Introduction
Bladder cancer is the ninth most common malignancy
in the world. In the US, ~15,000 people succumb to this disease
every year (1). As a common
malignancy, bladder cancer often exhibits the characteristics of
frequent recurrence and an undesirable clinical outcome when tumors
progress to invasive disease (2).
Approximately 90% of bladder cancer cases are urothelial
carcinomas, previously named transitional cell carcinomas. The
majority of bladder cancers are low-grade, non-muscle-invasive
cancers, which account for up to 70–80% of cases, and which seldom
progress to the muscle-invasive stage (3,4). However,
10–20% of patients are diagnosed with high-grade and
muscle-invasive tumors, exhibiting aggressive clinical behavior
(3). Although limited treatment
options are available, 31–78% of patients still suffer from disease
recurrence within 5 years. In addition, ~20% of the recurring
tumors have an increased degree of malignancy (5). Accumulating evidence suggests that
genetic and epigenetic factors contribute to uncontrolled cancer
cell proliferation and invasion, therefore mediating tumor cell
survival, treatment resistance and metastatic spread. Immunotherapy
and chemotherapy are commonly used to temporary restrain the
recurrence; however, eventually the tumor progresses (6). Methods of targeting these
chemotherapy-resistant cancer cells are now emerging as a new and
critical focus in the field.
miRNAs are a type of 21- to 25-nucleotide non-coding
RNAs which regulate gene expression at the level of mRNA stability
and translation (7). miRNAs are
post-transcriptional regulators that bind to the 3′-untranslated
regions (3′-UTRs) of target mRNAs, usually leading to translational
repression and gene silencing (8). It
is well established that a number of miRNAs are highly
tissue-specific and play significant roles in multiple
physiological processes, including cell differentiation and tissue
development. Therefore, aberrant expression of miRNA is involved in
cellular dedifferentiation, oncogenesis, tumor invasion and
metastasis (9). Identification of the
targets of miRNAs would help us to understand the function of
miRNAs in tumor development and progression. Among them, miR-143 is
of particular interest due to its constant downregulation in a wide
range of cancer cell lines and tumors, including bladder cancer
(10). However, the mechanism of
miR-143 action remains largely unknown.
Insulin-like growth factor-1 receptor (IGF-1R) and
its ligand play an essential role in regulating cellular
proliferation and apoptosis (11).
Upregulated expression of IGF-1R has been documented in numerous
malignancies, including lung, breast, liver and thyroid cancer
(12). Ligand binding to IGF-1R
triggers various downstream signaling pathways, including the
PI3K/Akt pathway, which is essential for cell survival (13,14). In
colorectal cancer, overexpression of miR-143 inhibits cell
proliferation, migration and tumor growth and increases
chemosensitivity to oxaliplatin treatment in an IGF-1R-dependent
manner, suggesting that IGF-1R is a functional target of miR-143
(15). Moreover, miR-143 levels in
human blood and tumor tissues are associated with cancer
occurrence, metastasis and drug resistance (15), indicating the potential of miR-143 as
a biomarker. The role of IGF-1R in cancerous transformation of
urothelial cells is not well established, but previous studies have
demonstrated that IGF-1R is overexpressed in bladder cancer
(16). Given the interrelationship
between IGF-1R and miR-143, we speculate that downregulation of
miR-143 in bladder cancer may be involved in tumor development via
the activation of IGF-1R and other downstream pathways (e.g.
PI3K/Akt and MAPK). To confirm this hypothesis, we examined the
levels of miR-143 and IGF-1R levels in benign bladder and cancer
tissues, as well as cancer cell lines. We also performed a
functional study to reveal the role of miR-143 and IGF-1R in
bladder cancer cell growth and chemoresistance. Finally, a large
bladder cancer data set from The Cancer Genome Atlas (TCGA) was
utilized to correlate the expression of IGF-1R to clinical
traits.
Materials and methods
Reagents and cell culture
Two human bladder cancer cell lines (T24 and 5637)
and an immortalized cell line (SV-HUC-1) from human urinary bladder
urothelium were obtained from the Shanghai Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). All cell lines were cultured
in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100
U/ml penicillin and 100 mg/l streptomycin in a humidified incubator
with 5% CO2 at 37°C.
