptidase inhibitor, clade B (ovalbumin), member 2
(SERPINB2, Fig. 2B).
Additionally, other genes displayed differences in expression
within all pairs, including serpin peptidase inhibitor, clade E,
member 1 (SERPINE1, Fig.
2C).
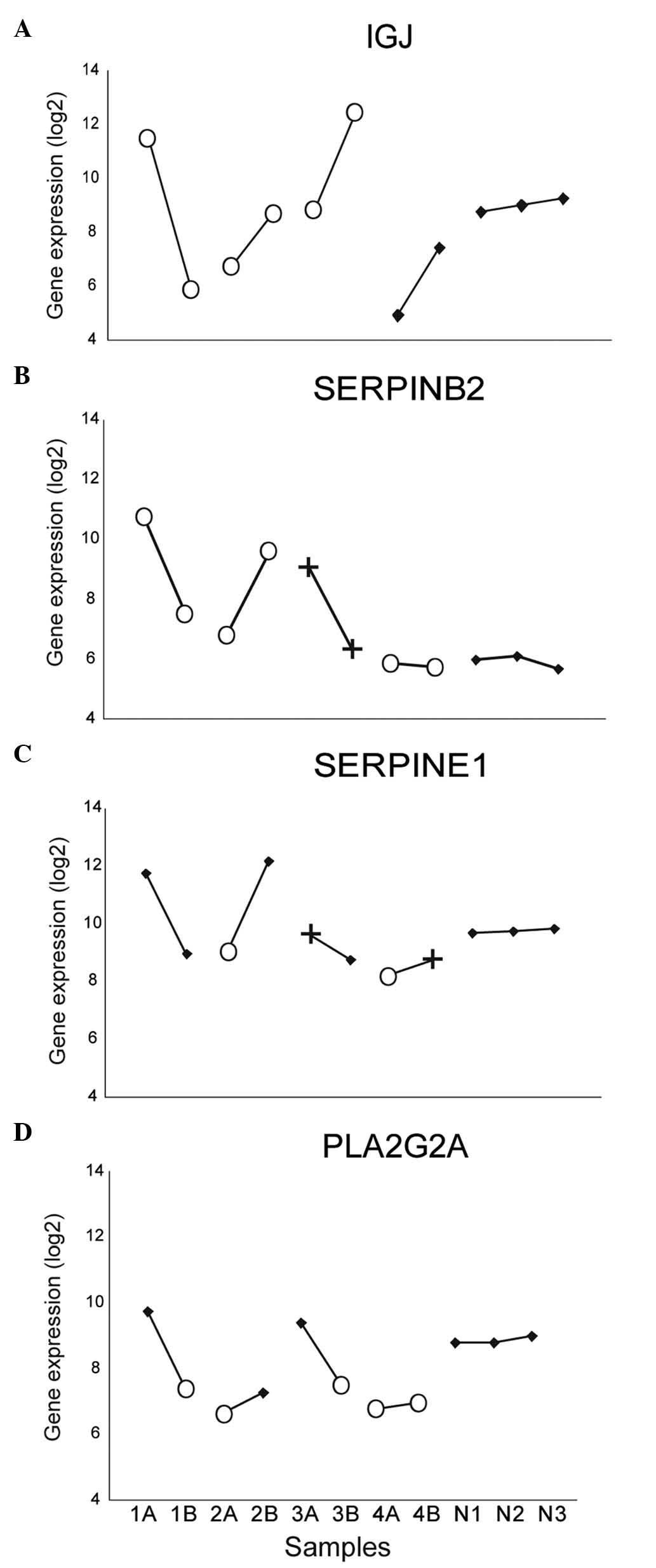 | Figure 2.Gene expression in three pairs of
HGSC (1–3), one pair of clear cell carcinoma
(4) and normal ovarian tissue
(replicates N1-3). Comparative genomic hybridization copy number
results are marked with O (loss) and + (gain). (A)
Immunoglobulin J exhibited >4-fold-change in expression
within all tumor pairs. (B) Serpin peptidase inhibitor, clade B
(ovalbumin), member 2 was differently expressed in all HGSC
pairs, (C) similarly to another member of the same gene family:
Serpin peptidase inhibitor, clade E, member 1. (D) For
phospholipase A2, group IIA (platelets, synovial fluid), an
association appeared to exist between DNA copy number loss and
reduced gene expression. HGSC, high-grade serous carcinoma; IGJ,
immunoglobulin J; SERPINB2, serpin peptidase inhibitor, clade B
(ovalbumin), member 2; SERPINE1, serpin peptidase inhibitor, clade
E, member 1; PLA2G2A, phospholipase A2, group IIA (platelets,
synovial fluid). |
 | Table II.Genes differently expressed within ≥2
pairs of HGSC. |
Table II.
