Introduction
Since glycolipids exhibit a tissue- and
cell-specific distribution, and their composition changes markedly
during processes such as carcinogenesis, differentiation and
proliferation, these molecules are considered to be important for
cellular functions (1). The number of
reports concerning the physiological role of glycolipids has
increased in the past years, and attention has been focused on
their functions (1). The present
authors have analyzed glycolipids in the human endometrium, where
differentiation and proliferation are controlled by sex hormones,
and have already reported that there is a marked increase of
sulfatides with sulfate groups in the secretory endometrium
(2), and that the concentrations of
glucosylceramide (GlcCer), lactosylceramide (LacCer) and
globotriaosylceramide (Gb3Cer) (which contain a ceramide
in which the fatty acids and sphingosine are hydroxylated)
exhibited a marked increase during the secretory phase of the
menstrual cycle (3). In a previous
study on breast cancer (4), it was
also reported that ceramide structures with specific chain lengths
are very different in estrogen receptor (ER)-positive tumors
compared with ER-negative tumors. Based on these observations, it
could be proposed that the ceramide expressed in cancer cells may
have specific functions influenced by sex steroid hormones.
Ceramide is synthesized from fatty acids and a long-chain base,
with the fatty acids being classified as α-hydroxy fatty acids if
they have a hydroxyl group, or non-hydroxy fatty acids if they lack
such a group (5). The long-chain base
is mostly composed of sphingosine, but it can be synthesized from
phytosphingosine (in which a hydroxyl group is attached to
sphingosine) or from dihydrosphingosine (in which the double bond
at position 4 of sphingosine is saturated) (6).
An association has been reported between the
prognosis of endometrial carcinoma and tumor differentiation (gland
formation) (7). Poorly differentiated
endometrial adenocarcinoma exhibits more rapid progression and is
less responsive to therapy than well-differentiated adenocarcinoma,
resulting in a worse prognosis (8).
Diagnosis of tumor differentiation is usually performed by
pathologists, and the tumor grade assigned may be the single most
important prognostic factor for endometrial cancer (9). However, little basic research has been
conducted on the process of transformation of endometrial cancer to
the well-differentiated or poorly differentiated phenotype.
Understanding the mechanism involved in the differentiation of
endometrial cancer may lead to novel therapies that induce tumor
differentiation.
Accordingly, the objectives of the present study
were to compare the expression of neutral glycolipids between
well-differentiated and poorly differentiated adenocarcinoma of the
endometrium, focusing on the ceramide moiety, and to investigate
the role of glycolipids in morphological differentiation (gland
formation), which is the most important prognostic factor for
endometrial cancer.
Materials and methods
Materials
Glycolipids from various sources were purified at
the Department of Obstetrics and Gynecology, Tokai University
(Tokai University School of Medicine, Isehara, Japan), including
GlcCer, LacCer, Gb3Cer and globotetraosylceramide
(Gb4Cer), from human erythrocytes, which were obtained
from the Japanese Red Cross (Tokyo, Japan).
Tumor tissues
Tumor tissues were obtained from the Department of
Obstetrics and Gynecology at Tokai University Hospital (Isehara,
Japan). Written informed consent for the use of tumor specimens in
the present study was obtained from all subjects, and the
experimental protocol was approved by the Ethics Committee of Tokai
University Hospital (Institutional Review Board approval no.
09R-097). The histological classification of the tumors was
performed according to the criteria of the International Federation
of Gynecology and Obstetrics (10).
Tumor differentiation was diagnosed histologically according to the
amount of glandular and solid areas in the cancerous tissue. A
total of 24 endometrial adenocarcinomas were studied, and 9
well-differentiated tumors, 11 moderately differentiated tumors and
4 poorly differentiated tumors were used for biochemical analysis.
Tumor samples were immediately stored at −70°C until use. For
histological examination, tissues were fixed in formalin and
embedded in paraffin, and subsequently, sections (4-μm-thick) were
cut and stained with hematoxylin and eosin.