Human specimens
Human bladder cancer tissues (n=23) and their
matched normal adjacent tissues (n=23) were obtained from patients
at the Huai'an First People's Hospital, Jiangsu, China. The study
was approved by the Academic and Ethics Advisory Board of Nanjing
Medical University with documented patient consent.
Lentivirus infection and transient
transfections
High-titer stocks of lentivirus carrying miR-143 or
scramble control (miR-SCR) were purchased from GenePharma
(Shanghai, China). The lentiviruses were used to infect the T24 and
5637 cells. Infected cells were selected by puromycin
(Sigma-Aldrich, St. Louis, MO, USA), and then stable T24 and 5637
cell lines expressing miR-143 and miR-SCR were established. Small
interfering RNA (siRNA) duplex oligonucleotides targeting IGF-1R or
scramble control were synthesized by GenePharma. T24 cells were
transfected with either siIGF-IR or siSCR using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNAs were extracted from cultured cells or
human tissues using TRIzol reagent (Invitrogen Life Technologies)
according to the manufacturer's instructions. To quantify the mRNA
levels of IGF-1R and GAPDH, RNAs were transcribed using a
PrimeScript RT reagent kit and oligo dT primer (Takara
Biotechnology Co., Ltd., Dalian, China). To measure miR-143 and U6
expression levels, RNAs were transcribed using stem-loop RT primer
and the PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.)
as previously described (17,18). RT-qPCR was performed using SYBR Premix
DimerEraser (Takara Biotechnology Co., Ltd.). Primers were
purchased from RiboBio (Shenzhen, China).
Antibodies and western blot
analysis
Tissues or cells were harvested and lysed on ice for
30 min in RIPA buffer (Beyotime Institute of Biotechnology, Haimen,