Genes differently expressed within ≥2
pairs of HGSC.
| Gene symbol | Ensembl gene
ID | Cytoband | 1a | 2a | 3a |
|---|
| ACTA2 |
ENSG00000107796 | 10q23.3 | 11.09 | 5.83 | 1.04 |
| BCAT1 |
ENSG00000060982 | 12p12.1 | 1.18 | 5.19 | 9.19 |
| BDKRB1 |
ENSG00000100739 | 14q32.1-q32.2 | 10.95 | 4.21 | 1.18 |
| C8orf4 |
ENSG00000176907 | 8p11.2 | 12.16 | 4.39 | 1.66 |
|
CRISPLD2 |
ENSG00000103196 | 16q24.1 | 5.54 | 5.05 | 1.34 |
| CXCL12 |
ENSG00000107562 | 10q11.1 | 9.59 | 5.12 | 1.80 |
| EFEMP1 |
ENSG00000115380 | 2p16 | 11.48 | 8.40 | 2.96 |
| EIF2S2 |
ENSG00000125977 | 20q11.2 | 3.21 | 4.06 | 4.07 |
| ENPP1 |
ENSG00000197594 | 6q22-q23 | 5.07 | 4.26 | 1.62 |
| EPYC |
ENSG00000083782 | 12q21 | 9.07 | 9.75 | 1.09 |
| FAP |
ENSG00000078098 | 2q23 | 11.13 | 3.34 | 2.69 |
| HAS2 |
ENSG00000170961 | 8q24.12 | 7.54 | 4.58 | 1.17 |
|
HIST1H1A |
ENSG00000124610 | 6p21.3 | 5.14 | 1.15 | 6.81 |
|
IGJb |
ENSG00000132465 | 4q21 | 41.81 | 3.62 | 13.20 |
| IL7R |
ENSG00000168685 | 5p13 | 5.55 | 3.47 | 1.34 |
| INHBA |
ENSG00000122641 | 7p15-p13 | 4.25 | 3.28 | 1.74 |
| PLA2G2A |
ENSG00000188257 | 1p35 | 5.48 | 1.45 | 4.07 |
| PTGIS |
ENSG00000124212 | 20q13.13 | 6.86 | 5.73 | 1.93 |
| S100A8 |
ENSG00000143546 | 1q21 | 7.16 | 1.77 | 7.09 |
| S100A9 |
ENSG00000163220 | 1q21 | 4.63 | 1.11 | 5.92 |
|
SERPINB2b |
ENSG00000197632 | 18q21.3 | 8.37 | 5.93 | 5.57 |
|
SERPINE1 |
ENSG00000106366 | 7q21.3-q22 | 7.35 | 8.38 | 1.99 |
Comparison with CCC and reference
tissue
Since the cell(s) of origin in ovarian carcinomas
is(are) uncertain, it is important to select the reference tissue
carefully. The different histotypes were analyzed separately, and
the results from normal whole ovary were used mainly as a control
of technical variation. Within the pair of CCC, 1.9% of the genes
differed in expression (≥2-fold-change) between the two sides. Of
the 100 most differently expressed genes within this pair, one gene
was also among the most differently expressed in all three HGSC
pairs: IGJ (Fig. 1C). In
addition to IGJ, 13 other genes were differently expressed
within ≥1 pair of HGSC [tenascin C, interleukin
(IL)-7 receptor, uroplakin 1B, chemokine (C-C
motif) ligand 2, small proline-rich protein 2A, eyes
absent homolog 4, cytochrome P450, family 24, subfamily
A, polypeptide 1, insulin-like growth factor 2,
lymphocyte antigen 96, lumican, paired related
homeobox 1 and chromosome 8 open reading frame
4]. RNA from normal whole ovary was analyzed in three separate
replicates, and only 0.9% of the total gene set differed >2-fold
when comparing the replicates.
