Introduction
Octamer-binding transcription factor 4 (Oct4)
protein is encoded by POU class 5 homeobox 1 (POU5F1), and belongs
to a small group of pluripotency transcription factors (1). Oct4 is a key component and is critical
in the maintenance of stem cell pluripotency and proliferation
(2,3).
It is overexpressed in multiple types of human tumor and has been
demonstrated to be associated with cancer progression. For example,
the expression of Oct4 was indicated to be significantly correlated
with early hepatocellular carcinoma recurrence (4). Oct4 has been identified as overexpressed
in human embryonic carcinoma (5),
prostate cancer (6), and bladder
cancer cells, and been associated with enhanced migration and
invasion (7). Oct4 was also
demonstrated to promote cell cycle progression in esophageal
carcinoma (8), promote colony
formation of glioma cells (9), and
increase transmigration capacity in melanoma cells (10). These previous studies suggest that
deregulation and dysfunction of Oct4 contribute to malignant
establishment of a cancer stem cell phenotype (5).
Osteosarcoma tends to have a poor prognosis, and is
the leading primary bone tumor in children and adolescents. Tumor
stem cells are hypothesized to promote tumor initiation, relapse
and metastasis. Thus, the poor prognosis of osteosarcoma likely
results in failure of treatment to target the osteosarcoma stem
cells (11). In previous studies
investigating the role of Oct4 in osteosarcoma, Oct4 was
overexpressed in stem-like cells isolated from the human
osteosarcoma MNNG/HOS cell line (12)
and promoted tumor formation when OCT4 was exogenously expressed in
the human osteosarcoma cell line (11). Overexpression of imprinted
tumor-suppressing subtransferable candidate 3, an
apoptosis-associated gene, efficiently downregulated the expression
of Oct4 as well as homeobox protein NANOG (NANOG) and SRY (sex
determining region Y)-box 2 (Sox2), which are two members of the
pluripotency transcription factor family, in tumor initiating
cells, decreased the clone formation rate, and downregulated
tumorigenesis in the MThFOB1.19 osteoblast cell line (13). This indicates Oct4 may be involved in
tumor promotion. However, the role of Oct4 in osteosarcoma requires
further investigation.
Long noncoding RNAs (lncRNAs) are functional RNAs
longer than 200 nucleotides. Increasing evidence suggests that
lncRNAs are involved in the regulation of diverse cellular
processes, including regulation of gene expression, imprinting,
chromatin modification, transcription and posttranslational
processing, particularly in carcinogenesis and metastasis (14,15). A
recent study demonstrated that Oct4 and Sox2 were increased in the
RWPE-1 immortalized human prostate cell line, which had concomitant
increase of lncRNA H19. Notably, targeted suppression of H19 with
small interfering RNA (siRNA) decreased Oct4 and Sox2 gene
expression. Conversely, overexpression of H19 notably increased
gene expression of these two transcription factors (16). Oct4 and Sox2 also had a marked
co-upregulation with lncRNA SOX2OT in the NTERA2 human embryonic
carcinoma stem cell line (17).
Thus, the present study aimed to investigate the
effect of Oct4 on the proliferation and invasion of osteosarcoma
cells via silencing Oct4 expression using siRNA. The current study
also investigated the possible association between Oct4 and a
number of lncRNAs.
Materials and methods
Reagents
3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Fetal bovine
serum (FBS), Lipofectamine 2000, non-essential amino acids,
RPMI-1640 medium, TRIzol, Annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection kit, and trypsin-EDTA solution were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Antibodies against Oct4 (ab109183) or GAPDH (ab181602) were
purchased from Abcam (Cambridge, UK). Horseradish
peroxidase-coupled secondary immunoglobulin G (sc-2004) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX USA).
Enhanced chemiluminescence reagent was purchased from Pierce
(Thermo Fisher Scientific, Inc.). SOSP-9607, MG63 and F5M2
osteosarcoma cell lines and the hFOB1.19 osteoblast cell line were
purchased from the American Type Culture Collection (Manassas, VA,
USA).
Patients and sample collection
A total of 10 patients who received osteosarcoma
surgery in The Second Hospital of Jilin University (Changchun,
China) between January 2012 and May 2014 were selected. These
patients did not receive adjuvant treatment, including chemotherapy
or radiotherapy, prior to the surgery. Biopsies were obtained from
the osteosarcoma tissues and normal tissues adjacent to the tumor
during surgery. The diagnosis was confirmed by pathological
examination of these tissues. All the patients provided written
informed consent and the present study was in strict accordance
with National Institutes of Health guidelines (18) and was approved by the ethics committee
of The Second Hospital of Jilin University.
Cell culture
SOSP-9607, MG63 and F5M2 osteosarcoma cell lines and
the hFOB1.19 osteoblast cell line were grown in RPMI-1640 medium,
supplemented with heat-inactivated FBS (10% v/v), 100 U/ml
penicillin, 100 µg/ml streptomycin and non-essential amino acids
(1% v/v), and cultured at 37°C in an atmosphere of 5%
CO2 with a relative humidity of 95%.