China) supplemented with 1 mM phenylmethylsulfonyl fluoride.
Lysates were subjected to western blot assay as described
previously (19). Antibodies against
IGF-1R, p-Akt (Ser-473), Akt, p-ERK (Thr202/Tyr204), ERK (Cell
Signaling Technology, Inc., Danvers, MA, USA) and GAPDH (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) were used to check the
protein expression.
Cell proliferation and
chemosensitivity assays
A total of 4,000 cells per well were plated in
96-well plates, and cultured at 37°C in an incubator with 5%
CO2. Cell proliferation was measured at 24, 48, 72 and
96 h using a Cell counting kit 8 (CCK8; Dojindo Molecular
Technologies, Inc., Tokyo, Japan) according to the manufacturer's
instructions. Results were obtained from three separate experiments
with six replications per experiment, and presented as the means ±
standard deviation (SD). For chemosensitivity assay, freshly
prepared gemcitabine (Sigma-Aldrich) was added at varying
concentrations (ranging from 0.64 to 10,000 nM). Cell viability was
determined 72 h later by CCK8 kit.
Statistical analysis
Results were mainly presented as mean ± SD, and
Graphpad Prism software (GraphPad Software, Inc., La Jolla, CA,
USA) was used to calculate the mean and standard deviation. The
statistical differences within results among the groups or
treatments were analyzed using an unpaired Student's t-test or
Pearson correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-143 is downregulated in bladder
cancer tissues and cell lines
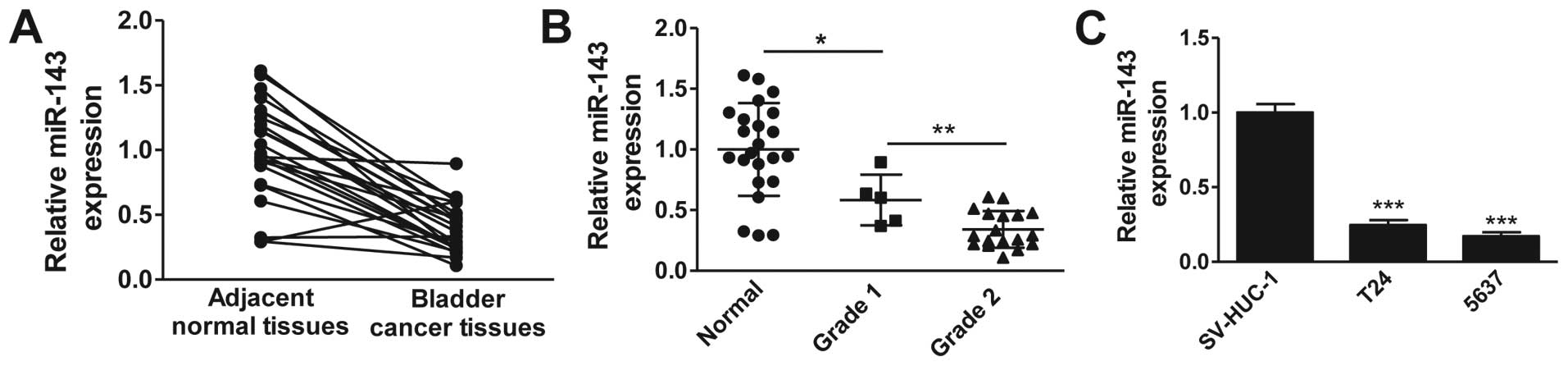
Accumulating evidence suggests a downregulation of
miR-143 in multiple cancers. To determine whether miR-143 is
downregulated in clinical bladder cancer tissues, we examined the
miR-143 expression levels in 20 pairs of bladder cancer tissues and
