Introduction
Despite various established and curatively intended
therapeutic regimens, new options for chemotherapy and novel modes
of androgen deprivation, >70,000 patients succumb to prostate
cancer each year in Europe (1).
Huggins et al (2) described
the dependence of prostate cancer on androgen levels in 1941. This
finding marked the beginning of systemic and targeted treatment for
advanced and metastasized adenocarcinoma of the prostate (3). To date, the backbone for the initial
systemic treatment of prostate cancer is androgen deprivation
therapy (ADT) (4). Androgen
suppression, however, is associated with adverse clinical effects
for the patient (5) and invariably
leads to the resistance to androgen deprivation and the progression
of the disease over time (6). The
term ‘hormone-refractory’ or ‘-resistant’ prostate cancer was used
to describe progressing prostate cancer under ADT, which appeared
to grow independently from androgen manipulation. With today's
understanding of prostate tumor biology, the term has eventually
been adapted to ‘castration-resistant prostate cancer’ (CRPC),
indicating that progression remains driven by androgen signaling at
the castration-resistant stage. CRPC is the current and recommended
term established by the Prostate Cancer Working Group 2 (PCWG 2)
(7). The new understanding of
castration-resistant disease has led to the development and
implementation of second-generation androgen ablative regimens, the
most established of which thus far are the two orally administered
substances abiraterone acetate and enzalutamide (8,9). Aside
from advances in hormone ablative therapy, chemotherapeutic options
have also expanded, including the introduction of cabazitaxel for
the treatment of docetaxel-resistant prostate cancer, which showed
a survival benefit in the preceding TROPIC trial (10). Prostate cancer at the metastatic CRPC
(mCRPC) stage progresses apparently independent of conventional
ADT. However, it is common practice that ADT is continued when
switching to chemotherapy or second-line hormone manipulation with
abiraterone acetate or enzalutamide. The monitoring of androgen
suppression is normally achieved by measuring total testosterone
levels, however, the biologically active androgen is free
testosterone (FT), which only comprises 1–2% of total testosterone
(11,12).
Discontinuation of luteinizing hormone-releasing
hormone (LHRH) therapy would reduce treatment costs, as well as the
incidence of adverse events attributed to LHRH therapy (5). The question of whether conventional ADT
may be omitted in progressive prostate cancer remains under debate.
This question will be addressed for abiraterone acetate in the
ongoing SPARE trial (13). To date,
there is no reliable clinical data on patients with
second-generation ADT and discontinuation of LHRH-analogue therapy.
The present study analyzed a series of patients with advanced mCRPC
receiving second-line chemotherapy and/or second generation ADT
with regard to FT serum levels and evaluated the effect of FT on
cancer-specific survival (CSS).
Patients and methods
Patient selection
Patients were followed up between March 2009 and
April 2014. Patients were deemed eligible for this retrospective
study is they had histologically confirmed mCRPC. All patients were
androgen ablated with an LHRH agonist, with the exception of 2
patients who underwent a bilateral subcapsular orchiectomy. ADT was
continued throughout the follow-up. FT represents the biologically
active fraction of total testosterone (11). Out of 4,642 patients from the
Departments of Urology and Urological Oncology, Hannover Medical
School (Hannover, Germany) database, only 34 exhibited CRPC and
were monitored with the inclusion of FT level. Levels of FT were
measured in the morning. Patients receiving 1,000 mg/day
abiraterone acetate received concomitant steroid medication with 10
mg prednisolone per day. The Eastern Cooperative Oncology Group
(ECOG) status at the beginning of the follow-up was 0 for all
patients (14), with the exception of
2 (1 patient with an ECOG score of 1 and 1 patient with an ECOG
score of 2). Patients received abiraterone acetate during the
compassionate use program, which was approved by the Hannover
Medical School Ethics Committee (Hannover, Germany). Carboplatin
AUC5 plus docetaxel at a dose of 35 mg/m2 was used as a
salvage chemotherapy option after failure of docetaxel chemotherapy
(15). Cabozantinib was administered
to patients participating in the COMET-1 trial (phase III,
cabozantinib vs. prednisone). Prostate-specific antigen (PSA)
measurements and testing of FT concentration was performed at
Hannover Medical School exclusively.
Laboratory measurements
For the measurement of FT concentration, an enzyme
immunoassay was applied for the quantitative determination of FT
(IBL International GmbH, Hamburg, Germany). To determine the serum
PSA concentration, an Electrochemiluminescence Immunoassay
(Cobas® 6000, Roche Diagnostics, Rotkreuz, Switzerland)
was used. The PCWG-2 criteria were applied to define the
progression of the cancer (7). Change
of therapy under follow-up was allowed on progression of the
disease while continuing constant androgen ablative therapy
(7).