Transient transfection of Oct4 and
AK055347 siRNAs
The transfection of Oct4 and AK055347 siRNA
into F5M2 cells was performed using Lipofectamine 2000 according to
the manufacturer's protocols. A total of 0.25×106 cells
were seeded into 6-well plates and 0.5 µg siRNA was used. For
transfection, Lipofectamine 2000 and siRNA were added directly to
cells, and subsequently incubated at 37°C. The sequences of the
siRNAs and scramble controls were as follows:
5′-GGAGGAAGCTGACAACAATG-3′ for Oct4-1; 5′-TTCAGCCAAACGACCATC-3′ for
Oct4-2; 5′-GTATTCAGCCAAACGACCAT-3′ for Oct4-3;
5′-GGAGCAATGCACAGATGGAA-3′ for Oct4 scramble;
5′-CACCTGAGTTGAATGAGGATCTACT-3′ for AK055347-1;
5′-GAGTTGAATGAGGATCTACTGTTAA-3′ for AK055347-2;
5′-CACGTACATGTGCACACACTGTCTA-3′ for AK055347-3; and
5′-CACGAGGTTTAAGAGTAGTCCTACT-3′ for AK055347-scramble.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay
as previously described (19).
Briefly, 1,000 cells from each group were plated in 96-well
microplates in 150 µl of medium. Following culture to different
time points (1–5 days), 20 µl of MTT substrate (5 mg/ml) was added
to each well, and the plates were incubated for an additional 4 h.
The medium was then removed, and the cells were solubilized in 150
µl of dimethyl sulfoxide. The culture plates were then agitated for
15 min and the optical absorbance values were read at a wavelength
of 490 nm.
Invasion assay
Cell invasion was evaluated using the Transwell
invasion assay. Three days following transfection, F5M2 cells were
trypsinized and resuspended in FBS-free RPMI-1640 medium. A total
of 2×104 cells were plated in the upper chamber of the
Transwell with a Matrigel-coated polycarbonate membrane. Fresh
medium with 10% FBS was added to the lower chamber as a
chemoattractant. Following incubation for a further 48 h at 37°C
with 5% CO2, cells on the lower surface of the membrane
were fixed with 5% formalin and stained with 0.2% crystal violet.
The non-migrated cells on the upper surface were wiped off with a
cotton swab. Images of the cells that migrated to the lower surface
were captured. The invaded cells were counted in five randomly
selected fields using a light microscope.
Flow cytometry analysis
Cell apoptosis was analyzed with an Annexin V-FITC
apoptosis detection kit according to the manufacturer's protocols.
Briefly, the cells were washed with cold phosphate-buffered saline
(PBS) and collected with a trypsin-EDTA solution. The cell
suspensions were then centrifuged at 95 × g for 5 min at 4°C
to remove the trypsin-EDTA solution. The cells
(3×106/ml) were then re-suspended and incubated with
Annexin V-FITC and Annexin V binding buffer for 15 min at room
temperature. The stained cells were analyzed with a BD LSR II flow
cytometer (BD Biosciences, San Jose, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA levels were measured using RT-qPCR as previously
described (20). Tumor and adjacent
normal tissue samples were used. Total RNA was extracted using
TRIzol reagent according to the manufacturer's protocols. The RNA
was reverse transcribed at 42°C for 1 h into cDNA using M-MLV
reverse transcriptase (Thermo Fisher Scientific, Inc.) and random
primers (Thermo Fisher Scientific, Inc.). PCR was performed using
SYBR GreenER qPCR SuperMix Universal kit (Thermo Fisher Scientific,
Inc.) with an ABI StepOnePlus Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions used were as follows: Initial denaturation at 95°C for 5
min; and 39 cycles of denaturation at 95°C for 30 sec, annealing at
60°C for 30 sec and extension at 72°C for 45 sec. Specific primer
sequences are listed in Table I.
Results are presented as the levels of expression relative to those
of the controls with GAPDH for normalization using the
2−∆∆Cq method (21).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| AK023948 |