matched adjacent normal tissues by RT-qPCR. As shown in Fig. 1A, the expression levels of miR-143 in
bladder cancer tissues were significantly lower than those in
adjacent normal tissues. We further analyzed the miR-143 expression
levels according to the pathological features of bladder cancer
patients. Consistently, miR-143 expression was notably decreased in
bladder cancer tissues compared with normal tissues, and the
expression of miR-143 was negatively correlated with the level of
malignancy, with grade 2 demonstrating the lowest expression
(Fig. 1B). We next evaluated the
expression levels of miR-143 in widely used bladder cancer cell
lines. The result clearly demonstrated that miR-143 expression was
markedly downregulated in bladder cancer cells (T24 and 5637)
compared with normal uroepithelial cells (SV-HUC-1) (Fig. 1C). Collectively, these data confirm
the downregulation of miR-143 in clinical bladder cancer tissues
and cancer cell lines.
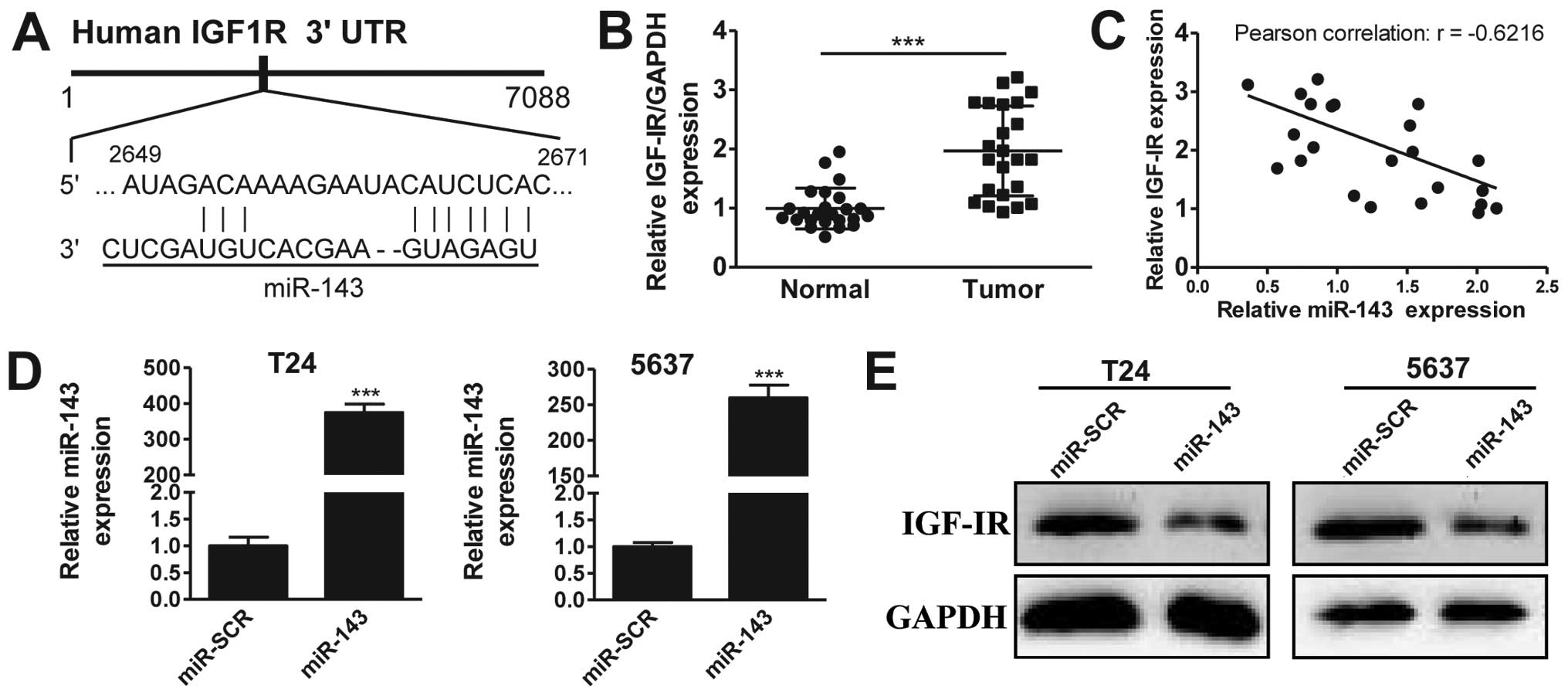
miR-143 targets IGF-1R
It has previously been suggested that IGF-1R may be
a target of miR-143 (20). To
investigate this further in bladder cancer, we first performed
in silico analysis using TargetScan, observing that miR-143
potentially targets a region of the 3′-UTR sequence of the IGF-1R
gene (Fig. 2A). Next, we analyzed the
expression of IGF-1R in 20 pairs of bladder cancer and matched
adjacent normal tissues. The RT-qPCR results revealed that IGF-1R
was overexpressed in bladder cancer tissues compared with normal
tissues (Fig. 2B), a pattern opposite
to that of miR-143. This suggested that IGF-1R may be a target of
miR-143. To strengthen this hypothesis, we plotted the expression
data from Figs. 1A and 2B, and observed that the expression levels
of miR-143 and IGF-1R were reversely correlated (Fig. 2C, Pearson's correlation scatter plots:
R=−0.6216, P<0.05). To confirm that miR-143 targets IGF-1R, we
performed overexpression experiments. As shown in Fig. 2C, transfection of miRNA mimics
successfully induced the upregulation of miR-143 in T24 (395-fold
higher) and 5637 (250-fold higher) cancer cell lines, compared with
the scrambled negative control (miR-SCR) group. Significantly,
IGF-1R protein levels were markedly decreased by miR-143
overexpression in the two cell lines (Fig. 2D). Together, our data suggest that
miR-143 inhibits IGF-IR expression in bladder cancer.
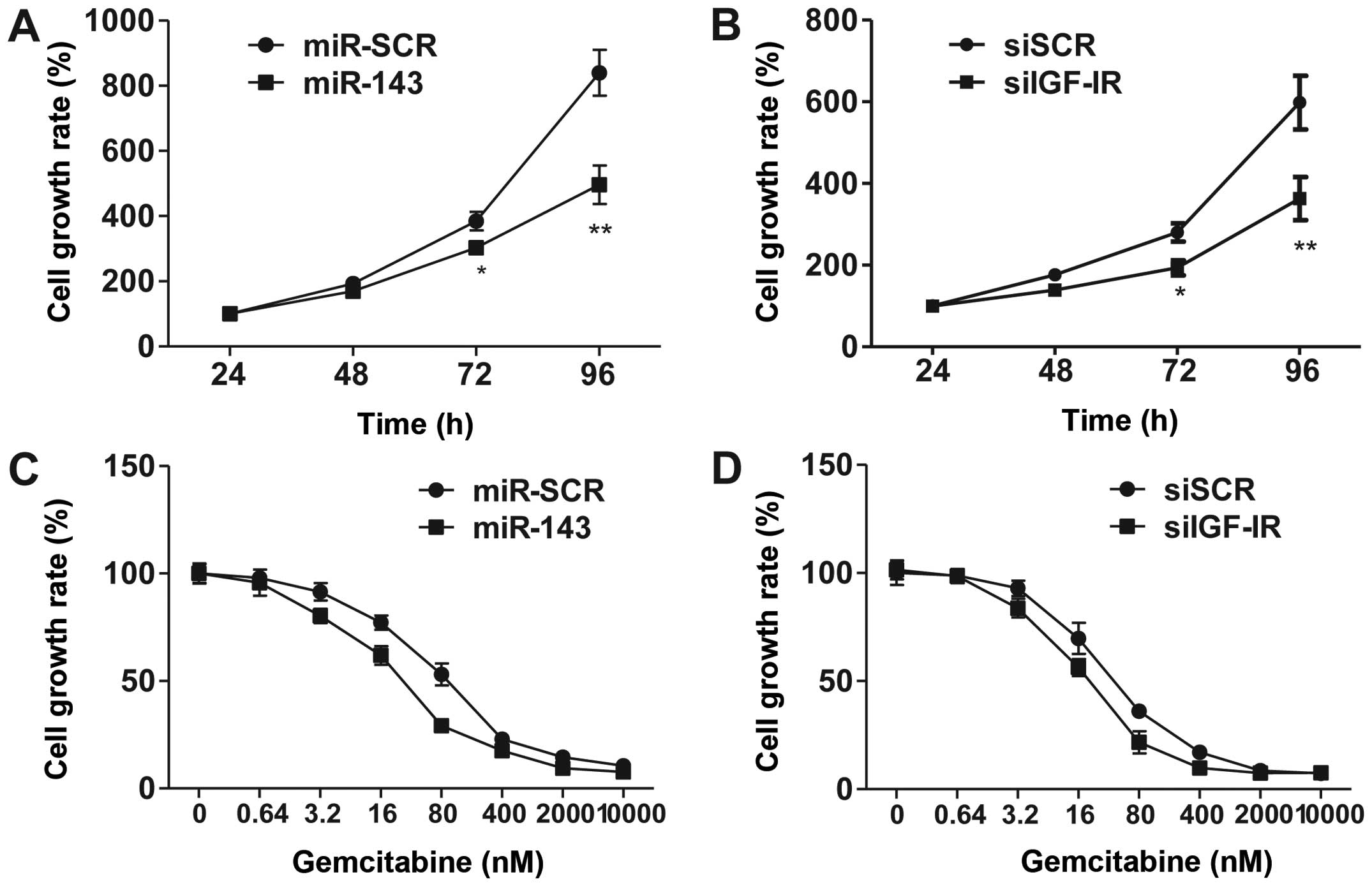
Overexpression of miR-143 and
knockdown of IGF-1R enhance chemosensitivity to gemcitabine
Several studies have suggested that miR-143
functions as a tumor suppressor (21–23). To
investigate the effects of overexpression of miR-143, we utilized
CCK-8 assay to demonstrate that forced miR-143 expression inhibited
5673 cell proliferation (Fig. 3A).
Consistently, knockdown of IGF-1R by siRNA phenocopied miR-143
overexpression in regulating cell proliferation. These results
suggest that miR-143 exhibits its function via the inhibition of
IGF-1R signaling.
IGF-1R is overexpressed in bladder cancer cells, and
IGF signaling plays a significant role in promoting cell survival
and proliferation. To explore the contribution of IGF-1R and
miR-143 in chemotherapy, we treated 5637 cells with various
concentrations of gemcitabine, a leading chemotherapy drug used in
the treatment of bladder cancer. The results revealed that
overexpression of miR-143 and siRNA-mediated knockdown of IGF-1R
significantly increased cell sensitivity to gemcitabine (Fig. 3C and D). These data suggested that
miR-143 enhances chemosensitivity to gemcitabine through, at least
partially, downregulation of IGF-1R.
