Introduction
Endometrial cancer is the fifth most common type of
cancer in females worldwide, accounting for 4.8% of all cancer
cases in women (1). The incidence of
endometrial cancer is higher in developed regions, such as North
America and Northern and Western Europe than in Asia and Africa
(2–4).
However, the number of newly diagnosed cases of endometrial cancer
have significantly increased between 2003 and 2013 in China
(5). Endometrial cancer has been
broadly classified into 2 groups based on histological examination
(6,7).
Type 1 endometrial cancers are mainly adenocarcinomas of
endometrioid origin, accounting for 70–80% of cases. Type 2 cancers
are of non-endometrioid origin and classified as serous, clear cell
and squamous cell cancers, accounting for 10–20% of all endometrial
tumors. The prognosis of type 1 endometrial cancers is usually more
favorable than type 2 cancers (8). In
addition to tumor histology type, survival of endometrial cancer is
strongly affected by tumor stage, grades, and invasive status. When
diagnosed at advanced stages, or with poorly differentiated tumors,
surgical treatment and chemotherapy are not curative for the two
types of endometrial cancer patients, and 5-year survival rate is
poor. Novel biomarkers and therapeutic targets are therefore
imperative for improving the survival of advanced endometrial
cancer patients.
The H19 gene is located on chromosome 11p15.5 and is
established as an imprinted gene (9,10). The H19
gene encodes a spliced and polyadenylated RNA that lacks conserved
open reading frames. Since no endogenous translation product has
been identified (11), the transcript
of H19 gene has been proposed as a long non-coding RNA (lncRNA).
Expression of lncRNA H19 is developmentally regulated, since it is
abundantly expressed in extra-embryonic and fetal tissues but
significantly repressed in the majority of adult organs following
birth. Deregulation of H19 is found in a variety of cancer tissues,
but its status and roles in cancer remains controversial. H19 is
initially suggested as a suppressor for tumor development and
metastasis (12,13). However, previously H19 has been
proposed as an oncogenic lncRNA in numerous cancers (14,15), and
is found to positively regulate the metastatic potential of cancer
cells (16,17). In endometrioid endometrial cancer
tissues (18–20), overexpression of H19 exists and is
associated with neoplastic cell invasion. However, the precise
function of H19 in cellular processes involved in progression of
endometrioid endometrial cancer is not yet understood.
H19 is associated with epithelial-mesenchymal
transition (EMT) in certain cancers (16,17), which
presents a novel mechanistic insight into the role of H19 in tumor
progression. EMT, first described in embryogenesis, is integrated
into pathological processes such as organ fibrosis and cancer
progression. EMT refers to the process by which epithelial
properties, including the compacted morphology and epithelial
marker expression, are replaced by the mesenchymal phenotype, such
as dispersed spindle-shaped morphology and mesenchymal gene
expression, accompanied by the enhancement of cell motility and
invasion. This provides evidence for EMT having a role in the
promotion of the migratory and invasive capabilities of cancer
cells. The most studied EMT-associated molecules are E-cadherin,
the adherens junction protein in epithelial cells, and its
transcriptional repressors, including Snail, Slug, Twist and Zeb.
These transcription factors are upregulated during EMT, binding to
the E-box in the promoter region of the gene encoding the
E-cadherin (CDH), repressing CDH transcription, and finally leading
to the loss of apical-basal polarity and cell-cell adhesion
junction.
In the present study, the level of H19 was increased
in cancerous tissues compared with paratumoral tissues. Suppression
of H19 by small interfering RNA (siRNA) inhibited cell motility and
invasion, reduced Snail expression and increased E-cadherin
expression, without affecting the vimentin level in HEC-1B
endometrial cancer cells, indicating partial reversion of EMT
process by H19 decrease. These results highlighted the pro-tumor
role of H19 in endometrial cancer progression.
Materials and methods
Tissue specimens
The study included 20 primary adenocarcinomas of
endometrioid origin and matched paratumoral normal tissues. Tissue
samples were acquired from the Department of Gynecology and
Obstetrics at the First Affiliated Hospital of Xi'an Jiaotong
University between January 2013 and October 2014. The patients
underwent radical hysterectomy with complete clinical history
records. All patients received no chemotherapy or radiotherapy
prior to surgery. Informed content was obtained from all patients
involved, and the study protocol was performed under approval of
the Ethics Committee of the First Affiliated Hospital, Xi'an
Jiaotong University.