CTTGTGAGCCCTCTTAGTC |
TTTAGTTCCAATCTGTCCC |
| AK027193 |
GGCTCAGGATCAAATGTC |
GCTCTTGGGTGAAGAAAC |
| AK055347 |
TGTCCCATACCACCAAGC |
AGGACCCACTGACTGAAC |
| BCAR4 |
GGACTCATTGTTGTTCTAC |
ACCTATGGCTATCATTGTT |
| CASC2 |
CTATTCCGAGTAAGAAGTG |
TCTGTGTTGATGTTGATT |
| GAPDH |
GGACCAATACGACCAAATCCG |
AGCCACATCGCTCAGACAC |
| HIF1A-AS1 |
AATGTGTTCCTTGCTCTT |
GTATGTCTCAGTTATCTTCCT |
| PCA3 |
AATCATACTGGTCACTTATCT |
TTAACAACTGGTCCTGAG |
| POU5F1 |
GGAGGAAGCTGACAACAATG |
CACTCGGTTCTCGATACTGG |
| PVT1 |
CTTGAGAACTGTCCTTACG |
CAGATGAACCAGGTGAAC |
| SNHG5 |
GACTGACTAGCAGCTTCAT |
ACTAGCCAGAAATCGTTG |
Western blot analysis
The protein expression levels of Oct4 in cells were
assessed using western blotting as described in a previous study
(20). Proteins were quantified by
the BCA assay. Whole cell proteins (2 µg) were separated
electrophoretically in 4–12% SDS-PAGE gels, and transferred to
nitrocellulose membranes. Following 30 min of blocking with 2.5%
nonfat milk, the membranes were incubated with primary antibodies
(1:2,000) at 4°C overnight. Subsequently the membranes were
incubated for 1 h at room temperature with horseradish
peroxidase-conjugated secondary antibody (1:2,000). The membranes
were then adequately washed with PBS containing 0.5% Tween 20. The
membranes were developed with enhanced chemiluminescence reagent
(Pierce, Rockford, IL, USA) and then exposed to X-ray film.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using one-way analysis
of variance followed by Bonferroni's post-hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
SPSS version 19 (IBM SPSS, Armonk, NY, USA)
Results
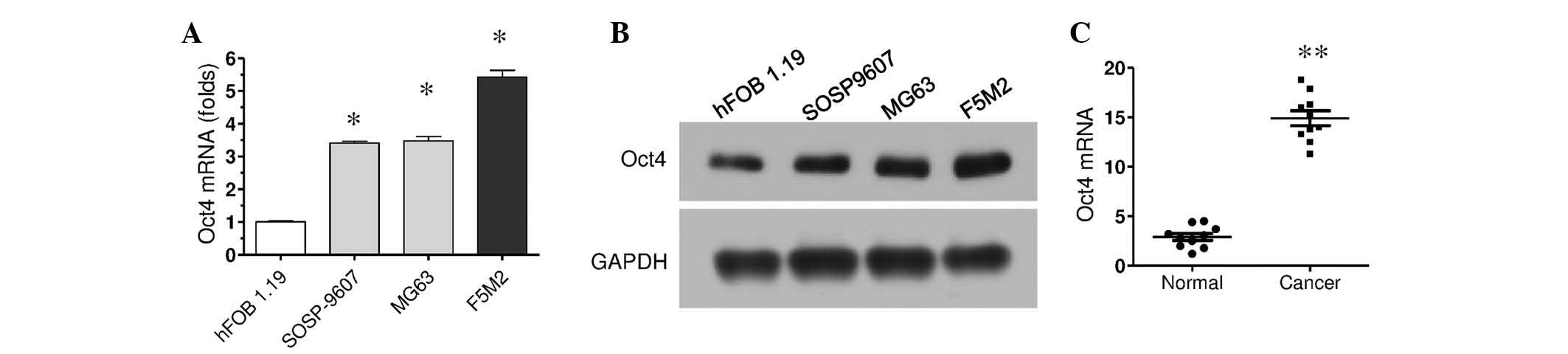
Oct4 expression is increased in
various osteosarcoma cell lines and human osteosarcoma tissues
Oct4 mRNA expression was investigated in various
osteosarcoma cells using RT-qPCR. The results demonstrated that
Oct4 expression was significantly increased in the three types of
osteosarcoma cells, SOSP9607, MG63 and F5M2, compared with the
hFOB1.19 osteoblast cell line (P<0.05; Fig. 1A). Of these cells, F5M2 cells
exhibited the highest Oct4 expression levels. Oct4 protein
expression levels in these cells were subsequently investigated
using western blotting. Consistent with mRNA expression levels, the
protein expression of Oct4 was also increased in these osteosarcoma
cells compared with normal cells (Fig.
1B).
It was also further examined whether the increased
Oct4 expression levels were also observed in human osteosarcoma
tissues using RT-qPCR. The mRNA was extracted from cancer tissue
samples and adjacent normal tissue samples from 10 patients with
osteosarcoma, and RT-qPCR was performed to measure Oct4 mRNA
expression levels. When compared with the normal tissues, Oct4
expression in cancer tissues was increased in each pair of samples,
and significantly higher collectively (P<0.01; Fig. 1C). These results demonstrate
POU5F1/Oct4 expression is increased in osteosarcoma, and suggest
POU5F1/Oct4 is associated with osteosarcoma progression.
Downregulation of Oct4 suppresses cell
proliferation and invasion
To investigate the possible role of increased Oct4
expression in osteosarcoma, F5M2 osteosarcoma cells were
transfected with siRNA against Oct4 or scrambled RNA to
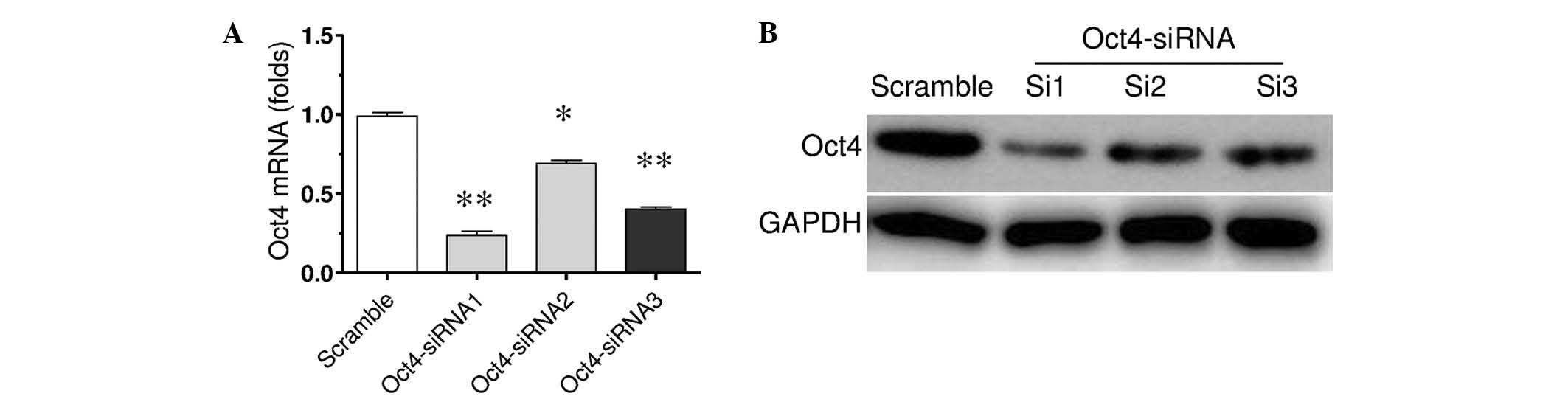
downregulate Oct4 expression. After 3 days transient transfection
with Oct4 siRNA, the mRNA and protein expression levels of Oct4 was
detected by RT-qPCR and western blotting (Fig. 2). The results demonstrated that the
mRNA expression levels were significantly decreased by three
different Oct4 siRNAs (P<0.05 and P<0.01; Fig. 2A). Among them, Oct4 siRNA-1 revealed
biggest inhibitive effect (P<0.01). This was consistent with the
protein expression level results. Thus, Oct4 siRNA-1 was used in
further experiments.