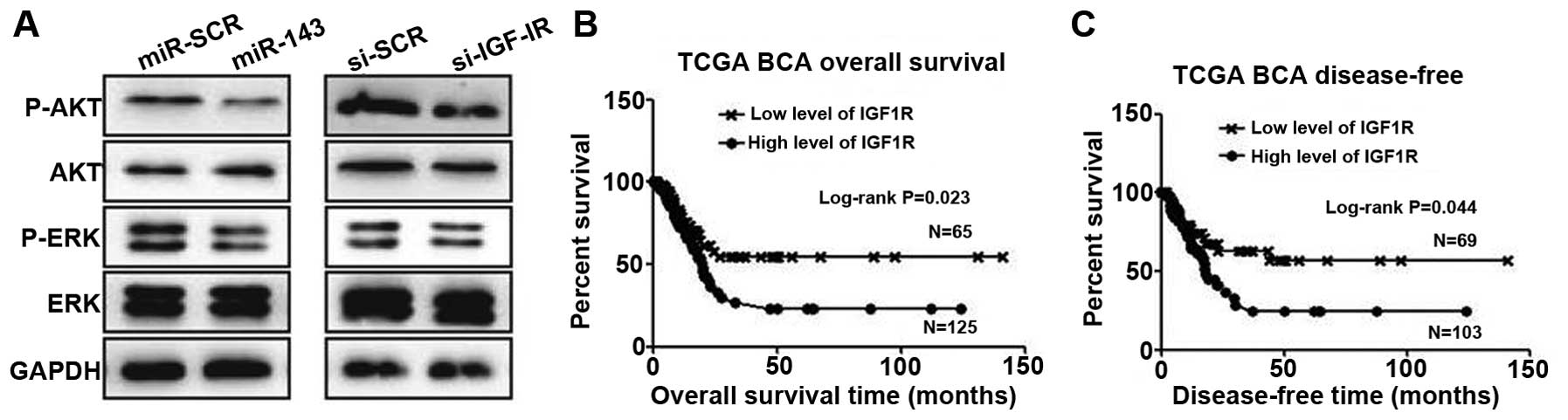
miR-143/IGF-1R axis regulates Akt and
extracellular signal-regulated kinase (ERK) pathway activation in
5637 cells
To assess the involvement of downstream signaling
molecules, we transfected 5637 cells with miR-143 or negative
control and examined Akt and ERK pathway activation. Overexpression
of miR-143 significantly inhibited p-Akt and p-ERK levels in 5637
cells (Fig. 4A). Similarly, knockdown
of IGF-1R also reduced the expression of these molecules (Fig. 4A). To put these findings into clinical
relevance, we speculate that downregulation of miR-134 would
benefit cancer cell growth through activation of the IGF-1R, Akt
and ERK pathways.
Expression of IGF-1R negatively
correlates with patient survival
A previous study (24)
and our data suggest that IGF-1R is upregulated in bladder cancer
cells. To investigate the possibility of IGF-1R as a biomarker for
prognosis, we employed the largest TCGA bladder cancer database.
After extracting and plotting IGF-1R expression against patient
clinical data, we observed that IGF-1R is a suitable marker for
predicting patient overall survival, with higher expression
demonstrating a short survival time (Fig.
4B). Significantly, IGF-1R levels also predict tumor recurrence
(Fig. 4C).
Discussion
It is well known that miRNAs are involved in diverse
processes, including apoptosis, proliferation, differentiation and
chemosensitivity. Thus, a number of miRNAs have been linked to
distinct development defects or cancers (25,26).
Previous studies have revealed low miR-143 expression in various
types of tumors (27,28). In the present study, we observed
decreased expression of miR-143 in bladder cancer tissues and cell
lines compared with normal tissues, consistent with the findings of
Noguchi et al (28). We
observed a negative correlation between miR-143 and IGF-1R mRNA
expression in bladder cancer tissues and cell lines, suggesting
that the tumor-suppressive role of miR-143 may be attributed to its
suppression of IGF-1R. Subsequently, we identified IGF-1R as a
functional target for miR-143. Furthermore, chemosensitivity to
gemcitabine was improved by upregulating miR-143 and knocking down
IGF-1R in 5637 cells.
Notably, miR-143 has been suggested as a biomarker
for various types of cancer (29,30). Qian
et al (15) indicated that low
levels of miR-143 in colorectal cancer tissues were closely
associated with cancer stage and metastasis. Consistently, the
results of the present study revealed a decreasing expression of
miR-143 with the increasing bladder cancer grade, suggesting that
miR-143 has a tumor suppressive role in bladder cancer
progression.