Cell line and culture
The human endometrial cancer cell line HEC-1-B was
obtained from the Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China). Cells were grown in RPMI 1640 supplemented with
10% newborn bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a humidified atmosphere with 5% CO2
at 37°C.
siRNA transfection
Transfection of siRNA was conducted using the
X-tremeGENE siRNA transfection reagent (Roche Applied Science,
Madison, WI, USA) according to the manufacturer's protocol. siRNAs
specific to the human H19 gene were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China), and the sequences were as
follows siH19-a, 5′-UAAGUCAUUUGCACUGGUUdTdT-3′ and siH19-b,
5′-CCAACAUCAAAGACACCAUdTdT-3′. A scrambled siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) was used in parallel experiments as a
negative control. Cells were plated onto 6-well plates and grew to
40–50% confluence at the time of transfection. For each sample, 1
µg of the siRNA and 5 µl of transfection reagent were incubated in
100 µl of serum- and antibiotics- free medium for 5 min,
respectively. This was followed by mixing the solutions together
and incubating at room temperature for another 20 min, and the
resultant solution was layered over cells at 37°C for 48 h for RNA
extraction and 72 h for protein extraction.
RNA isolation and reverse
transcription
Isolation of total RNA was performed using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), in accordance
with the manufacturer's protocol. Quality and concentration of
total RNA were assessed using a UV spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a 260/280 nm absorbance
ratio and at the 260 nm absorbance, respectively. First strand cDNA
was synthesized using RevertAid first strand cDNA synthesis kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The reverse transcription reactions were performed at
25°C for 5 min, followed by 42°C for 60 min and 70°C for 5 min. The
cDNAs were stored at −80°C for later use.
Quantitative (q)PCR
qPCR was performed using a CFX-96 qPCR system
(Bio-Rad Laboratories, Inc.) and SYBR Green Master Mix (Takara
Biotechnology Co. Ltd., Dalian, China). The gene β-actin was used
as an internal control. The PCR reaction mixture was prepared
following the manufacturer's protocol for the SYBR®
Premix Ex Taq™ (Takara Biotechnology Co., Ltd., Dalian, China). The
temperature cycle profile for the PCR reactions was 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
Each measurement was performed in triplicate, and no template
controls were included for each assay. Subsequent to PCR, a
dissociation curve analysis was performed. The relative quantity of
gene expression was calculated automatically by the
2−ΔΔCq method (21).
Primers were synthesized by Shanghai Shenggong Biology Engineering
Technology Service, Ltd. (Shanghai, China), as follows: H19
forward, 5′-TGCTGCACTTTACAACCACTG-3′ and reverse,
5′-ATGGTGTCTTTGATGTTGGGC-3′; E-cadherin forward,
5′-GCTGCTCTTGCTGTTTCTTCG-3′ and reverse,
5′-CCGCCTCCTTCTTCATCATAG-3′; vimentin forward,
5′-AAGTTTGCTGACCTCTCTGAGGCT-3′ and reverse
5′-CTTCCATTTCACGCATCTGGCGTT-3′; Snail forward,
5′-TCCAGAGTTTACCTTCCAGCA-3′ and reverse
5′-CTTTCCCACTGTCCTCATCTG-3′; β-actin forward,
5′-TCCCTGGAGAAGAGCTACGA-3′ and reverse
5′-AGCACTGTGTTGGCGTACAG-3′.
Western blot analysis
Total protein was isolated from cells in RIPA lysis
buffer on ice. Protein concentration was determined using Bradford
Protein Assay kit (Bio-Rad Laboratories, Inc.). Proteins were
boiled, then separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membranes (Pall
Life Sciences, Port Washington, NY, USA). The membranes were
blocked with 5% non-fat milk at room temperature for 1 h, incubated
with primary antibodies, including monoclonal rabbit-anti human
E-cadherin (dilution, 1:1,000; #3195), monoclonal rabbit-anti human
vimentin (dilution, 1:1,000; #5741), monoclonal rabbit-anti human
Snail (dilution, 1:500; #3879) and monoclonal mouse-anti human
β-actin (dilution, 1:1,000; #3700) (Cell Signaling Technology,
Inc., Danvers, MA, USA) overnight at 4°C. The membranes were then
exposed to horseradish peroxidase (HRP) conjugated goat anti-rabbit
immunoglobulin (IgG) (dilution, 1:1,000; #31460; Pierce
Biotechnology, Inc., Rockford, IL, USA) for detection of
E-cadherin, vimentin and Snail, or HRP conjugated goat anti-mouse
IgG (dilution, 1:1,000; #31430; Pierce, Biotechnology, Inc.) for
detection of β-actin. Immunodetection was performed using ECL
reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
luminescence image system (Bio-Rad Laboratories, Inc.). The protein
amounts were semi-quantified by analyzing blot intensity using
β-actin as loading control. In total, 3 independent experiments
were performed.
Cell viability
Cell viability was assessed using Cell Counting
kit-8 (CCK-8) (7sea; Pharmatech Co., Ltd., Shanghai, China) assays.