Comparison with genomic data
The present gene expression results were compared
with previously reported genomic analyses of the same material
(7). CGH analysis provided copy
number information at a resolution level of ~300 cytobands. All
HGSC tumors exhibited identical status in 39 cytobands,
specifically, all six had either copy number gain, copy number loss
or were balanced/had no alteration. In total, ≥1 tumor sample(s)
differed in copy number from the rest in the remaining cytobands.
In eight of them (1p35, 1p32, 4p16, 7q11, 11p11, 12q14, 19p13 and
20q11), all three HGSC pairs displayed different results within
pairs. In three of these, the same combination was detected in all
pairs: 1p35 (loss/no alteration), 11p11 (loss/no alteration) and
20q11 (gain/no alteration). The present study attempted next to
explore whether the copy number imbalances could partly explain the
deregulation of gene expression in the selected genes of interest.
For the phospholipase A2, group IIA (platelets, synovial
fluid) (PLA2G2A) gene, which maps to 1p35, alterations
at DNA and RNA level pointed in the same direction (Fig. 2D); in all HGSC pairs, one tumor had
concurrent genomic loss of 1p35 and lower expression of
PLA2G2A compared with the gene expression in the
contralateral tumor with balanced CGH result. In the CCC pair, both
tumors had copy number loss in 1p35 and PLA2G2A expression
similar to the HGSC tumors with copy number loss (Fig. 2D). The normal ovarian sample used as
the control exhibited PLA2G2A expression similar to that of
tumor samples with balanced copy number.
However, for the majority of genes examined, no
clear associations were observed between DNA imbalances and RNA
expression, including the IGJ gene. For IGJ (mapping
to 4q21), the gene expression varied greatly within all tumor
pairs, whereas the CGH results within all tumor pairs were
identical (Fig. 2A); therefore, the
genomic analysis provided no indication regarding the mechanism
behind the identified differences in gene expression.
Chromosome 19
The genes mapping to chromosome 19 (1,211 genes)
were subjected to additional analysis. In general, the expression
was more similar within the pairs for these genes than for the
global gene expression data set; only 6.4% of the genes from
chromosome 19 differed >2-fold in expression compared with 9.5%
for the total set. The comparison of the most differently expressed
genes within each HGSC pair revealed three recurrent genes: Zinc
finger protein 36 (ZFP36, mapping on 19q13.1), zinc
finger protein 585B (mapping on 19q13.12) and growth
differentiation factor 15 (mapping on 19p13.11) (Fig. 3A). None of these genes were
differently expressed within the CCC pair. ZFP36 was also
included among the most differently expressed genes for the total
data set and exhibited >2-fold-change in expression within all
three HGSC pairs (Fig. 3B).
Known ovarian cancer-related
genes
In the Cancer Gene Census database, 19 genes are
reported to be associated with ovarian cancer; all of which
displayed similar expression within all four tumor pairs
(<2-fold-change difference).
Discussion
We hypothesize that bilateral ovarian carcinomas may
possibly represent two primary tumors, a primary tumor and a
metastasis, or two metastases. In the present study, the gene
expression profiles in the bilateral carcinoma pairs from four
patients were compared to assess the association between the two
tumors. To the best of our knowledge, this is the first time that
bilateral ovarian carcinomas have been compared in this manner. An
overall similarity between the two lesions was noticed in all
patients examined, thus indicating that the two tumors in each
patient are clonally related. This is in agreement with former
conclusions based on studies performed at the genomic level, which
suggest that bilateral ovarian carcinomas have a unicentric origin
(6,7,19). The
small number of cases examined in the present study precludes
statistical testing of this conclusion, however.