Quantitative determination of neutral
glycolipids from tumor tissues
Tumor tissues were homogenized by Polytron
homogenizer (Kinematica, Luzern, Switzerland) with water and were
lyophilized. Total lipids were extracted from the lyophilized
powder with chloroform/methanol/water (20:10:1, 10:20:1 and 1:1,
v/v). Then, the lipid extracts were fractionated into neutral and
acidic lipids on a DEAE Sephadex™ A-25 column in acetate form
(Pharmacia Biotech; GE Healthcare Life Sciences, Uppsala, Sweden).
Preparation of neutral glycolipids was performed as described
previously (11–13). The neutral glycolipids were separated
from the unabsorbed neutral lipid fraction by acetylation,
separation of the acetylated derivatives, deacetylation and
desalting. The neutral glycolipids obtained were developed on
thin-layer chromatography (TLC) plates (Merck, Darmstadt, Germany)
with chloroform/methanol/water (65:35:8, v/v/v), and then
visualized with orcinol-H2SO4 (Wako Pure
Chemical Industries, Tokyp, Japan). The density of the spots was
determined at an analytical wavelength of 420 nm for
orcinol-H2SO4-positive spots, using a
dual-wavelength TLC densitometer (CS-9000; Shimadzu Corporation,
Kyoto, Japan). Standard glycolipids (Funakoshi Co., Ltd., Tokyo,
Japan), which were N-stearoyl derivatives of GlcCer, LacCer and
Gb3Cer (0.1–1.5 µg), were developed on the same TLC
plates to generate standard curves for quantitation.
Isolation and structural analysis of
specific neutral glycolipids
Isolation
Only 1 well-differentiated case (lane 1 in Fig. 1) and 1 poorly-differentiated case
(lane 17 in Fig. 1) were selected for
analysis. The neutral glycolipids from well-differentiated and
poorly differentiated adenocarcinoma were purified on a column
(1.8-cm inner diameter and 55.0-cm length) packed with
Iatrobeads® (Iatron Lab. Inc., Tokyo, Japan), with a
linear gradient system formed from chloroform/methano1/water
(85:15:0.5 and 20:80:5, v/v/v) (3).
Under these conditions, individual bands
corresponding to Gb3Cer and Gb4Cer
specifically expressed in both adenocarcinomas were successfully
isolated in a pure form. The homogeneity of the isolated neutral
glycolipids was examined by TLC with
orcinol-H2SO4 reagent.
Fatty acid and long-chain base compositions of
ceramides in neutral glycolipids specifically expressed in
endometrial adenocarcinoma
The isolated glycolipids were treated with 0.75 M
methanolic HCl at 80°C for 20 h (4),
and then the fatty acid methyl esters were extracted from the
hydrolyzates with n-hexane. The samples were analyzed by gas-liquid
chromatography using 3% OV-101 (GL Science, Inc., Tokyo, Japan) on
ChroLite (100–120 mesh; Shimadzu Corporation), with a programmed
temperature increase of 1°C/min from 150–250°C, and were
characterized with non-hydroxy fatty acids and α-hydroxy fatty
acids (Wako Pure Chemical Industries). The peak areas obtained were
corrected by comparison with the peak areas of an authentic mixture
of fatty acid methyl esters (Applied Science Labs., State College,
PA, USA). The long-chain bases were also extracted from the
hydrolyzates with diethyl ether after changing the pH to 11 with 1
M NaOH, and were developed on a TLC plate with sphingosine,
dihydrosphingosine and phytosphingosine (Sigma-Aldrich, St. Louis,
MO, USA) using chloroform/methano1/2 N ammonia (40:10:1, v/v)
(11), and visualized with ninhydrin
reagent.