The cells were inoculated into 96-well plates, and then transfected
with siRNA or negative control, respectively, for 24–96 h. The
cells were then incubated with CCK-8 for 4 h at 37°C, and
absorbance at 450 nm was determined using an EnSpire multimode
plate reader (PerkinElmer, Inc., Waltham, MA, USA).
Wound healing assay
Cells were seeded into 6-well plate and transfected
with siRNA specific to H19 or scrambled siRNA control for 24 h.
When cells reached 90% confluence, wounds were generated by
scratching the monolayers using a 200 µl pipette tip. Cells were
washed to remove the detached cells and then maintained in media
without serum. Images were captured on an inverted microscope
(Leica Biosystems, Wetzlar, Germany) installed with a ToupCam
digital camera and ToupView 3.7 software (ToupTek, Hangzhou, China)
of the wounded areas following incubation for 72 h.
Transmigration and invasion
assays
Cellular migration and invasion were tested using
Millicell modified Boyden chambers (EMD Millipore, Billerica, MA,
USA) with (invasion assay) or without (migration assay)
Matrigel-coated membrane. Cells were seeded in serum-free medium
into the upper chamber and allowed to migrate or invade toward
media containing 20% fetal bovine serum (FBS) as a chemoattractant
in the lower chamber for 24 h (migration assay) or 48 h (invasion
assay). Cells on the upper surface of the membrane were removed
with a cotton swab, and cells at the bottom of the membrane were
fixed with 5% glutaric dialdehyde and stained with Giemsa
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). The number of
transmigrated cells was counted in 5 random fields under an
inverted microscope (Leica Biosystems) installed with the ToupCam
digital camera and ToupView software (ToupTek) (magnification,
×200). Each experiment was performed in triplicate.
Statistical analysis
Statistical differences were determined by Student's
two-tailed t-test. All statistical analyses were conducted using
SPSS statistical software (SPSS, Inc., Chicago, IL, USA). Values
were considered to indicate a statistically significant difference
at P<0.05 and highly significant difference at P<0.01.
Results
Expression of H19 in endometrial
cancer and paratumoral tissues
In total, 20 informative cases were analyzed. The
ages of the patients ranged between 38 and 70 years (median age, 54
years). The clinicopathological characteristics of the patients
were summarized in Table I. The
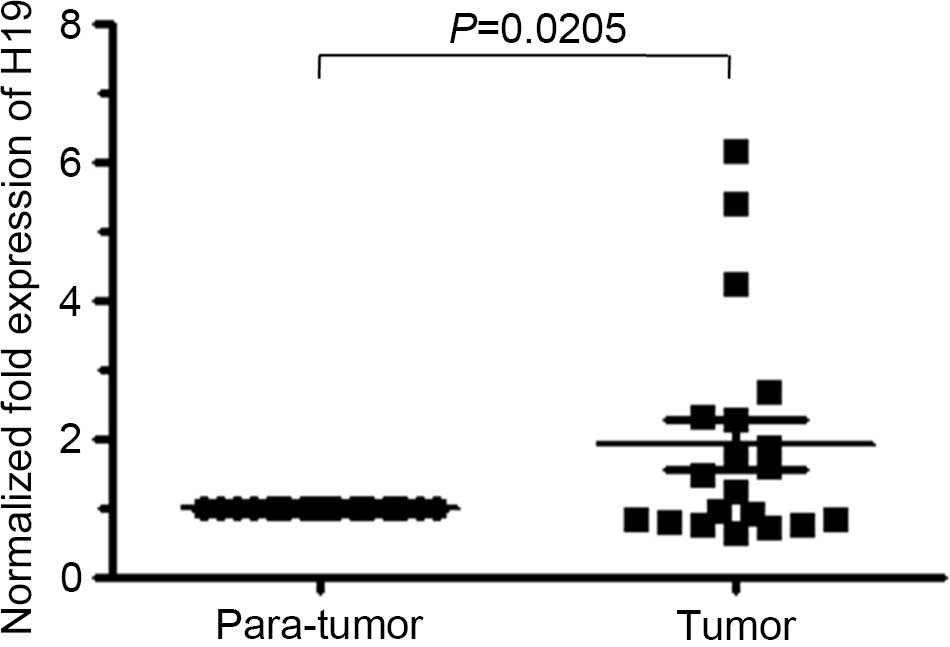
average level for H19 RNA in endometrial cancer was almost 2-fold
that of paratumoral tissues (Fig. 1),
and the differential H19 expression status between paratumoral and
tumor tissues was statistically significant (P=0.0205). The H19
transcript level increased with the lack of cellular
differentiation. However, no significant association was identified
between H19 level and other clinicopathological parameters of
endometrial cancer patients (P>0.05; Table II).
 | Table I.Clinicopathological parameters of
patients with endometrial cancer. |
Table I.