The present study focused on the differently
expressed genes within tumor pairs and, notably, identified among
them several genes previously linked to the metastatic process in
ovarian and other cancers (20–24). These
results, therefore, indicate that the two tumor lesions represent
different stages of tumor progression; however, since the debate on
whether ovarian cancer originates from the ovarian surface
epithelium or from the tube is still ongoing, it is impossible to
conclude, based on the present findings, whether the ovarian
lesions represent a primary tumor and its metastasis or two
clonally-related metastases.
The SERPINB2 and SERPINE1 genes were
among the most differentially expressed genes in all three pairs of
HGSC. Both genes belong to the same gene family, encode proteins
that participate in the regulation of fibrinolysis and have been
linked to cancer and metastasis (23). SERPINE1 has been proposed to
have a pro-tumorigenic function by protecting tumor cells from
apoptosis, since inhibition of SERPINE1 has been
demonstrated to increase spontaneous apoptosis in cancer cell lines
(25,26). High expression of SERPINE1 is
associated with poor outcome in several cancer types, including
ovarian (27), gastric (28), colorectal (29), renal (30) and breast (31) cancer. In studies of ovarian
carcinomas, both SERPINB2 and SERPINE1 were
upregulated in omental metastases compared with the corresponding
ovarian tumors (11,21,24). To
the best of our knowledge, the present study demonstrates for the
first time that SERPINE1 and SERPINB2 are also
differently expressed within bilateral HGSC pairs.
IGJ was among the most differently expressed
genes in all four bilateral tumor pairs (three HGSC pairs and one
CCC pair). According to the Universal Protein Resource (UniProt;
www.uniprot.org), the IGJ gene maps to
4q21 and encodes a protein that links two monomer units of either
IgM or IgA. Rearrangements involving IGJ have been
associated with overexpression of the oncogene v-myc avian
myelocytomatosis viral oncogene homolog in multiple myeloma,
possibly leading to a more aggressive disease phenotype (32). IGJ has not previously been
linked to ovarian cancer or metastasis, and its role and the
mechanism behind the deregulation of gene expression require
further analysis.
The gene expression data from the present study were
compared with genomic aberrations. Gene expression can be altered
by several mechanisms, including genomic copy number gains or
losses (33). Earlier studies have
reported that, in cancer, ≥12% of variation in gene expression is
attributable to underlying variation in gene copy number (33,34). The
current study identified eight cytobands with different CGH results
within bilateral tumor pairs, and attempted to identify an
association between genetic copy number variation and expression.
In the case of PLA2G2A, the tumors with loss of 1p35 (one in
each HGSC pair and both CCC tumors) exhibited lower gene expression
than the bilateral samples without genomic alterations. According
to UniProt, this gene encodes an extracellular enzyme considered to
participate in the regulation of phospholipid metabolism in
biomembranes. The role of PLA2G2A in cancer is not fully
understood. It has been suggested that PLA2G2A prevents
carcinogenesis and metastasis by inhibiting metastasis-related
genes (22). The effect seems to
differ among cancer types; overexpression of PLA2G2A has
been associated with both improved survival in gastric cancer
(22) and poor prognosis in prostate
cancer (35). Metastasis has been
associated with loss of PLA2G2A expression. Ganesan et
al (22) observed an inverse
correlation between gene expression and cancer stage in several
cancer types (gastric, colon and prostate), and suggested that
genomic deletion could be one mechanism causing lower gene
expression in advanced stages. To the best of our knowledge, the
present study is the first to report that PLA2G2A is linked
to ovarian cancer. Although cytoband 1p35 was lost in one of the
bilateral HGSC in each patient, it is important to remember that
the resolution of CGH does not provide precise information on
single genes. In line with our findings, the results reported by
The Cancer Genome Atlas regarding a cohort of 489 HGSC demonstrated
that the genomic region harboring PLA2G2A was significantly
and recurrently deleted (36).