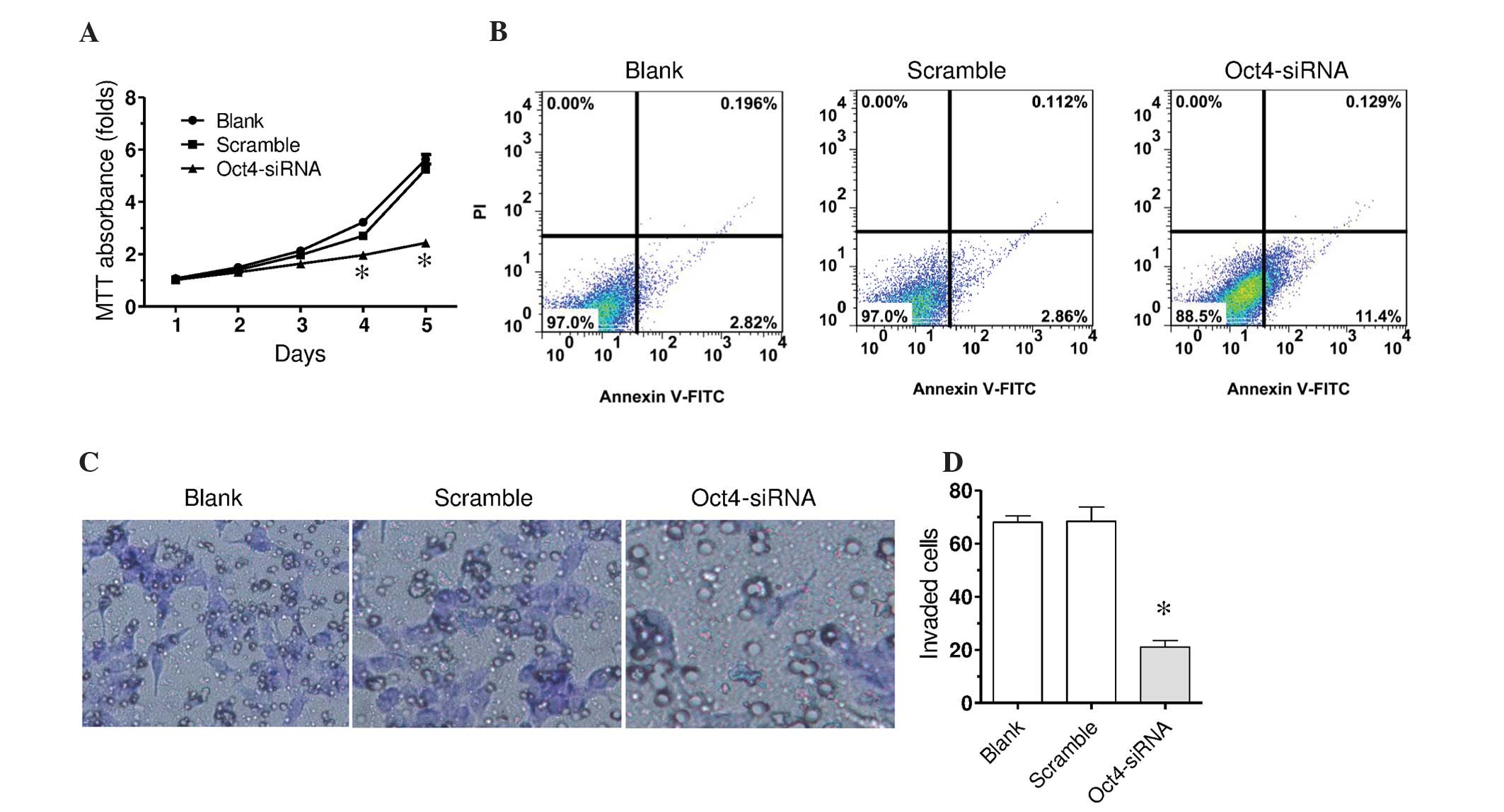
It was subsequently investigated whether the
downregulation of Oct4 affected the proliferation of F5M2 cells
using the MTT assay. F5M2 cells were transfected with Oct4 siRNA-1
or scramble siRNA. In the MTT assay, Oct4 siRNA transfection
significantly decreased cell proliferation in a time-dependent
manner compared with the scramble control, which was indicated by
decreased MTT absorbance value (Fig.