Clinicopathological parameters of
patients with endometrial cancer.
| Clinicopathological
parameter | Value, % (n) |
|---|
| Age, years |
|
|
<50 | 65.0 (13/20) |
| ≥50 | 35.0 (7/20) |
| FIGO |
|
| I | 70.0 (14/20) |
| II | 15.0 (3/20) |
|
III | 15.0 (3/20) |
| Grade |
|
| Well
differentiated | 25.0 (5/20) |
|
Moderately differentiated | 60.0 (12/20) |
| Poorly
differentiated | 15.0 (3/20) |
| Invasion
deptha |
|
|
<1/3 | 75.0 (15/20) |
|
≥1/3 | 25.0 (5/20) |
| Invasion to cervix
uterus, ovary or oviduct |
|
| No | 80.0 (16/20) |
|
Yes | 20.0 (4/20) |
 | Table II.Clinicopathological parameters of
patients with endometrial cancer according to H19 expression. |
Table II.
Clinicopathological parameters of
patients with endometrial cancer according to H19 expression.
| Clinicopathological
parameters | H19 level | P-value |
|---|
| Age, years |
| 0.637 |
|
<50 | 1.662±0.475 |
|
|
≥50 | 2.028±0.494 |
|
| FIGO |
| 0.374 |
| I | 1.790±0.384 |
|
| II | 1.271±0.272 |
|
|
III | 3.041±1.613 |
|
| Grade |
| 0.014 |
| Well
differentiated | 0.965±0.229 |
|
|
Moderately differentiated | 1.742±0.291 |
|
| Poorly
differentiated | 4.092±1.676 |
|
| Invasion
deptha |
| 0.192 |
|
<1/3 | 1.627±0.359 |
|
|
≥1/3 | 2.718±0.911 |
|
| Invasion to cervix
uterus, |
| 0.427 |
| ovary or
oviduct |
|
Negative | 1.754±0.336 |
|
|
Positive | 2.485±1.269 |
|
Downregulation of H19 exerts no effect
on cell viability
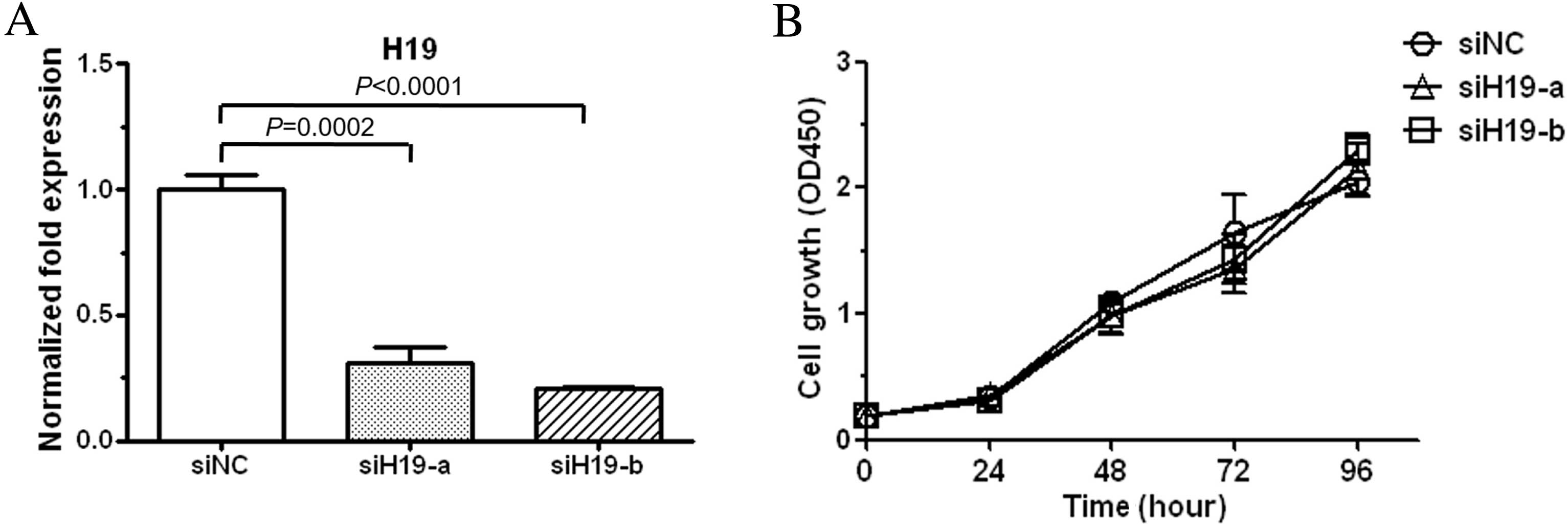
To elucidate the roles of H19 in endometrial cancer
progression, HEC-1-B cells were transduced with siRNAs specific to
H19 (siH19-a and siH19-b). As determined by qPCR analysis, siH19-a
and siH19-b could effectively knockdown H19 level (Fig. 2A). The effect of H19 under-expression
on cell viability was subsequently examined, and the results showed
that the viability of H19-suppressed cells was almost the same as
negative control cells during 96 h of siRNA transfection (Fig. 2B). These results showed that H19
overexpression did not confer proliferation advantage on HEC-1-B
cells.