Statistical analysis
Survival rates were estimated using the Kaplan-Meier
method. The log-rank test was applied for comparing survival
between patients with different mean FT concentrations. Hazard
ratios for the prediction of CSS were calculated using multivariate
Cox regression with Efron's approximation. Likelihood ratio-, Wald-
and score (log-rank) tests were applied to test the effect of
covariates of the Cox regression model. Proportionality of all
predictor variables were tested using Pearson's product-moment
correlation between the scaled Schoenfeld residuals and time for
each covariate. All mortalities during observation were
attributable to the underlying prostate cancer disease, hence no
patient had to be censored for competing causes of mortality.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses and graphical illustrations were
performed using R statistical software (R version 3.0.3; R
Foundation for Statistical Computing, Vienna, Austria). Patient
characteristics are depicted in Table
I.
 | Table I.Patient characteristics: Pathology and
previous treatment. |
Table I.
Patient characteristics: Pathology and
previous treatment.
| Characteristic | Value |
|---|
| Patients, n (%) | 34 (100.0) |
| Median
age (range), years | 72 (51–86) |
| Primary therapy, n
(%) |
|
|
Retropubic prostatectomy | 19 (55.9) |
|
Laparoscopic
prostatectomy | 2 (5.9) |
| External
beam radiation | 3 (8.8) |
| LDR
brachytherapy | 1 (2.9) |
| No
primary therapy | 8
(23.5) |
| NA | 1 (2.9) |
| TNM stage, n (%) |
|
| T2a | 1 (2.9) |
| T2b | 1 (2.9) |
| T2c | 2 (5.9) |
|
T3a | 4
(11.8) |
|
T3b | 13 (38.2) |
|
T4a,b | 4
(11.8) |
| NA | 9
(26.5) |
| Gleason score, n
(%) |
|
| ≤6 | 1 (2.9) |
| 7 | 10 (29.4) |
| 8 | 12 (35.3) |
| 9 | 5
(14.7) |
| 10 | 3 (8.8) |
| NA | 3 (8.8) |
| Hormonal therapy, n
(%) |
|
|
Orchiectomy | 2 (5.9) |
|
ADT | 1 (2.9) |
|
CAB | 27 (79.4) |
|
Abiraterone | 25 (73.5) |
|
Enzalutamide | 24 (70.6) |
| Chemotherapy, n
(%) |
|
|
Docetaxel | 31 (91.2) |
|
Carboplatin+docetaxel | 19 (55.9) |
|
Cabazitaxel | 8
(23.5) |
|
Cabozantinib | 3 (8.8) |
| Mean
PSA (range), pg/ml | 182.8
(1.9–1486.2) |
Ethics and approval
The present study was performed in accordance with
all ethical standards laid down in the 1964 Declaration of Helsinki
and its later amendments. Ethics board approval was obtained for
this observational retrospective study. All patient data was
anonymized prior to statistical analysis. No additional data was
created nor used aside from the retrospective evaluation of our
database.
Results
In total, 34 patients with mCRPC were eligible and
had sufficient follow-up of serum FT values. The median follow-up
time was 16.1 months (range, 0.7–55.6 months) and the median
patient age was 72 years (range, 51–86 years). The mean FT
concentration in the cohort was 0.328 pg/ml. Despite the fact that
all patients were under continuous ADT, mean FT levels for each
patient varied, ranging from 0.01–9.1 pg/ml, with a variance of 0.4
pg/ml. A total of 17 patients succumbed during follow-up. Median
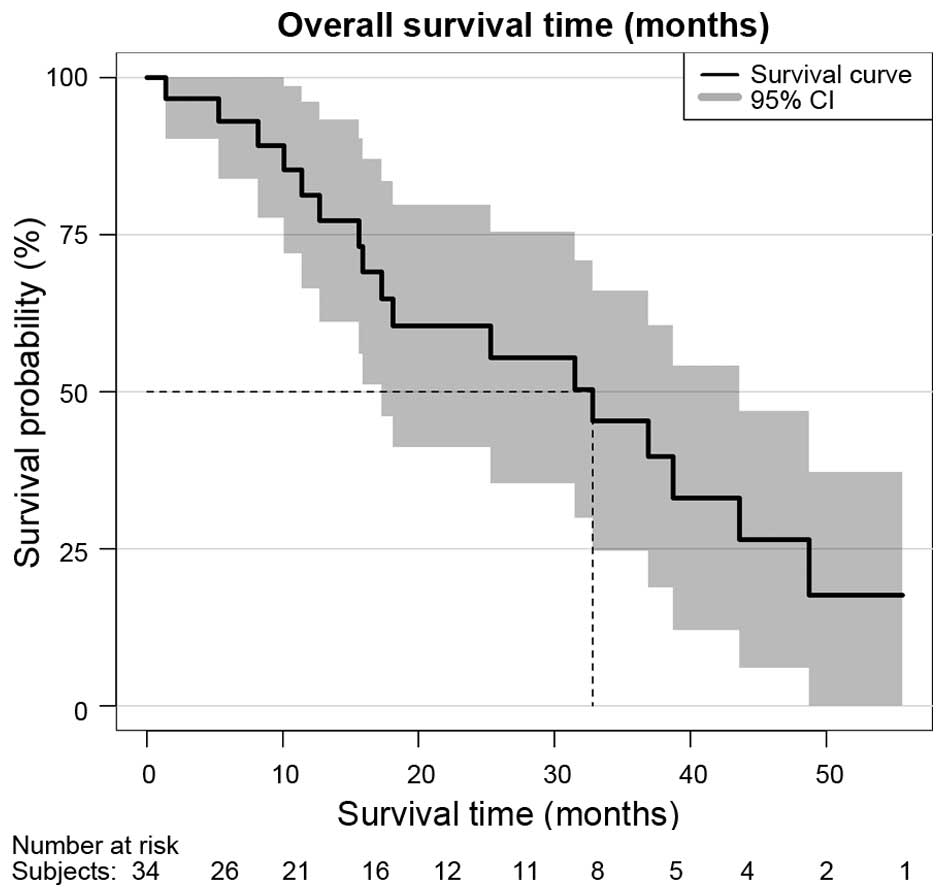
survival over all patients according to Kaplan-Meier survival
estimation was 32.8 months [95% confidence interval (CI), 17-not
available (NA)], as shown in Fig. 1.