3A). The scramble control indicated a similar change in the MTT
absorbance value when compared with the blank control. Furthermore,
the current study investigated whether the decreased proliferation
by downregulation of Oct4 was associated with cell apoptosis. Cell
surface expression of Annexin V was measured using flow cytometry.
As presented in Fig. 3B, scramble RNA
did not markedly affect surface expression of Annexin V. Oct4 siRNA
markedly increased the surface expression of Annexin V, suggesting
increased apoptosis by Oct4 downregulation.
Invasion is an important characteristic of tumor
metastasis. The present study examined whether Oct4 is involved in
osteosarcoma metastasis using the Transwell invasion assay. In F5M2
cells with Oct4 downregulation, fewer cells invaded through the
Matrigel-coated polycarbonate membrane as compared with the
scramble control and blank control (Fig.
3C and D; P<0.01). This indicates that Oct4 is involved in
promotion of invasion of osteosarcoma cells.
Downregulation of Oct4 suppresses
AK055347 expression levels
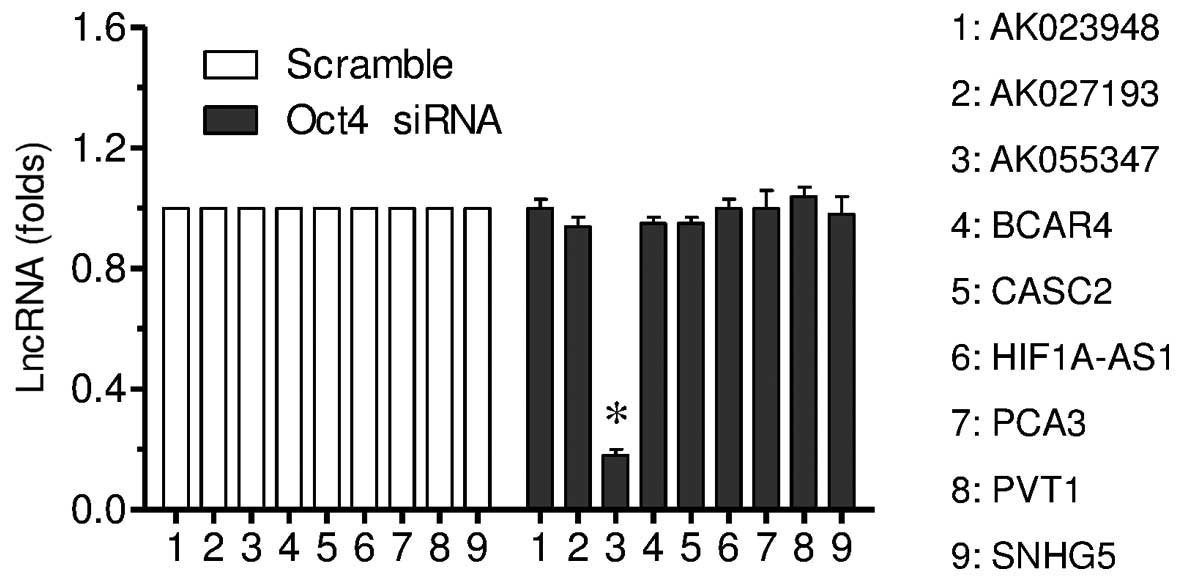
RT-qPCR was conducted to investigate the effect of
Oct4 downregulation on lncRNA expression. The current study
observed downregulation of Oct4 significantly decreased AK055347
expression, but not the other eight lncRNAs investigated
(P<0.01; Fig. 4).
Downregulation of AK055347 suppresses
cells proliferation and invasion
To investigate whether the decreased expression of
Ak055347 by Oct4 is the underlying mechanism of Oct4 in
osteosarcoma, the effect of downregulated AK055347 on proliferation
and invasion ability of F5M2 cells was investigated. F5M2 cells
were treated with AK055347 siRNA or scramble control for 3 days.