Suppression of H19 inhibits cell
migration and invasion ability
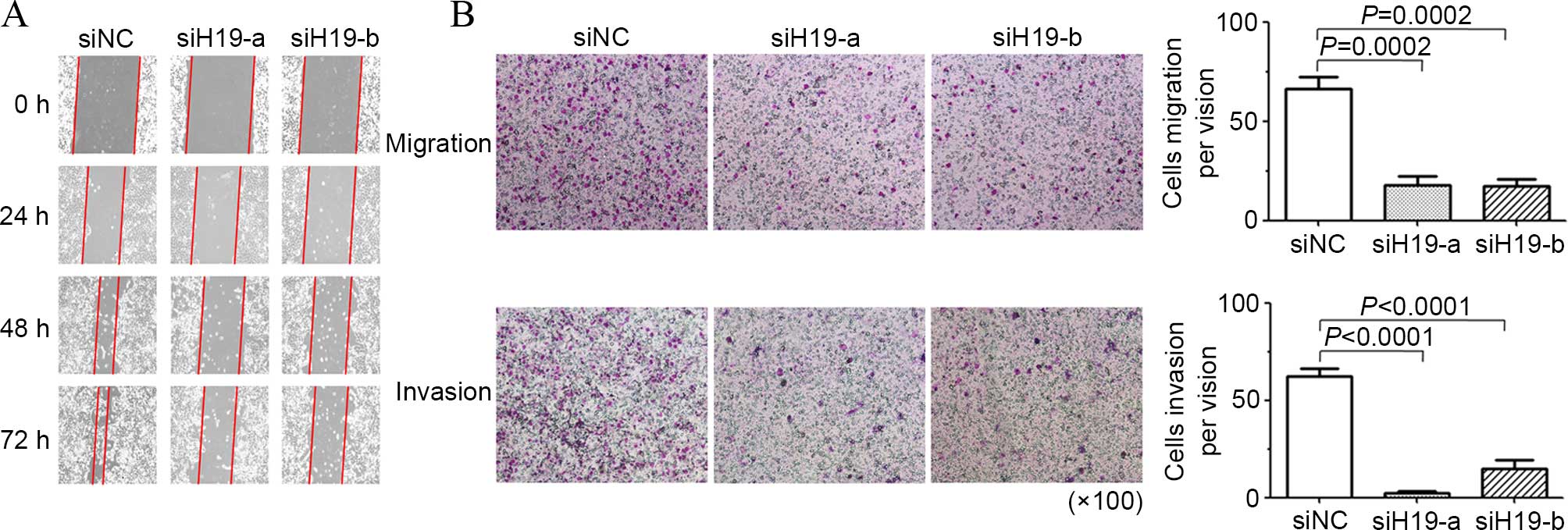
The present study examined the effect of H19
suppression on cell mobility with a wound-healing assay. As shown
in Fig. 3A, downregulation of H19
impeded wound closure of HEC-1-B cells over 72 h of H19 siRNA
transfection. Transmigration and invasion assays additionally
showed that H19 under-expression significantly lessened the in
vitro migration and invasion ability of HEC-1-B cells (Fig. 3B). These results indicated H19 was
involved in the metastatic spread of endometrial cancer cell.