Mean PSA correlated with CSS (log-rank test, P=0.0063), which was
expected since all patients succumbed during the progression of the
disease. Mean PSA was 264.2 ng/ml (range, 1.9–1,486.2 ng/ml). All
mortalities were associated with a rising PSA at the end of
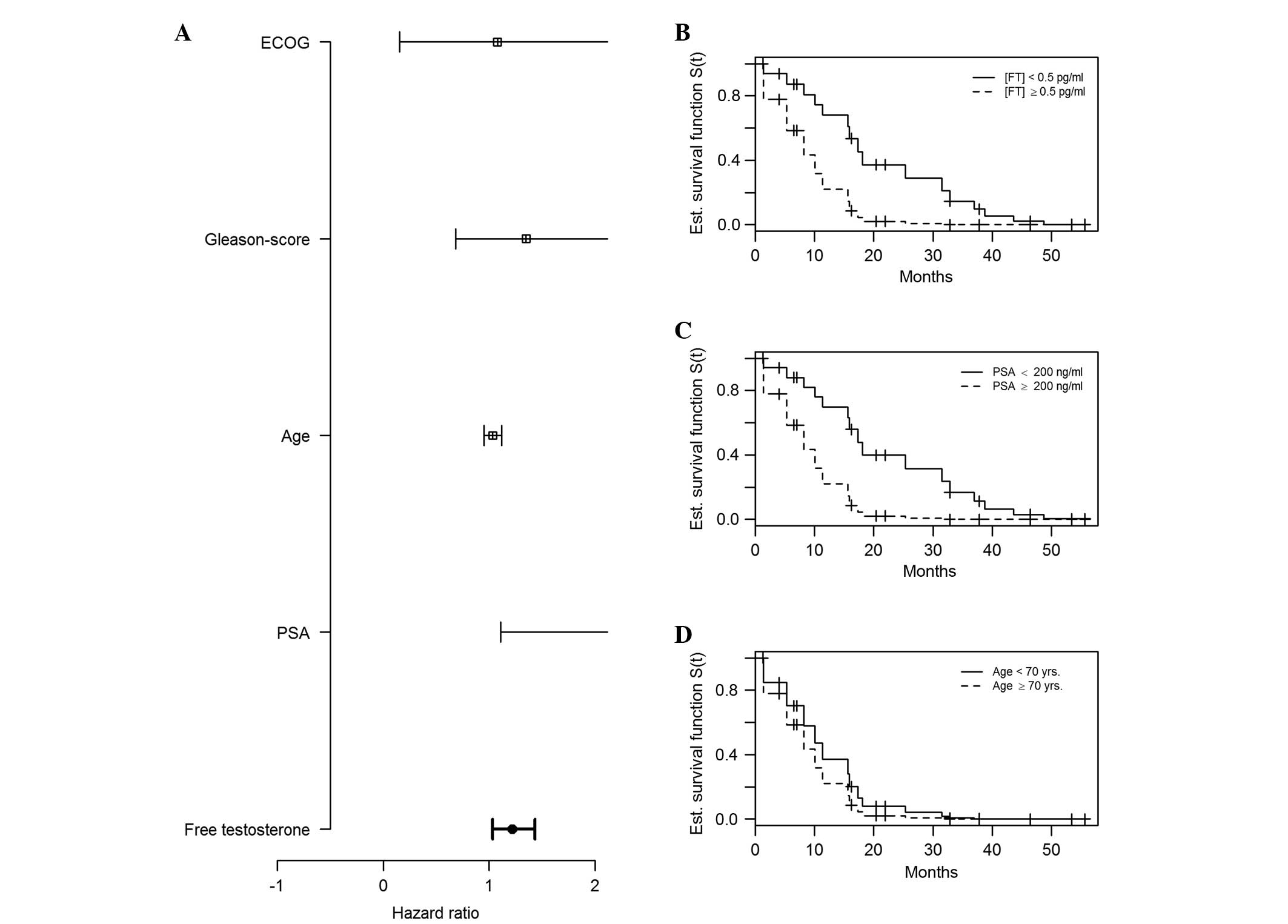
follow-up. The mean PSA value was associated with mortality in the
multivariate Cox model for patients with a mean PSA of ≥200 ng/ml
[hazard ratio (HR), 4.6; 95% CI, 1.1–19.3; P=0.0354). A notable
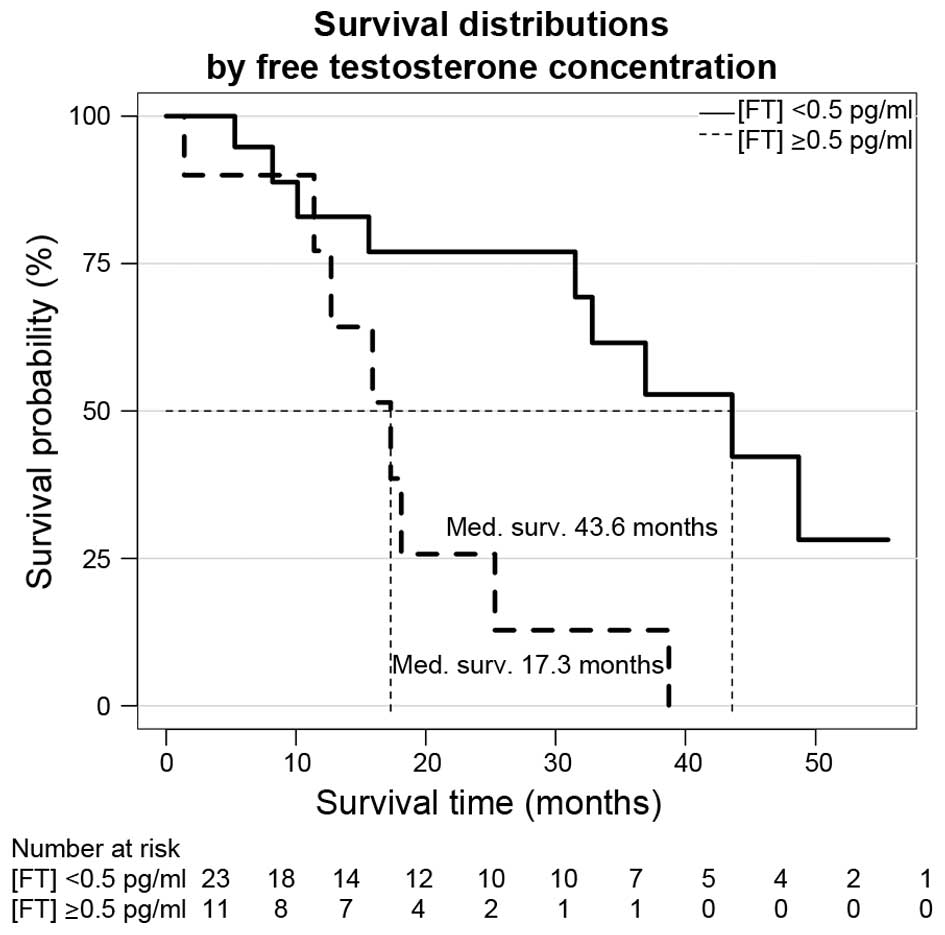
difference with regard to CSS was observed for patients with FT
concentrations below and above 0.5 pg/ml: Median survival for
patients with a FT level below and above the cutoff were 43.6
months (range, 31.5-NA months) and 17.3 months (range, 12.7-NA
months), respectively (log-rank test, P=0.0063). The Kaplan-Meier
estimation of survival according to FT serum concentration is
depicted in Fig. 2. When applying
multivariate Cox regression analysis, the HR for the risk of
mortality for patients with FT concentrations ≥0.5 pg/ml was 1.2