The analysis of genes mapping on chromosome 19
identified ZFP36 as differently expressed within all three
HGSC pairs. According to UniProt, the protein that this gene
encodes is involved in post-transcriptional gene regulation by
binding adenylate-uridylate-rich elements-containing messenger RNAs
and promoting their degradation. ZFP36 has been suggested to have a
protective function in cancer by inhibiting metastasis-related
genes, including matrix metalloproteinase (MMP)2,
MMP9 and IL-6 (20,37). Loss
of ZFP36 expression has been associated with metastasis
(20). A study by Veskimäe et
al (38) identified ZFP36
as one of the most downregulated genes when the authors compared
the gene expression in BRCA1/2 germline mutated tubal
and ovarian tissues (removed by risk-reducing
salpingo-oophorectomy) with that in healthy control tissues, thus
supporting a role for ZFP36 in ovarian carcinogenesis.
The present study has demonstrated that genes
previously linked to metastasis are differently expressed within
HGSC pairs in bilateral ovarian cancer. Deregulation of
SERPINE1, SERPINB2, PLA2G2A and ZFP36
has previously been associated with invasion and advancing disease.
However, it cannot be concluded whether bilateral tumors represent
one primary tumor and a metastasis or two metastases. It would have
been interesting to analyze gene expression in healthy ovarian
tissue and tubal epithelium from the same patients; however, these
materials were not available. The results of this study indicate
that bilateral ovarian tumors represent different stages of
progression during a single clonal process. However, the
significance of these findings requires further analysis in larger
studies.
Acknowledgements
The present study was supported by grants from the
Norwegian Cancer Society (Oslo, Norway), the Inger and John
Fredriksen Foundation for Ovarian Cancer Research (Oslo, Norway)
and the Research Council of Norway (Oslo, Norway) through its
Centers of Excellence funding scheme (project no. 179571).
References
|
1
|
Stewart B and Wild C: Cancer by organ
site: Cancers of the female genital organs. World Cancer Report
2014Cancer by Organ Site: Cancers of the female genital Organs.
Lyon: International Agency for Research on Cancer; pp. 465–481.
2014
|
|
2
|
Auersperg N: The origin of ovarian
cancers-hypotheses and controversies. Front Biosci (Schol Ed).
5:709–719. 2013.PubMed/NCBI
|
|
3
|
Prat J: Ovarian carcinomas: Five distinct
diseases with different origins, genetic alterations, and
clinicopathological features. Virchows Arch. 460:237–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ, Ellenson L Hendrick and Ronnett
BM: Blaustein's Pathology of the Female Genital Tract. Springer;
New York, NY: 2011
|
|
5
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pejovic T, Heim S, Mandahl N, Elmfors B,
Furgyik S, Flodérus UM, Helm G, Willén H and Mitelman F: Bilateral
ovarian carcinoma: Cytogenetic evidence of unicentric origin. Int J
Cancer. 47:358–361. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Micci F, Haugom L, Ahlquist T, Abeler VM,
Trope CG, Lothe RA and Heim S: Tumor spreading to the contralateral
ovary in bilateral ovarian carcinoma is a late event in clonal
evolution. J Oncol. 2010:6463402010.PubMed/NCBI
|
|
8
|
Khalique L, Ayhan A, Whittaker JC, Singh
N, Jacobs IJ, Gayther SA and Ramus SJ: The clonal evolution of
metastases from primary serous epithelial ovarian cancers. Int J
Cancer. 124:1579–1586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adib TR, Henderson S, Perrett C, Hewitt D,
Bourmpoulia D, Ledermann J and Boshoff C: Predicting biomarkers for
ovarian cancer using gene-expression microarrays. Br J Cancer.