Knockdown of H19 partially reverses
the EMT process
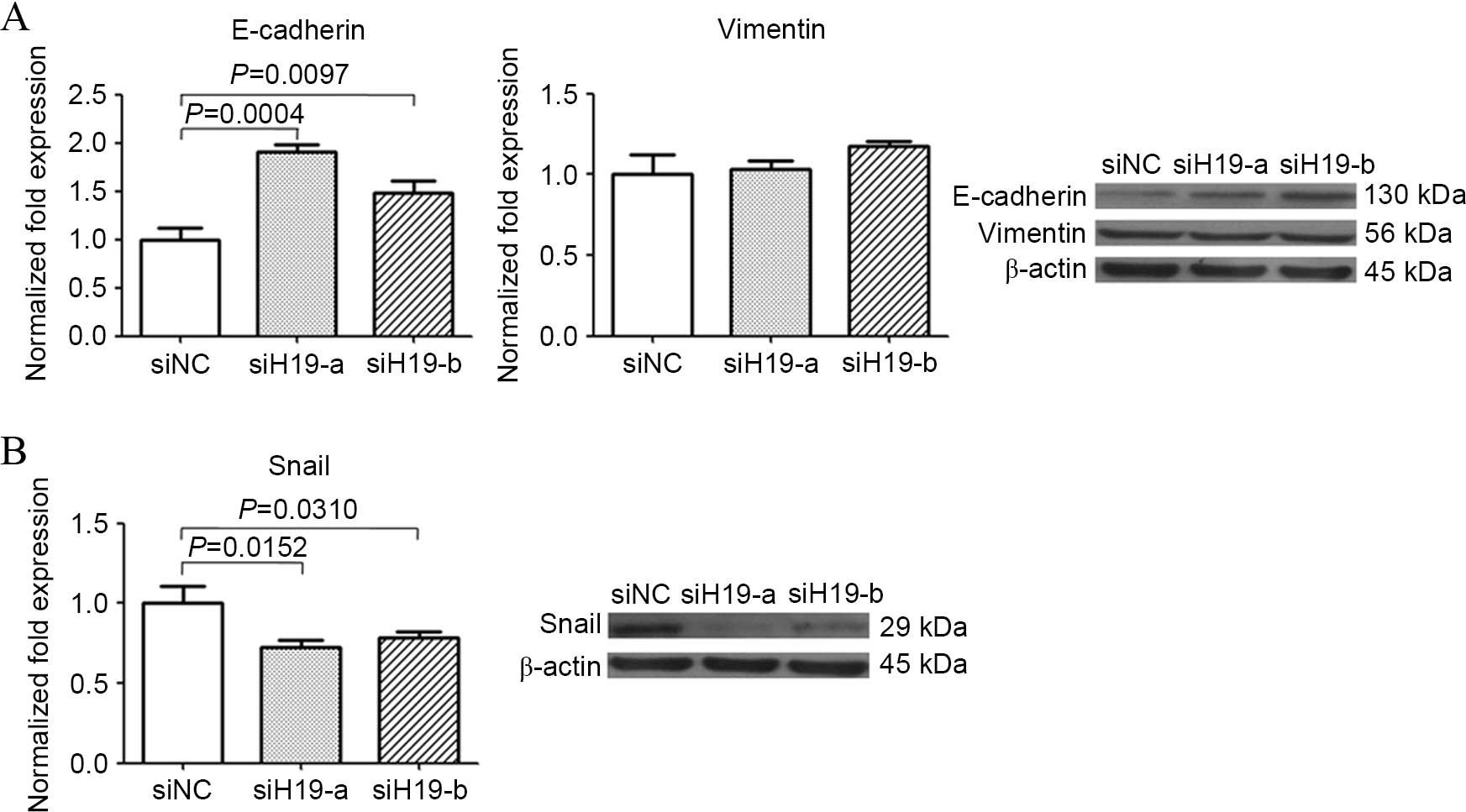
To explore the possible mechanism of H19 in cancer
cell migration and invasion, occurrence of EMT was examined by
detecting the expression changes of EMT molecules. Knocking-down of
H19 increased E-cadherin mRNA and protein, but caused no evident
change in vimentin expression (Fig.
4A). Increase of E-cadherin mRNA indicated H19 suppression may
release transcription of E-Cadherin coding gene from repression.
Expression of E-cadherin transcription repressor Snail was then
examined in H19-suppressed cells, and reduction of Snail at mRNA
and protein level was observed (Fig.
4B). These results suggested H19 decrease resulted in a partial
reverse of EMT.
Discussion
Relative survival for endometrial cancer has not
substantially improved over past decades (22,23). It is
necessary to mechanistically study endometrial cancer progression
and identify novel markers to monitor disease progression or to
develop gene-oriented drugs. The H19 gene is transcribed in a long
non-coding RNA molecule that accumulates in fetal tissues but is
repressed in the majority of adult organs (24). Dysregulation of H19 is associated with
cancer, but inconsistency exists about the role of H19 in tumor
development and progression (25–27). The
observations in certain cancers support an oncogenic role of H19,
since it is overexpressed and regulates genes involved in tumor
growth, metastasis and angiogenesis (15,24,28,29).
However, in other cases, H19 was not considered an
oncodevelopmental marker (13,30). At
present, the function of H19 in endometrial cancer invasion has not
been well established. In the present study, overexpressed H19 was
found to be associated with EMT in endometrial cancer, without
conferring a growth advantage on endometrial cancer cells. This
supports the conclusion that H19 is actively linked with cell
aggressiveness and positively effects the progression of
endometrial cancer.