Structural analysis of specific glycolipids
The structures of the further purified glycolipids
were also determined by negative ion fast atom bombardment mass
spectrometry (FABMS) and glycosidase treatment. Approximately 5 µg
of an isolated neutral glycolipid in 5 µl of chloroform/methanol
(1:1, v/v) was mixed with ~5 µl of triethanolamine, and the
resultant solution was placed on a stainless steel sample holder
for FABMS. Analysis was performed by bombardment with a neutral
xenon beam with a kinetic energy of 4 keV, and detection of
negative ions was performed with a mass spectrometer (JMSHX-110;
JEOL, Ltd., Tokyo, Japan) equipped with a JMA-5500 computer system
(JEOL, Ltd.). Assignment of mass numbers was achieved by comparing
the spectrum with that of perfluoroalkyl phosphazine (Ultramark
1621; PCR, Inc., Gainesville, FL, USA).
TLC upon treatment with α-galactosidase
(Sigma-Aldrich) was performed to confirm the sequence of the sugar
chains.
Results
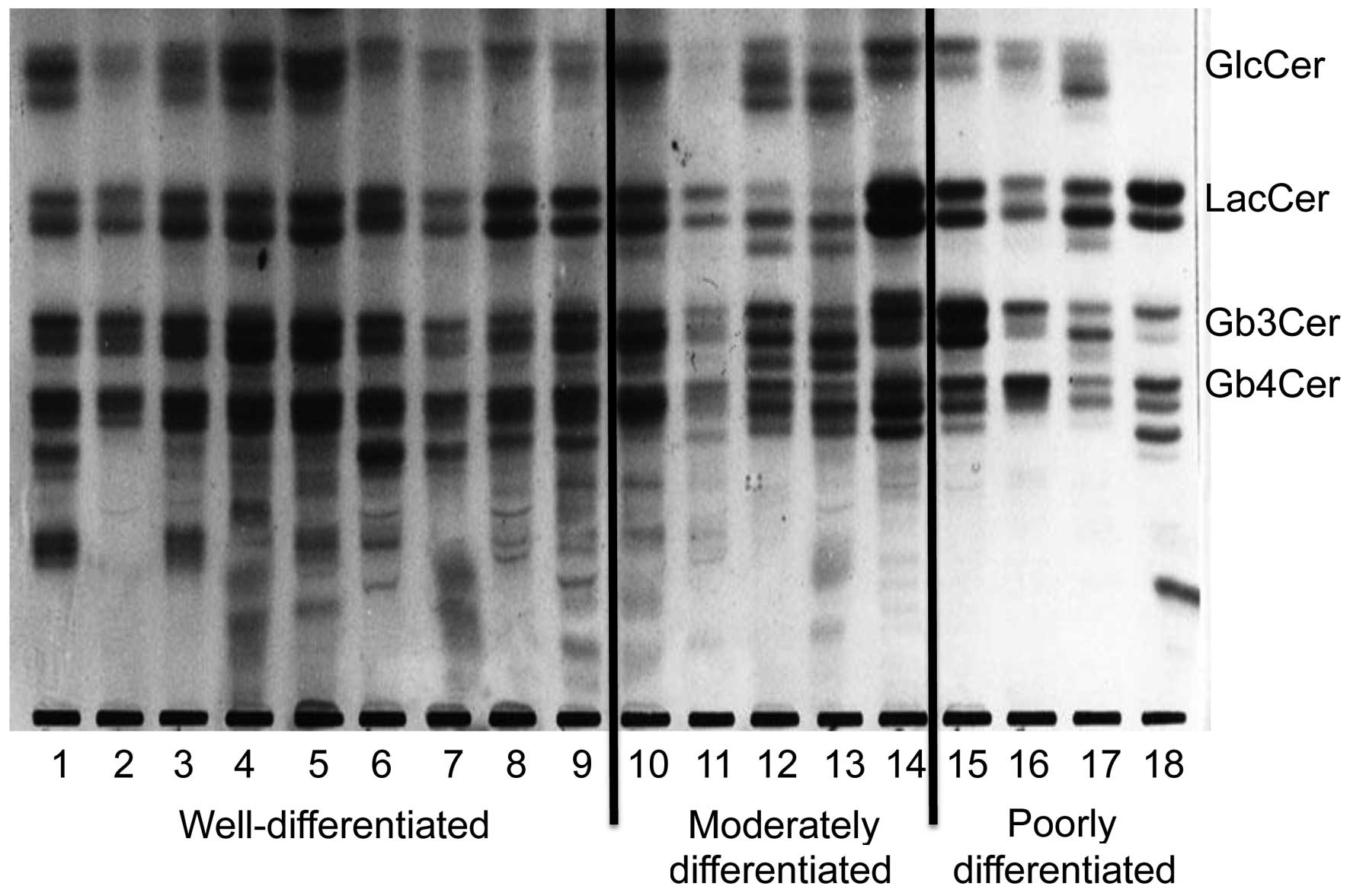
Expression of neutral glycolipids in
well-, moderately and poorly differentiated adenocarcinoma of the
endometrium
GlcCer, LacCer, Gb3Cer and
Gb4Cer were the principal neutral glycolipids identified
in endometrial cancer (Fig. 1). In
well-differentiated tumors, numerous structurally unknown