RT-qPCR was performed to validate the downregulation of AK055347 by
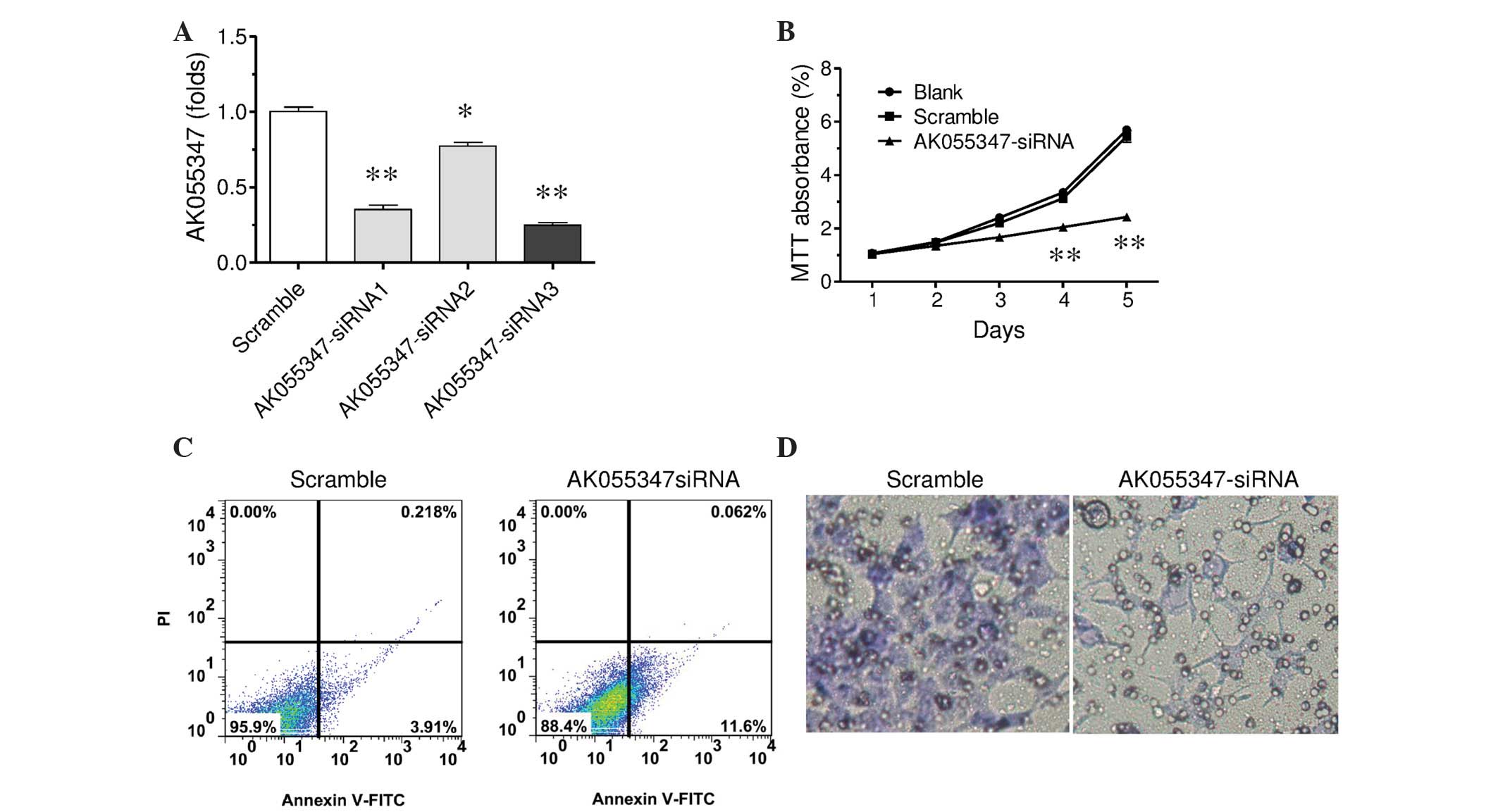
three different AK055347 siRNAs (P<0.05 and P<0.01; Fig. 5A). Among them, AK055347 siRNA3
exhibited the most marked inhibitive effect (P<0.01). Thus,
AK055347 siRNA-3 was used in further experiments.
Similarly to experiments for Oct4, the effect of
AK055347 downregulation on cell proliferation, apoptosis and
invasion was investigated. F5M2 cells were transfected with
AK055347 siRNA-3 or scramble control, and the cells were cultured
for 1–5 days, and the MTT assay was subsequently performed. The
results indicated that AK055347 downregulation significantly
decreased MTT absorbance value in a time-dependent manner as
compared with the scramble control (Fig.
5B). AK055347 downregulation markedly increased the surface
expression of Annexin V compared with the scramble control
(Fig. 5C). Further, AK055347
downregulation decreased cell invasion ability in Transwell
invasion assay (Fig. 5D). Thus, these
results suggest AK055347 promotes cell proliferation and invasion,
and has a suppressive effect on apoptosis.
Discussion
The present study aimed to investigate the role of
POU5F1/Oct4 in the regulation of osteosarcoma progression and
elucidate possible underlying mechanisms. It was observed that Oct4
expression was significantly increased in various osteosarcoma cell
lines and osteosarcoma tissues. Oct4 downregulation significantly
decreased osteosarcoma cell proliferation and invasion ability, and
increased cell apoptosis. The downregulation of Oct4 reduced the
expression of AK055347, a novel identified lncRNA. AK055347, in a
siRNA-mediated downregulation model, suggesting a role in tumor
promotion in osteosarcoma cells. Thus, the results of the present
study suggest Oct4 is involved in the promotion of osteosarcoma,
which may be mediated via AK055347.
POU5F1/Oct4 is a recently identified proto-oncogene
that serves as a marker of aggressive phenotypes in a number of
types of cancer. In a previous study, strong expression of Oct4 was
observed in recurrent prostate cancer when compared with adjacent
normal tissue samples (6). In
patients with head and neck squamous cell carcinoma, increased
expression levels of Oct4, in addition to cytoplasmic/nuclear
β-catenin, was considered to be associated with a worse prognosis
(22). In the present study, the
results indicated that Oct4 expression was increased in the
osteosarcoma tissues obtained from 10 osteosarcoma patients
compared with adjacent normal tissues. This finding is consistent
with these previous studies (6,22).
Furthermore, in three types of osteosarcoma cells, SOSP-9607, MG63
and F5M2, the mRNA expression levels of Oct4 were significantly
increased and protein expression levels were also increased
compared with the hFOB1.19 osteoblast cell line. Thus, the results
of the present study demonstrate that Oct4 is highly expressed in
osteosarcoma.