Several lines of evidence have been presented for
the reasons why H19 is dysregulated in adult cancer tissues
(15,16,31,32). H19
is responsive to the induction of pro-tumor factors in the cancer
microenvironment including transforming growth factor-β and hypoxia
(16). In addition, H19 is under the
control of promoter regulation. It has been reported that the H19
promoter is activated by oncogenic transcription factors such as
c-myc (31), but negatively modulated
by the tumor suppressor p53 (33).
Methylation in the promoter region is another regulatory mechanism
of H19 expression. In ovarian cancer cells, overexpression of
histone H1 variant H1.3 increases occupancy of H1.3 at the H19
regulator region, leading to increase of DNA methylation and H19
knockdown (15). Additional
investigation is required to determine whether similar regulatory
mechanisms for H19 expression exist in endometrial cancer.
The importance of EMT has been well documented in
cancer metastasis; however, published literature about the
implication of H19 in EMT is inconsistent. H19 promotes EMT via
antagonizing let-7 and contributing to HMGA2-mediated EMT in
pancreatic ductal adenocarcinoma (17), or by being associated with enhancer of
zeste homolog 2 (EZH2) and activating wingless/β-catenin to
decrease E-cadherin in bladder cancer (34). H19 has been identified to encode
microRNA-675 (miR-675), and a positive feedback loop between
H19/miR-675 and Slug has been reported to attenuate E-cadherin and
lead to EMT in certain cancer cell lines (16). However, H19 is found to reverse EMT by
activating the miR-200 family in hepatocellular carcinoma (35). In addition, overexpressed miR-675
downregulates Twist1 and reverses EMT in AFP-secreting
hepatocellular carcinoma (30).
Furthermore, no effect of H19 reduction on proliferation is
observed in endometrial cancer cells. Although certain published
studies have shown that H19 affects cancer cell proliferation
(36,37), it also has been found that H19 RNA has
conferred growth advantage for the cells when cultured in
serum-poor medium rather than in 10% FBS, partly due to the
inability of the H19-expressing cells to induce the
cyclin-dependent kinase inhibitor p57 (kip2) in response to serum
stress (38). Therefore, the tumor
microenvironment or cell type may determine the expression and the
role of H19 in cancer. It is necessary to verify the cellular
evidence in the in vivo systems.
In summary, the present study provides key molecular
insights into endometrial cancer biology and a possibility for
gene-oriented drug development. Nevertheless, the detailed
mechanism merits additional investigation to contribute to a
decrease in endometrial cancer mortality.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiderpass E, Antoine J, Bray FI, Oh JK
and Arbyn M: Trends in corpus uteri cancer mortality in member
states of the European Union. Eur J Cancer. 50:1675–1684. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duong LM, Wilson RJ, Ajani UA, Singh SD
and Eheman CR: Trends in endometrial cancer incidence rates in the
United States, 1999–2006. J Womens Health (Larchmt). 20:1157–1163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual Report to the Nation on the status of cancer,
1975–2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Zheng S, Chen S, Qin F, Lau S and
Chen Q: Trends in gynaecological cancers in the largest obstetrics
and gynaecology hospital in China from 2003 to 2013. Tumour Biol.
36:4961–4966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
7
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Setiawan VW, Yang HP, Pike MC, McCann SE,
Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, et al:
Type I and II endometrial cancers: Have they different risk
factors? J Clin Oncol. 31:2607–2618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zemel S, Bartolomei MS and Tilghman SM:
Physical linkage of two mammalian imprinted genes, H19 and
insulin-like growth factor 2. Nat Genet. 2:61–65. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Tycko B: Monoallelic
expression of the human H19 gene. Nat Genet. 1:40–44. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pachnis V, Brannan CI and Tilghman SM: The
structure and expression of a novel gene activated in early mouse
embryogenesis. EMBO J. 7:673–681. 1988.PubMed/NCBI
|
|
12
|
Hao Y, Crenshaw T, Moulton T, Newcomb E
and Tycko B: Tumour-suppressor activity of H19 RNA. Nature.
365:764–767. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng
R, Wang Y, Huang J, Xu M, Yan J and Yu J: lncRNA H19/miR-675 axis
represses prostate cancer metastasis by targeting TGFBI. FEBS J.
281:3766–3775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berteaux N, Lottin S, Monté D, Pinte S,
Quatannens B, Coll J, Hondermarck H, Curgy JJ, Dugimont T and
Adriaenssens E: H19 mRNA-like noncoding RNA promotes breast cancer
cell proliferation through positive control by E2F1. J Biol Chem.