glycolipids exhibiting slower migration than Gb4Cer were
also identified, although there was individual variation.
Gb3Cer (indicated by the top arrow in Fig. 2A and B) had two components in
well-differentiated cancer, while it had only one component in
poorly differentiated cancer. Gb4Cer (indicated by the
bottom arrow in Fig. 2A and B) was
also composed of two bands in well-differentiated cancer, while it
was composed of only one band in poorly differentiated cancer.
Thus, a band that was not noted in poorly differentiated cancer was
observed to be present in Gb3Cer and Gb4Cer
from well-differentiated cancer. Fig.
3 represents the relative amount of these characteristic bands.
These observations suggest that specific bands of Gb3Cer
and Gb4Cer were characteristically more expressed in
well-differentiated adenocarcinoma than in poorly differentiated
adenocarcinoma.
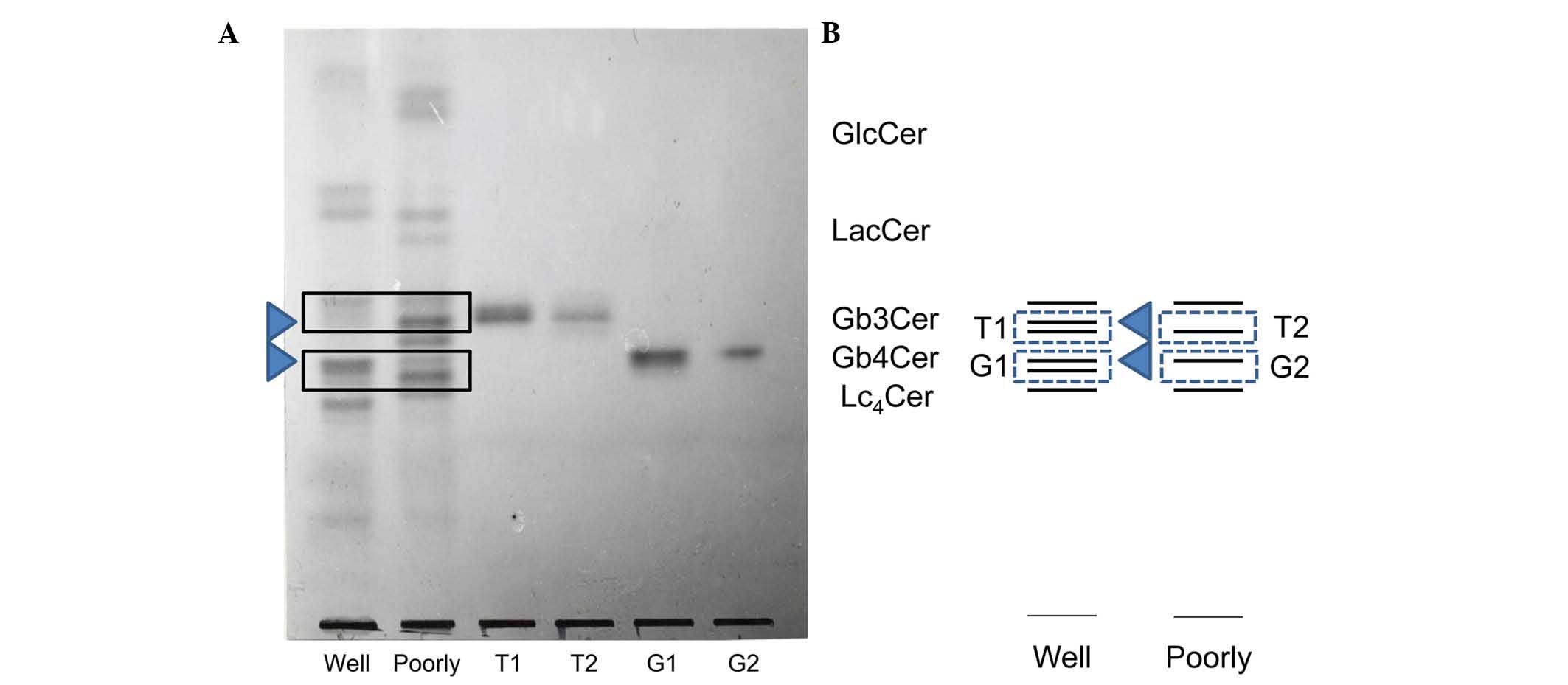 | Figure 2.(A) TLC of the constituents of the
Gb3Cer and Gb4Cer bands isolated from well-
(bands T1 and G1) and poorly (bands T2 and G2) differentiated
endometrial adenocarcinoma. Neutral glycolipids were isolated from
the total neutral lipid fraction as described in Materials and
methods. The total neutral glycolipids and the isolated glycolipids