In order to elucidate whether the increased Oct4
expression is associated with poor prognosis of osteosarcoma, Oct4
was downregulated by specific siRNA in the present study. The
results indicated Oct4 downregulation markedly reduced cell
proliferation and induced cell apoptosis. In addition, the present
study also demonstrated that Oct4 downregulation reduced cell
invasion ability in a Transwell invasion assay. These results
suggest a promotive role of Oct4 in osteosarcoma progress.
Proliferation and invasion, in addition to migration, are important
characteristics of cancer. Particularly, invasion is involved in
cancer cell metastasis, which ultimately leads to a poor prognosis.
The promotive effect of Oct4 on cancer cell proliferation was
reported in certain types of cancer cells, including hepatocellular
carcinoma (23) and esophageal cancer
(8). The identification of
Oct4-positive putative stem cells may result in uncontrolled cell
proliferation and treatment failure in esophageal squamous cell
carcinoma (24). The promoting effect
of Oct4 on migration and invasion has also been observed in
numerous types of cancer. In gastric cancer, only positive
expression of Oct4, but not other proteins, including NANOG, Sox2,
proliferating cell nuclear antigen, Ki67 and epithelial cadherin
was reported to be correlated with lymphatic invasion (25). Oct4 was also reported to enhance lung
cancer cell invasion and adhesion accompanied by the downregulation
of epithelial marker cytokeratin and upregulation of the
mesenchymal markers vimentin and neural cadherin (26). The percentage of OCT4-positive cells
was significantly increased in higher grade, higher non-classic
differentiation number and more invasive urothelial bladder cancer
cases (27). Oct4 was also suggested
to promote proliferation and invasion of esophageal cancer cells
(8). The data in the present study
further supports this role for Oct4 in cancer progression. Notably,
a controversial effect of Oct4 was observed in breast cancer. Oct4
was determined to exert a suppressive effect on cell migration and
invasion in vitro and the formation of metastatic lung
nodules in vivo (28).
Alterations in the expression of a number of lncRNAs
have been observed in certain types of cancer, and this has been
indicated to be important in carcinogenesis and metastasis
(14). Li et al (29) demonstrated that 25,733 lncRNAs were
expressed in osteosarcoma tissue samples from a microarray
analysis. Among them, 403 lncRNAs were consistently upregulated,
while 798 lncRNAs were consistently downregulated in all samples
analyzed. In the present study, AK055347, an lncRNA, was identified
to be markedly decreased by Oct4 downregulation in osteosarcoma
cells. Previous studies have demonstrated that lncRNAs may exert
different effects in osteosarcoma. Metastasis-associated lung
adenocarcinoma transcript 1 was reported to promote the
proliferation and metastasis of osteosarcoma cells (30). Downregulation of taurine upregulated 1
inhibited osteosarcoma cell proliferation and promoted apoptosis
(31). Genetic deletions of LOC285194
and BC040587 were determined to be associated with poor survival of
osteosarcoma patients (32). These
lncRNAs were indicated to promote osteosarcoma progress. By
contrast, a novel lncRNA, hypoxia-inducible factor-2α promoter
upstream transcript, was indicated to function as an inhibitor of
osteosarcoma stem cells in vitro (33). In the present study, AK055347
downregulation markedly decreased cell proliferation, increased
apoptosis and reduced invasion ability. Thus, the data suggests
that AK055347, similar to the majority of identified lncRNAs, has a
promotive effect on osteosarcoma progression.
In conclusion, the results presented in the present
study demonstrate increased expression of POU5F1/Oct4 in
osteosarcoma, which may exert a promotive effect in osteosarcoma
progression via regulating AK055347 expression levels. These
findings provide potential targets for clinical applications.
References
|
1
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bi Y, He Y, Huang J, Su Y, Zhu GH, Wang Y,
Qiao M, Zhang BQ, Zhang H, Wang Z, et al: Functional
characteristics of reversibly immortalized hepatic progenitor cells
derived from mouse embryonic liver. Cell Physiol Biochem.
34:1318–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeineddine D, Hammoud AA, Mortada M and
Boeuf H: The Oct4 protein: More than a magic stemness marker. Am J
Stem Cells. 3:74–82. 2014.PubMed/NCBI
|
|
4
|
Chang TS, Wu YC, Chi CC, Su WC, Chang PJ,
Lee KF, Tung TH, Wang J, Liu JJ, Tung SY, et al: Activation of
IL6/IGFIR confers poor prognosis of HBV-related hepatocellular
carcinoma through induction of Oct4/NANOG expression. Clin Cancer
Res. 21:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SW, Do HJ, Ha WT, Han MH, Park KH,
Song H, Kim NH and Kim JH: Transcriptional activation of Oct4 by
the ETS transcription factor PEA3 in NCCIT human embryonic
carcinoma cells. FEBS Lett. 588:3129–3136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guzel E, Karatas OF, Duz MB, Solak M,
Ittmann M and Ozen M: Differential expression of stem cell markers
and ABCG2 in recurrent prostate cancer. Prostate. 74:1498–1505.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL
and Wu CL: Oct-3/4 expression reflects tumor progression and
regulates motility of bladder cancer cells. Cancer Res.