IGF-1R has been identified as a key regulator of
tumor development by regulating cell proliferation, differentiation
and survival (31,32). Upregulated expression of IGF-1R has
been observed in numerous human malignancies (12). Previous studies have revealed that
IGF-1R is also upregulated in human invasive bladder cancer and
promotes cell migration and invasion (33). Based on the signaling pathway analysis
results, we speculated that miR-143 exerts a tumor suppressor
function through the inhibition of IGF-1R, consistent with previous
studies demonstrating that miR-143 directly targets the 3′-UTR
region to suppress IGF-1R expression (20). Aberrant expression of IGF-1R affects a
number of downstream signaling cascades, including the PI3K/Akt and
MAPK/ERK pathways (34,35), which are frequently activated in
cancer cells. These pathways play critical roles in tumor growth
and progression (36,37). The present study indicated that
overexpression of miR-143 significantly inhibits Akt and ERK
activity, as evidenced by the decreased levels of p-Akt and p-ERK.
Consequently, forced expression of miR-143 enhanced the
chemosensitivity of 5637 cells to gemcitabine in vitro. To
further strengthen the clinical relevance of our study, we analyzed
TCGA bladder cancer data, observing that expression of IGF-1R
stratifies patients with higher levels of IGF-1R, predicting a
poorer patient survival time.
In conclusion, our study demonstrated that miR-143
inhibits bladder cancer cell proliferation and improves
chemosensitivity to gemcitabine, at least in part through the
inactivation of IGF-1R and downstream pathways in vitro. The
expression of IGF-1R negatively correlates with a beneficial
clinical outcome of patients. The findings of the present study
provide new information to support the possible usage of
miR-143/IGF-1R-based therapeutic strategies to treat bladder cancer
patients in the future.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
miR-143
|
microRNA-143
|
|
IGF-1R
|
insulin-like growth factor-1
receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
UTR
|
untranslated region
|
|
mRNA
|
messenger RNA
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dinney CP, McConkey DJ, Millikan RE, Wu X,
Bar-Eli M, Adam L, Kamat AM, Siefker-Radtke AO, Tuziak T, Sabichi
AL, et al: Focus on bladder cancer. Cancer Cell. 6:111–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jebar AH, Hurst CD, Tomlinson DC, Johnston
C, Taylor CF and Knowles MA: FGFR3 and Ras gene mutations are
mutually exclusive genetic events in urothelial cell carcinoma.
Oncogene. 24:5218–5225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McConkey DJ, Lee S, Choi W, Tran M,
Majewski T, Lee S, Siefker-Radtke A, Dinney C and Czerniak B:
Molecular genetics of bladder cancer: emerging mechanisms of tumor
initiation and progression. Urol Oncol. 28:429–440. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A and Palou-Redorta J: European Association of
Urology (EAU): EAU guidelines on non-muscle-invasive urothelial
carcinoma of the bladder. Eur Urol. 54:303–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamm DL, Riggs DR, Traynelis CL and Nseyo
UO: Apparent failure of current intravesical chemotherapy
prophylaxis to influence the long-term course of superficial
transitional cell carcinoma of the bladder. J Urol. 153:1444–1450.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dyrskjøt L, Ostenfeld MS, Bramsen JB,
Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL,
Andersen CL, Zieger K, et al: Genomic profiling of microRNAs in
bladder cancer: miR-129 is associated with poor outcome and
promotes cell death in vitro. Cancer Res. 69:4851–4860. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubin R and Baserga R: Insulin-like growth
factor-I receptor. Its role in cell proliferation, apoptosis, and
tumorigenicity. Lab Invest. 73:311–331. 1995.PubMed/NCBI
|
|
12
|
Macaulay VM: Insulin-like growth factors
and cancer. Br J Cancer. 65:311–320. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baserga R: Controlling IGF-receptor
function: a possible strategy for tumor therapy. Trends Biotechnol.