were developed with chloroform/methanol/water (65:35:8, v/v) and
visualized with orcinol-H2S04 reagent. Lanes
Well and Poorly correspond to the total neutral glycolipids in
well- and poorly differentiated adenocarcinoma, respectively. Lanes
T1, T2, G1 and G2 are the isolated neutral glycolipids from the
three bands of Gb3Cer and the band of Gb4Cer,
respectively. (B) TLC schema of T1, T2, G1 and G2. TLC, thin-layer
chromatography; GlcCer, glucosylceramide; LacCer, lactosylceramide;
Gb3Cer, globotriaosylceramide; Gb4Cer,
globotetraosylceramide; Lc4Cer, lactotetraosyl
ceramide. |
Structural analysis
The Gb3Cer and Gb4Cer bands
exhibited differences between well- and poorly differentiated
cancer, and were isolated and purified using a Iatrobeads column
(Fig. 2A and B). The
Gb3Cer bands with a lower rate of migration on TLC
isolated from well- and poorly differentiated cancer were
designated as T1 and T2, respectively, while the Gb4Cer
bands isolated from well- and poorly differentiated cancer were
called G1 and G2, respectively. Differences between T1 and T2 or
between G1 and G2 were investigated with respect to sugar chains,
fatty acids and the long-chain base as constituents of
ceramide.
α-Galactosidase was reacted with T1 and T2 prior to
structural analysis by TLC (Fig. 4A).