90:686–692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lancaster JM, Dressman HK, Clarke JP,
Sayer RA, Martino MA, Cragun JM, Henriott AH, Gray J, Sutphen R,
Elahi A, et al: Identification of genes associated with ovarian
cancer metastasis using microarray expression analysis. Int J
Gynecol Cancer. 16:1733–1745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brodsky AS, Fischer A, Miller DH, Vang S,
MacLaughlan S, Wu HT, Yu J, Steinhoff M, Collins C, Smith PJ, et
al: Expression profiling of primary and metastatic ovarian tumors
reveals differences indicative of aggressive disease. PloS One.
9:e944762014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pejovic T, Heim S, Mandahl N, Baldetorp B,
Elmfors B, Flodérus UM, Furgyik S, Helm G, Himmelmann A, Willén H,
et al: Chromosome aberrations in 35 primary ovarian carcinomas.
Genes Chromosomes Cancer. 4:58–68. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pejovic T, Heim S, Mandahl N, Elmfors B,
Flodérus UM, Furgyik S, Helm G, Willén H and Mitelman F: Consistent
occurrence of a 19p+ marker chromosome and loss of 11p material in
ovarian seropapillary cystadenocarcinomas. Genes Chromosomes
Cancer. 1:167–171. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jenkins RB, Bartelt D Jr, Stalboerger P,
Persons D, Dahl RJ, Podratz K, Keeney G and Hartmann L: Cytogenetic
studies of epithelial ovarian carcinoma. Cancer Genet Cytogenet.
71:76–86. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taetle R, Aickin M, Yang JM, Panda L,
Emerson J, Roe D, Adair L, Thompson F, Liu Y, Wisner L, et al:
Chromosome abnormalities in ovarian adenocarcinoma: I. Nonrandom
chromosome abnormalities from 244 cases. Genes Chromosomes Cancer.
25:290–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smebye ML, Sveen A, Haugom L, Davidson B,
Tropé CG, Lothe RA, Heim S, Skotheim RI and Micci F: Chromosome 19
rearrangements in ovarian carcinomas: Zinc finger genes are
particularly targeted. Genes Chromosomes Cancer. 53:558–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dysvik B and Jonassen I: J-Express:
Exploring gene expression data using Java. Bioinformatics.
17:369–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bashashati A, Ha G, Tone A, Ding J,
Prentice LM, Roth A, Rosner J, Shumansky K, Kalloger S, Senz J, et
al: Distinct evolutionary trajectories of primary high-grade serous
ovarian cancers revealed through spatial mutational profiling. J
Pathol. 231:21–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Souhibani N, Al-Ahmadi W, Hesketh JE,
Blackshear PJ and Khabar KS: The RNA-binding zinc-finger protein
tristetraprolin regulates AU-rich mRNAs involved in breast
cancer-related processes. Oncogene. 29:4205–4215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorn J, Harbeck N, Kates R, Gkazepis A,
Scorilas A, Soosaipillai A, Diamandis E, Kiechle M, Schmalfeldt B
and Schmitt M: Impact of expression differences of
kallikrein-related peptidases and of uPA and PAI-1 between primary
tumor and omentum metastasis in advanced ovarian cancer. Ann Oncol.
22:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganesan K, Ivanova T, Wu Y, Rajasegaran V,
Wu J, Lee MH, Yu K, Rha SY, Chung HC, Ylstra B, et al: Inhibition
of gastric cancer invasion and metastasis by PLA2G2A, a novel
beta-catenin/TCF target gene. Cancer Res. 68:4277–4286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heit C, Jackson BC, McAndrews M, Wright
MW, Thompson DC, Silverman GA, Nebert DW and Vasiliou V: Update of
the human and mouse SERPIN gene superfamily. Hum Genomics.
7:222013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmalfeldt B, Kuhn W, Reuning U, Pache L,
Dettmar P, Schmitt M, Jänicke F, Höfler H and Graeff H: Primary
tumor and metastasis in ovarian cancer differ in their content of
urokinase-type plasminogen activator, its receptor and inhibitors
types 1 and 2. Cancer Res. 55:3958–3963. 1995.PubMed/NCBI
|
|
25
|
Fang H, Placencio VR and DeClerck YA:
Protumorigenic activity of plasminogen activator inhibitor-1
through an antiapoptotic function. J Natl Cancer Inst.