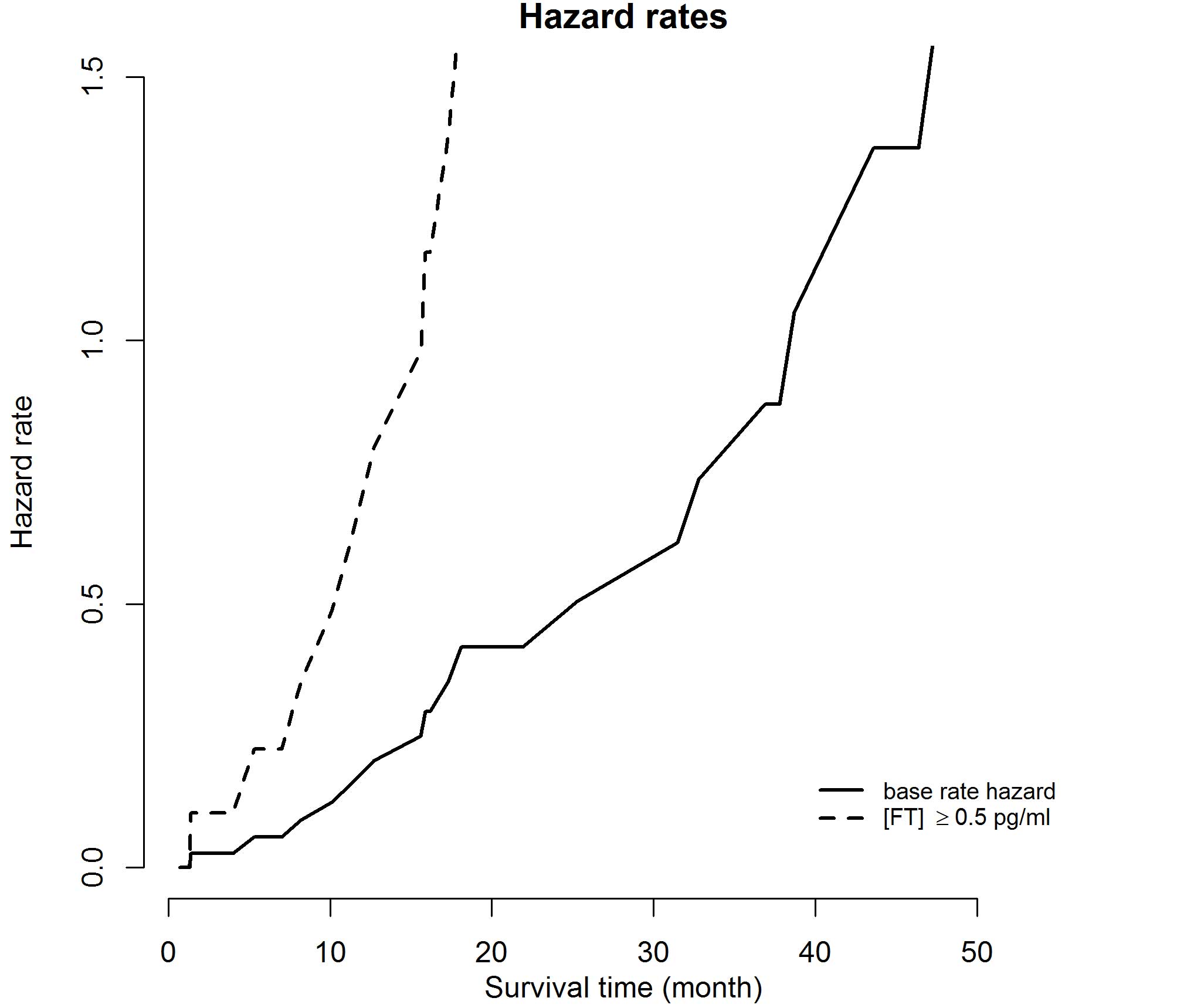
(95% CI, 1–1.4; P=0.0182); hazard rates and HRs are depicted in
Figs. 3 and 4, respectively. For the covariates of
patient age, primary Gleason score and ECOG performance status
(14,16,17), there
was no significant statistical association for the risk of
mortality (P=0.4293, P=0.3854 and P=0.9391, respectively), as shown
in Table II.
 | Table II.HRs, 95% CIs and P-values for the
multivariate Cox regression analysis on cancer-specific survival
for patients with metastatic castration-resistant prostate
cancer. |
Table II.
HRs, 95% CIs and P-values for the
multivariate Cox regression analysis on cancer-specific survival
for patients with metastatic castration-resistant prostate
cancer.
| Variable | HR | 95% CI | P-value |
|---|
| FT | 1.22 |
1.03–1.43 |
0.0182a |
| PSA | 4.63 |
1.11–19.28 |
0.0354a |
| Age | 1.03 |
0.95–1.12 | 0.4293 |
| Gleason score | 1.35 |
0.69–2.66 | 0.3854 |
| ECOG | 1.08 | 0.15–7.6 | 0.9391 |
Discussion
When prostate cancer progresses apparently
independent of conventional hormonal manipulation, the question
arises as to whether a conventional ADT regimen should be continued
during second-line ADT. Abiraterone acetate and enzalutamide exert
their effects on intracellular signaling by more substantial and
firm effects on androgen biosynthesis and androgen receptor binding
than their predecessors: LHRH analogues cause a downregulation of
LHRH receptors in the pituitary gland, thereby decreasing the
release of gonadotropins and consecutively the production of
testosterone; however, this effect on prostate neoplastic cells is
confined to the hypothalamic-pituitary-gonadal axis (18). Abiraterone decreases serum
testosterone and androgen levels by inhibiting
17α-hydroxylase/C17,20-lyase in steroid biosynthesis, and is not
only limited to the testicular Leydig cells, but is also exerting
its effect in the adrenal gland and in prostate cancer cells
(19). First-generation non-steroidal
anti-androgens, including flutamide and bicalutamide, block the
androgen receptor, thereby inhibiting intracellular signaling.