14:150–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian X, Yu J, Yin Y, He J, Wang L, Li Q,
Zhang LQ, Li CY, Shi ZM, Xu Q, et al: MicroRNA-143 inhibits tumor
growth and angiogenesis and sensitizes chemosensitivity to
oxaliplatin in colorectal cancers. Cell Cycle. 12:1385–1394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rochester MA, Patel N, Turney BW, Davies
DR, Roberts IS, Crew J, Protheroe A and Macaulay VM: The type 1
insulin-like growth factor receptor is over-expressed in bladder
cancer. BJU Int. 100:1396–1401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X: A PCR-based platform for microRNA
expression profiling studies. RNA. 15:716–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL,
Barnett J, Rojanasakul Y, Agani F and Jiang BH: Cadmium increases
HIF-1 and VEGF expression through ROS, ERK, and AKT signaling
pathways and induces malignant transformation of human bronchial
epithelial cells. Toxicol Sci. 125:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He J, Qian X, Carpenter R, Xu Q, Wang L,
Qi Y, Wang ZX, Liu LZ and Jiang BH: Repression of miR-143 mediates
Cr (VI)-induced tumor angiogenesis via IGF-IR/IRS1/ERK/IL-8
pathway. Toxicol Sci. 134:26–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao
Y, Feng Y, Li L, Wang Y, Liu X, et al: MicroRNA-143 (miR-143)
regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol
Chem. 287:23227–23235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gregersen LH, Jacobsen A, Frankel LB, Wen
J, Krogh A and Lund AH: MicroRNA-143 down-regulates Hexokinase 2 in
colon cancer cells. BMC Cancer. 12:2322012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kent OA, Fox-Talbot K and Halushka MK:
RREB1 repressed miR-143/145 modulates KRAS signaling through
downregulation of multiple targets. Oncogene. 32:2576–2585. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Metalli D, Lovat F, Tripodi F, Genua M, Xu
SQ, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, et al:
The insulin-like growth factor receptor I promotes motility and
invasion of bladder cancer cells through Akt- and mitogen-activated
protein kinase-dependent activation of paxillin. Am J Pathol.
176:2997–3006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sekar D, Islam VI Hairul,
Thirugnanasambantham K and Saravanan S: Relevance of miR-21 in HIV
and non-HIV-related lymphomas. Tumour Biol. 35:8387–8393. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iida K, Fukushi J, Matsumoto Y, Oda Y,
Takahashi Y, Fujiwara T, Fujiwara-Okada Y, Hatano M, Nabashima A,
Kamura S and Iwamoto Y: miR-125b develops chemoresistance in Ewing
sarcoma/primitive neuroectodermal tumor. Cancer Cell Int.
13:212013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noguchi S, Mori T, Hoshino Y, Maruo K,
Yamada N, Kitade Y, Naoe T and Akao Y: MicroRNA-143 functions as a
tumor suppressor in human bladder cancer T24 cells. Cancer Lett.
307:211–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ak S, Tunca B, Tezcan G, Cecener G, Egeli
U, Yilmazlar T, Ozturk E and Yerci O: MicroRNA expression patterns
of tumors in early-onset colorectal cancer patients. J Surg Res.
191:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ng EK, Li R, Shin VY, Siu JM, Ma ES and
Kwong A: MicroRNA-143 is downregulated in breast cancer and
regulates DNA methyltransferases 3A in breast cancer cells. Tumour
Biol. 35:2591–2598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hart LS, Dolloff NG, Dicker DT, Koumenis
C, Christensen JG, Grimberg A and El-Deiry WS: Human colon cancer
stem cells are enriched by insulin-like growth factor-1 and are
sensitive to figitumumab. Cell Cycle. 10:2331–2338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao D, Bakirtzi K, Zhan Y, Zeng H, Koon
HW and Pothoulakis C: Insulin-like growth factor-1 receptor
transactivation modulates the inflammatory and proliferative
responses of neurotensin in human colonic epithelial cells. J Biol
Chem. 286:6092–6099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Z, Xu T, Wang L, Wang X, Zhong S, Xu C
and Shen Z: MicroRNA-145 directly targets the insulin-like growth
factor receptor I in human bladder cancer cells. FEBS Lett.
588:3180–3185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berns K, Horlings HM, Hennessy BT,
Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM,
Stemke-Hale K, Hauptmann M, et al: A functional genetic approach
identifies the PI3K pathway as a major determinant of trastuzumab
resistance in breast cancer. Cancer Cell. 12:395–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park BH and Davidson NE: PI3 kinase
activation and response to trastuzumab therapy: what's neu with
herceptin resistance? Cancer Cell. 12:297–299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|