68:6281–6291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Li X, Li C, Su Y, Fang W, Zhong C,
Ji W, Zhang Q and Su C: Transcription factor OCT4 promotes cell
cycle progression by regulating CCND1 expression in esophageal
carcinoma. Cancer Lett. 354:77–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y,
Cao X, Ling EA and Hao A: October4 is expressed in human gliomas
and promotes colony formation in glioma cells. Glia. 57:724–733.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borrull A, Ghislin S, Deshayes F, Lauriol
J, Alcaide-Loridan C and Middendorp S: Nanog and Oct4
overexpression increases motility and transmigration of melanoma
cells. J Cancer Res Clin Oncol. 138:1145–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN, Kang T and Wang J: Salinomycin
inhibits osteosarcoma by targeting its tumor stem cells. Cancer
Lett. 311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martins-Neves SR, Lopes ÁO, do Carmo A,
Paiva AA, Simões PC, Abrunhosa AJ and Gomes CM: Therapeutic
implications of an enriched cancer stem-like cell population in a
human osteosarcoma cell line. BMC Cancer. 12:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Dai H and Guo QN: TSSC3
overexpression reduces stemness and induces apoptosis of
osteosarcoma tumor-initiating cells. Apoptosis. 17:749–761. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Huang J, Zhou N, Zhang Z, Zhang A,
Lu Z, Wu F and Mo YY: LncRNA loc285194 is a p53-regulated tumor
suppressor. Nucleic Acids Res. 41:4976–4987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Isin M and Dalay N: LncRNAs and neoplasia.
Clin Chim Acta. 444:280–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauderlique-Le Roy H, Vennin C,
Brocqueville G, Spruyt N, Adriaenssens E and Bourette RP:
Enrichment of human stem-like prostate cells with s-SHIP promoter
activity uncovers a role in stemness for the long noncoding RNA
H19. Stem Cells Dev. 24:1252–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shahryari A, Rafiee MR, Fouani Y, Oliae
NA, Samaei NM, Shafiee M, Semnani S, Vasei M and Mowla SJ: Two
novel splice variants of SOX2OT, SOX2OT-S1 and SOX2OT-S2 are
coupregulated with SOX2 and Oct4 in esophageal squamous cell
carcinoma. Stem Cells. 32:126–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chinese National Institutes of Health, .
Ethics examination in biomedicine studies involving the human body.
Chinese National Institutes of Health; Beijing, China: 2007
|
|
19
|
Hu J, Fang Y, Cao Y, Qin R and Chen Q:
miR-449a regulates proliferation and chemosensitivity to cisplatin
by targeting cyclin D1 and BCL2 in SGC7901 cells. Dig Dis Sci.
59:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Wang J, Wang Y, Fu Q, Lei YH, Nie
ZY, Qiu J and Bao TY: Protective effect of exogenous matrix
metalloproteinase-9 on chronic renal failure. Exp Ther Med.
7:329–334. 2014.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SH, Koo BS, Kim JM, Huang S, Rho YS,
Bae WJ, Kang HJ, Kim YS, Moon JH and Lim YC: Wnt/β-catenin
signalling maintains self-renewal and tumourigenicity of head and
neck squamous cell carcinoma stem-like cells by activating
October4. J Pathol. 234:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao H, Jiang N, Zhou B, Liu Q and Du C:
TAZ regulates cell proliferation and epithelial-mesenchymal
transition of human hepatocellular carcinoma. Cancer Sci.
106:151–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaiphei K, Sinha SK and Kochhar R:
Comparative analysis of Oct4 in different histological subtypes of
esophageal squamous cell carcinomas in different clinical
conditions. Asian Pac J Cancer Prev. 15:3519–3524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li N, Deng W, Ma J, Wei B, Guo K, Shen W,
Zhang Y and Luo S: Prognostic evaluation of nanog, Oct4, Sox2,
PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol.
32:4332015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen ZS, Ling DJ, Zhang YD, Feng JX, Zhang
XY and Shi TS: Octamer-binding protein 4 affects the cell biology
and phenotypic transition of lung cancer cells involving
β-catenin/E-cadherin complex degradation. Mol Med Rep.
11:1851–1858. 2015.PubMed/NCBI
|
|
27
|
Jóźwicki W, Brożyna AA and Siekiera J:
Expression of Oct4A: The first step to the next stage of urothelial
bladder cancer progression. Int J Mol Sci. 15:16069–16082. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen L, Qin K, Wang D, Zhang Y, Bai N,
Yang S, Luo Y, Xiang R and Tan X: Overexpression of Oct4 suppresses
the metastatic potential of breast cancer cells via Rnd1
downregulation. Biochim Biophys Acta. 1842:2087–2095. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JP, Liu LH, Li J, Chen Y, Jiang XW,
Ouyang YR, Liu YQ, Zhong H, Li H and Xiao T: Microarray expression
profile of long noncoding RNAs in human osteosarcoma. Biochem
Biophys Res Commun. 433:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP
and Yao J: Down-regulation of long non-coding RNA TUG1 inhibits
osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J
Cancer Prev. 14:2311–2315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pasic I, Shlien A, Durbin AD, Stavropoulos
DJ, Baskin B, Ray PN, Novokmet A and Malkin D: Recurrent focal
copy-number changes and loss of heterozygosity implicate two
noncoding RNAs and one tumor suppressor gene at chromosome 3q13.31
in osteosarcoma. Cancer Res. 70:160–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Yao J, Meng H, Yu Z, Wang Z, Yuan
X, Chen H and Wang A: A novel long non-coding RNA,
hypoxia-inducible factor-2α promoter upstream transcript, functions
as an inhibitor of osteosarcoma stem cells in vitro. Mol Med Rep.
11:2534–2540. 2015.PubMed/NCBI
|