Enzalutamide has a higher binding affinity to the
androgen-receptor, and it not only acts competitively at the
receptor level, but also blocks the activation of
androgen-responsive genes and inhibits the preceding translocation
of the homodimerized receptor-ligand (20). Applying LHRH analogues or
non-steroidal anti-androgens while administering abiraterone
acetate or enzalutamide may therefore appear redundant when
considering modes of action for these substances. The concept of
continued conventional ADT and serum testosterone level monitoring
with regard to clinical parameters and overall survival (OS),
originates from a notable study by Perachino et al, showing
a clear association between OS and serum testosterone levels
measured 6 months after initiation of ADT (21). The study was based on previous results
of a study by Morote et al, in which it was deduced that
from a cohort of 73 patients, ~25% of all men being treated with
LHRH-depot injection exhibited testosterone levels higher than the
formerly recommended serum level of 0.5 ng/ml. The study found a
direct correlation between ‘androgen-independent’ (originally used
expression) progression and serum testosterone levels. It was also
able to show that breakthrough increases of testosterone levels
during LHRH agonist therapy exhibited a markedly negative effect on
‘androgen-independent’ progression. The mean survival time, free
from ‘androgen-independent’ progression, was 137 months for the
subgroup of patients without breakthrough increases of testosterone
and it decreased to 88 months for patients with breakthrough
increases of >32 ng/dl (22).
The study by Byar was also able to show a
contributing effect of insufficient androgen suppression on overall
mortality, however, this effect was observed with the
administration of diethylstilbestrol (23), and unlike our current study, not with
LHRH analogues in conjunction with abiraterone acetate or
enzalutamide.
Other retrospective studies evaluating the positive
effects of continued ADT therapy in patients with CRPC have also
shown survival advantages for patients who sustained LHRH analogue
therapy (24,25). These findings clearly emphasize the
requirement for laboratory monitoring of ADT therapy, however, the
aforementioned studies were undertaken a long time prior to the
advent of second-line anti-androgens. Also, the previously
mentioned studies by Morote et al (22) and others, used total testosterone,
which is easier to measure than FT. In the current study, an
emphasis was placed on FT, which is the active fraction responsible
for biological activity (11).
Notably, there is not yet much data on FT with regard to prostate
cancer.
A more recent finding that does potentially support
the continuation of LHRH therapy, including serum testosterone
measurements on a regular schedule, was derived from the COU-AA-301
study itself (26). Data from the
trial, initially comparing the efficacy of abiraterone acetate plus
low-dose prednisone versus prednisone only, was subsequently
analyzed with regard to androgen dynamics in correlation with serum
PSA: In an ultra-sensitive assay, PSA measurements showed a
reduction to undetectable levels in 47% of patients in the
abiraterone arm, while none of the patients continuing regular
androgen deprivation exhibited serum testosterone levels below the
detection threshold. The study compared androgen levels with
radiographic progression-free survival and time to PSA progression,
but found no significant correlation. However, unlike the present
study, the measurements were timed exclusively 12 weeks after the
initiation of therapy. Additionally, the focus was on total
testosterone concentration and not FT levels. Nonetheless, these
findings show that inadequate androgen suppression may and does
occur in patients with inhibition of the
hypothalamic-pituitary-gonadal axis, even when combined with
inhibitors of precursor steroid biosynthesis (27). Notably, 13–42% of patients under
therapy with LHRH analogues fail to reach serum testosterone levels
of <0.5 ng/ml (28). These
involuntary elevations of serum testosterone may provide an insight
as to what extent circulating androgens play a role in the advanced
mCRPC setting when next-generation ADT is in place. The reason for
the significant increases of androgen levels under therapy is not
fully understood. Certain men may experience a surge in serum
testosterone concentration while under long-term therapy with LHRH
analogues. This phenomenon was previously described as the
‘acute-on-chronic response’ (29).