280:29625–29636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medrzycki M, Zhang Y, Zhang W, Cao K, Pan
C, Lailler N, McDonald JF, Bouhassira EE and Fan Y: Histone h1.3
suppresses h19 noncoding RNA expression and cell growth of ovarian
cancer cells. Cancer Res. 74:6463–6473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matouk IJ, Raveh E, Abu-lail R, Mezan S,
Gilon M, Gershtain E, Birman T, Gallula J, Schneider T, Barkali M,
et al: Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys
Acta. 1843:1414–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan
Z and Ai K: H19 promotes pancreatic cancer metastasis by
derepressing let-7's suppression on its target HMGA2-mediated EMT.
Tumour Biol. 35:9163–9169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanos V, Ariel I, Prus D, De-Groot N and
Hochberg A: H19 and IGF2 gene expression in human normal,
hyperplastic and malignant endometrium. Int J Gynecol Cancer.
14:521–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lottin S, Adriaenssens E, Berteaux N,
Lepretrê A, Vilain MO, Denhez E, Coll J, Dugimont T and Curgy JJ:
The human H19 gene is frequently overexpressed in myometrium and
stroma during pathological endometrial proliferative events. Eur J
Cancer. 41:168–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mokhtar NM, Ramzi NH, Yin-Ling W, Rose IM,
Mohd Dali AZ Hatta and Jamal R: Laser capture microdissection with
genome-wide expression profiling displayed gene expression
signatures in endometrioid endometrial cancer. Cancer Invest.
30:156–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abu-lail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PloS one. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Sun Y, Yi J, Wang X, Liang J, Pan
Z, Li L and Jiang G: Targeting H19 by lentivirus-mediated RNA
interference increases A549 cell migration and invasion. Exp Lung
Res. 1–8. 2016.(Epub ahead of print).
|
|
26
|
Zhang E, Li W, Yin D, De W, Zhu L, Sun S
and Han L: c-Myc-regulated long non-coding RNA H19 indicates a poor
prognosis and affects cell proliferation in non-small-cell lung
cancer. Tumour Biol. 37:4007–4015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshimizu T, Miroglio A, Ripoche MA,
Gabory A, Vernucci M, Riccio A, Colnot S, Godard C, Terris B,
Jammes H and Dandolo L: The H19 locus acts in vivo as a tumor
suppressor. Proc Natl Acad Sci USA. 105:12417–12422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Cai Y, Zhao X, Jia X, Zhang J, Liu
J, Zhen H, Wang T, Tang X, Liu Y and Wang J: Down-regulated long
non-coding RNA H19 inhibits carcinogenesis of renal cell carcinoma.
Neoplasma. 62:412–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi
Y and Guo J: Long noncoding RNA associated-competing endogenous
RNAs in gastric cancer. Sci Rep. 4:60882014.PubMed/NCBI
|
|
30
|
Hernandez JM, Elahi A, Clark CW, Wang J,
Humphries LA, Centeno B, Bloom G, Fuchs BC, Yeatman T and Shibata
D: miR-675 mediates downregulation of Twist1 and Rb in
AFP-secreting hepatocellular carcinoma. Ann Surg Oncol. 20:Suppl 3.
S625–S635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo G, Kang Q, Chen Q, Chen Z, Wang J, Tan
L and Chen JL: High expression of long non-coding RNA H19 is
required for efficient tumorigenesis induced by Bcr-Abl oncogene.
FEBS Lett. 588:1780–1786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Min HY, Lee SC, Woo JK, Jung HJ, Park KH,
Jeong HM, Hyun SY, Cho J, Lee W, Park JE, et al: Essential role of
DNA methyltransferase 1-mediated transcription of insulin-like
growth factor 2 in resistance to histone deacetylase inhibitors.
Clin Cancer Res. pii: clincanres.0534.2016, 2016. (Epub ahead of
print).
|
|
33
|
Dugimont T, Montpellier C, Adriaenssens E,
Lottin S, Dumont L, Iotsova V, Lagrou C, Stéhelin D, Coll J and
Curgy JJ: The H19 TATA-less promoter is efficiently repressed by
wild-type tumor suppressor gene product p53. Oncogene.
16:2395–2401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Yang F, Yuan JH, Yuan SX, Zhou
WP, Huo XS, Xu D, Bi HS, Wang F and Sun SH: Epigenetic activation
of the MiR-200 family contributes to H19-mediated metastasis
suppression in hepatocellular carcinoma. Carcinogenesis.
34:577–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang EB, Han L, Yin DD, Kong R, De W and
Chen J: c-Myc-induced, long, noncoding H19 affects cell
proliferation and predicts a poor prognosis in patients with
gastric cancer. Med Oncol. 31:9142014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ayesh S, Matouk I, Schneider T, Ohana P,
Laster M, Al-Sharef W, De-Groot N and Hochberg A: Possible
physiological role of H19 RNA. Mol Carcinog. 35:63–74. 2002.
View Article : Google Scholar : PubMed/NCBI
|