104:1470–1484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mashiko S, Kitatani K, Toyoshima M,
Ichimura A, Dan T, Usui T, Ishibashi M, Shigeta S, Nagase S, Miyata
T and Yaegashi N: Inhibition of plasminogen activator inhibitor-1
is a potential therapeutic strategy in ovarian cancer. Cancer Biol
Ther. 16:253–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuhn W, Schmalfeldt B, Reuning U, Pache L,
Berger U, Ulm K, Harbeck N, Späthe K, Dettmar P, Höfler H, et al:
Prognostic significance of urokinase (uPA) and its inhibitor PAI-1
for survival in advanced ovarian carcinoma stage FIGO IIIc. Br J
Cancer. 79:1746–1751. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakakibara T, Hibi K, Koike M, Fujiwara M,
Kodera Y, Ito K and Nakao A: Plasminogen activator inhibitor-1 as a
potential marker for the malignancy of gastric cancer. Cancer Sci.
97:395–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berger DH: Plasmin/plasminogen system in
colorectal cancer. World J Surg. 26:767–771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zubac DP, Wentzel-Larsen T, Seidal T and
Bostad L: Type 1 plasminogen activator inhibitor (PAI-1) in clear
cell renal cell carcinoma (CCRCC) and its impact on angiogenesis,
progression and patient survival after radical nephrectomy. BMC
Urol. 10:202010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Offersen BV, Alsner J, Ege Olsen K,
Riisbro R, Brünner N, Sørensen FB, Sørensen BS, Schlemmer BO and
Overgaard J: A comparison among HER2, TP53, PAI-1, angiogenesis and
proliferation activity as prognostic variables in tumours from 408
patients diagnosed with early breast cancer. Acta Oncol.
47:618–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Affer M, Chesi M, Chen WD, Keats JJ,
Demchenko YN, Tamizhmani K, Garbitt VM, Riggs DL, Brents LA, et al:
Promiscuous MYC locus rearrangements hijack enhancers but mostly
super-enhancers to dysregulate MYC expression in multiple myeloma.
Leukemia. 28:1725–1735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pollack JR, Sørlie T, Perou CM, Rees CA,
Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Børresen-Dale AL
and Brown PO: Microarray analysis reveals a major direct role of
DNA copy number alteration in the transcriptional program of human
breast tumors. Proc Natl Acad Sci USA. 99:12963–12968. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hyman E, Kauraniemi P, Hautaniemi S, Wolf
M, Mousses S, Rozenblum E, Ringnér M, Sauter G, Monni O, Elkahloun
A, et al: Impact of DNA amplification on gene expression patterns
in breast cancer. Cancer Res. 62:6240–6245. 2002.PubMed/NCBI
|
|
35
|
Graff JR, Konicek BW, Deddens JA, Chedid
M, Hurst BM, Colligan B, Neubauer BL, Carter HW and Carter JH:
Expression of group IIa secretory phospholipase A2 increases with
prostate tumor grade. Clin Cancer Res. 7:3857–3861. 2001.PubMed/NCBI
|
|
36
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Van Tubergen EA, Banerjee R, Liu M, Broek
R Vander, Light E, Kuo S, Feinberg SE, Willis AL, Wolf G, Carey T,
et al: Inactivation or loss of TTP promotes invasion in head and
neck cancer via transcript stabilization and secretion of MMP9,
MMP2 and IL-6. Clin Cancer Res. 19:1169–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Veskimäe K, Staff S, Tabaro F, Nykter M,
Isola J and Mäenpää J: Microarray analysis of differentially
expressed genes in ovarian and fallopian tube epithelium from
risk-reducing salpingo-oophorectomies. Genes Chromosomes Cancer.
54:276–287. 2015. View Article : Google Scholar : PubMed/NCBI
|