Also, obese patients tend to have higher testosterone concentration
levels under LHRH therapy than men with a normal body mass index
(30). Other reasons for insufficient
androgen suppression are a faulty preparation of the LHRH depot
injection or inadvertent discontinuation of therapy. While FT and
total testosterone concentrations can frequently be assessed, the
aforementioned reasons for insufficient androgen suppression cannot
adequately be identified or monitored. The present study showed a
variance in FT serum concentration. However, it did not provide a
clear explanation for these surges in FT levels. One possible
incentive for the continuation of conventional ADT may lay in the
assumption of a broader mode of therapy in the castration-resistant
state. The mechanisms that lead to the castration-resistant state
are numerous and have been subjected to extensive research in the
past. Intracellular cell signaling promoting growth and tumor
progression may continue by means of ‘bypass’ or ‘outlaw’ pathways,
even without the binding of the androgen receptor ligand. One
example for these mechanisms is the expression of B-cell lymphoma
2, which is a critical anti-apoptotic protein in CRPC and prostate
cancer in general (31). Another
example is the Akt signaling cascade (32) or the overexpression of human epidermal
growth factor receptor-2/neu tyrosine kinase. The latter is able to
boost prostate cancer growth and androgen receptor signaling
independently of the androgen ligand binding to the receptor
(33). These models for the alternate
activation of prostate cancer cells appear to be independent of
androgen signaling and do not give a clear justification for the
continuation of LHRH therapy in the mCRPC state. Androgen receptor
splice variant-7 (AR-V7), presented at the 2014 Genitourinary
Cancer Symposium annual meeting, lacks the ligand-binding domain
for enzalutamide, but it remains active as a transcription factor.
PSA response rates were 0% for abiraterone and enzalutamide in
patients with the AR-V7 splice variant, which directly translated
into shorter progression-free survival times (34). However, these data do neither support
nor negate the beneficial effect of an ongoing conventional ADT
while starting with enzalutamide or abiraterone acetate. Free
androgen levels appear to significantly affect CSS, as shown in the
current study. This indicates that progression in a cohort of
patients with mCRPC is not merely driven by escape mechanisms and
resistance completely independent of androgen signaling, but is
dependent on serum FT levels, even with the combination of
conventional ADT and second-generation hormone manipulation. This
finding can be explained through clonal heterogeneity or by
resistance mechanisms that rely on FT, such as androgen receptor
overexpression (35,36). We hypothesize that one possible
argument in favor for continuing conventional ADT, while
administering enzalutamide or abiraterone acetate, may be an
overlap in treatment that could potentially reduce the risk of FT
surges due to accidental pauses of treatment. One of the most
notable studies with regard to LHRH therapy during second-line
hormonal manipulation was conducted by Pinski et al, which
showed that LH receptors exist on prostate cancer cells and
stimulate cancer growth by increasing intrinsic steroidogenesis
(37). This finding would be an
argument in favor for the continuation of conventional LHRH
analogue therapy. The inhibition of steroid biosynthesis in
addition to LHRH analogue therapy is not new; it was used even
prior to the advent of abiraterone acetate, when ketoconazole was
combined with complete androgen blockade resulting in a markedly
lower testosterone concentration when compared to complete androgen
blockade alone (38). Probably the
most important argument in favor of the continued use of LHRH
analogue therapy in the mCRPC state is, however, the lack of
clinical studies with regard to survival. The current study,
therefore, may represent one of the first pieces of clinical
evidence on the topic.
The current study was limited by its retrospective
design and the heterogeneity of treatments that, however, reflect
the therapeutic reality of patients with mCRPC today.
In conclusion, patients with advanced mCRPC who have
progressed under conservative ADT have FT as a significant
predictor of CSS, even in the sequence of second-generation ADT
(abiraterone acetate or enzalutamide) and chemotherapy. The present
findings support the recommendation that LHRH-analogue therapy and
measurements of androgen suppression on a regular basis should not
be omitted in this setting.
References
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huggins C, Stevens RE and Hodges CV:
Studies on prostate cancer. II. The effects of castration on
advanced carcinoma of the prostate gland. Arch Surg. 43:209–223.
1941. View Article : Google Scholar
|
|
3
|
Walsh PC: Physiologic basis for hormonal
therapy in carcinoma of the prostate. Urol Clin North Am.
2:125–140. 1975.PubMed/NCBI
|
|
4
|
Merseburger AS, Hammerer P, Rozet F,
Roumeguère T, Caffo O, da Silva FC and Alcaraz A: Androgen
deprivation therapy in castrate-resistant prostate cancer: How
important is GnRH agonist backbone therapy? World J Urol.
33:1079–1085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faris JE and Smith MR: Metabolic sequelae
associated with androgen deprivation therapy for prostate cancer.
Curr Opin Endocrinol Diabetes Obes. 17:240–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the prostate cancer clinical
trials working group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al: Abiraterone acetate for treatment of metastatic
castration-resistant prostate cancer: Final overall survival
analysis of the COU-AA-301 randomised, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mendel CM: The free hormone hypothesis: A
physiologically based mathematical model. Endocr Rev. 10:232–274.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreno SA, Shyam A and Morgentaler A:
Comparison of free testosterone results by analog radioimmunoassay
and calculated free testosterone in an ambulatory clinical
population. J Sex Med. 7:1948–1953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trial of Abiraterone Acetate Plus
LHRH-therapy Versus Abiraterone Acetate Sparing LHRH-therapy in
Patients With Progressive Chemotherapy-naïve Castration-resistant
Prostate Cancer (SPARE) (SPARE). https://clinicaltrials.gov/ct2/show/NCT02077634Accessed
June 12, 2016.
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reuter CWM, Morgan MA, Ivanyi P, Fenner M,
Ganser A and Grünwald V: Carboplatin plus weekly docetaxel as
salvage chemotherapy in docetaxel-resistant and
castration-resistant prostate cancer. World J Urol. 28:391–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974.PubMed/NCBI
|
|
17
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. 1941. J Urol. 167:948–952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attard G, Belldegrun AS and de Bono JS:
Selective blockade of androgenic steroid synthesis by novel lyase
inhibitors as a therapeutic strategy for treating metastatic
prostate cancer. BJU Int. 96:1241–1246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tran C, Ouk S, Clegg NJ, Chen Y, Watson
PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al:
Development of a second-generation antiandrogen for treatment of
advanced prostate cancer. Science. 324:787–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perachino M, Cavalli V and Bravi F:
Testosterone levels in patients with metastatic prostate cancer
treated with luteinizing hormone-releasing hormone therapy:
Prognostic significance? BJU Int. 105:648–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morote J, Orsola A, Planas J, Trilla E,
Raventós CX, Cecchini L and Catalán R: Redefining clinically
significant castration levels in patients with prostate cancer
receiving continuous androgen deprivation therapy. J Urol.
178:1290–1295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Byar DP: Proceedings: The veterans
administration cooperative urological research group's studies of
cancer of the prostate. Cancer. 32:1126–1130. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain M, Wolf M, Marshall E, Crawford ED
and Eisenberger M: Effects of continued androgen-deprivation
therapy and other prognostic factors on response and survival in
phase II chemotherapy trials for hormone-refractory prostate
cancer: A southwest oncology group report. J Clin Oncol.
12:1868–1875. 1994.PubMed/NCBI
|
|
25
|
Taylor CD, Elson P and Trump DL:
Importance of continued testicular suppression in
hormone-refractory prostate cancer. J Clin Oncol. 11:2167–2172.
1993.PubMed/NCBI
|
|
26
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryan CJ, Peng W, Kheoh T, Welkowsky E,
Haqq CM, Chandler DW, Scher HI and Molina A: Androgen dynamics and
serum PSA in patients treated with abiraterone acetate. Prostate
Cancer Prostatic Dis. 17:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oefelein MG and Cornum R: Failure to
achieve castrate levels of testosterone during luteinizing hormone
releasing hormone agonist therapy: The case for monitoring serum
testosterone and a treatment decision algorithm. J Urol.
164:726–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharifi R and Browneller R: Leuprolide
Study Group: Serum testosterone suppression and potential for
agonistic stimulation during chronic treatment with monthly and
3-month depot formulations of leuprolide acetate for advanced
prostate cancer. J Urol. 168:1001–1004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith MR: Obesity and sex steroids during
gonadotropin-releasing hormone agonist treatment for prostate
cancer. Clin Cancer Res. 13:241–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colombel M, Symmans F, Gil S, O'Toole KM,
Chopin D, Benson M, Olsson CA, Korsmeyer S and Buttyan R: Detection
of the apoptosis-suppressing oncoprotein bc1-2 in
hormone-refractory human prostate cancers. Am J Pathol.
143:390–400. 1993.PubMed/NCBI
|
|
32
|
Sun M, Yang L, Feldman RI, Sun XM, Bhalla
KN, Jove R, Nicosia SV and Cheng JQ: Activation of
phosphatidylinositol 3-kinase/Akt pathway by androgen through
interaction of p85alpha, androgen receptor, and src. J Biol Chem.
278:42992–43000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the hER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-v7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egan A, Dong Y, Zhang H, Qi Y, Balk SP and
Sartor O: Castration-resistant prostate cancer: Adaptive responses
in the androgen axis. Cancer Treat Rev. 40:426–433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Merseburger AS, Kuczyk MA and Wolff JM:
Pathophysiology and therapy of castration-resistant prostate
cancer. Urologe A. 52:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinski J, Xiong S, Wang Q, Stanczyk F,
Hawes D and Liu SV: Effect of luteinizing hormone on the
steroidogenic pathway in prostate cancer. Prostate. 71:892–898.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mostaghel EA, Nelson PS, Lange P, Lin DW,
Taplin ME, Balk S, Ellis W, Kantoff P, Marck B, Tamae D, et al:
Targeted androgen pathway suppression in localized prostate cancer:
A pilot study. J Clin Oncol. 32:229–237. 2014. View Article : Google Scholar : PubMed/NCBI
|