Following treatment with the enzyme, both T1 and T2 changed to
LacCer. Both were noticed to be Gb3Cer with a terminal
α-galactose, and the sugar chain of the band in T1 that was not
observed in poorly differentiated cancer was also identified as
Gb3Cer. Fig. 5 displays
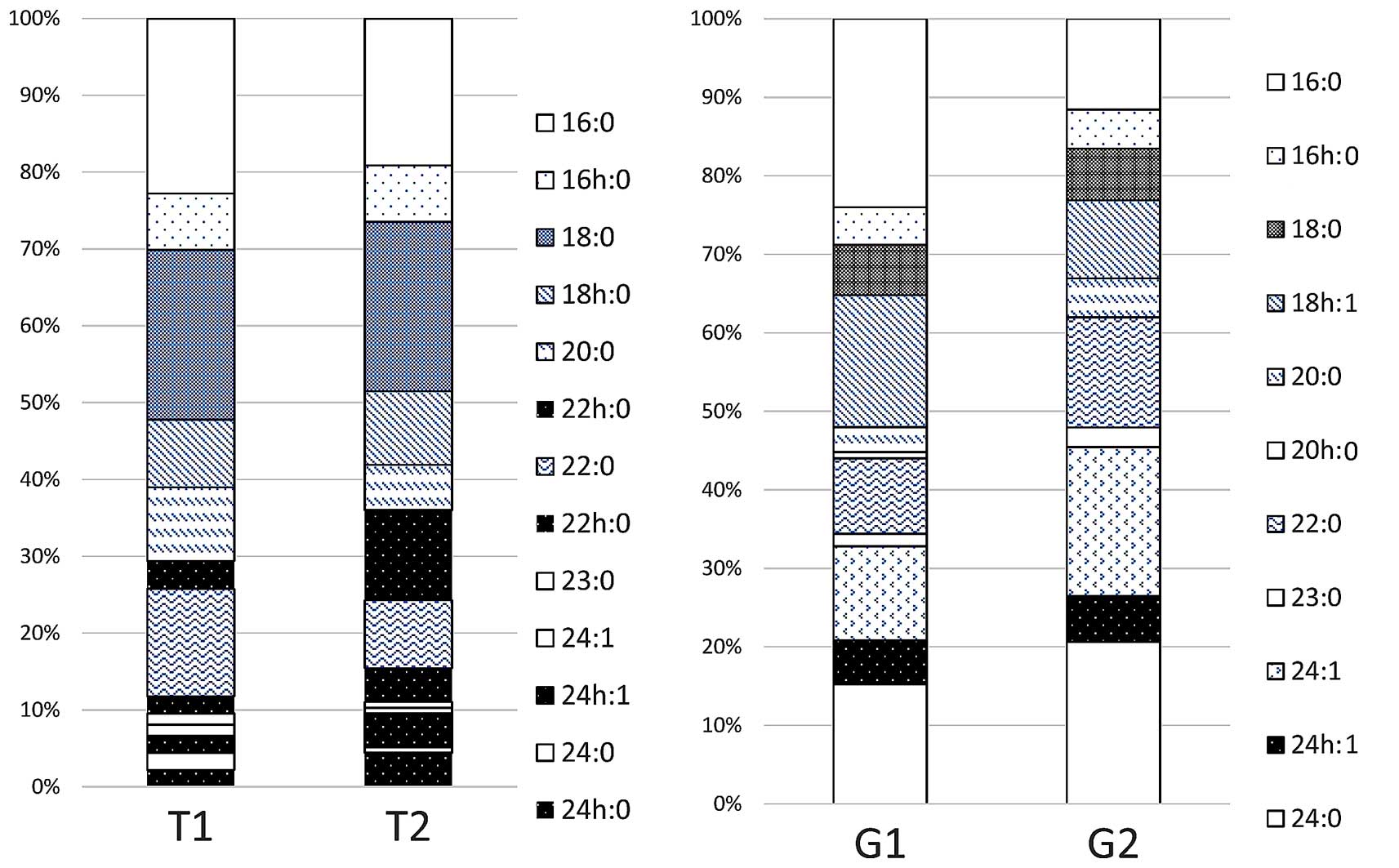
the results of the analysis of the fatty acid composition of
ceramide. There were no appreciable differences between T1 and T2
when the number of carbon atoms was 16 or 18. When the number of
carbon atoms was ≥20, however, the proportion of non-hydroxy fatty
acids without hydroxyl groups was higher in T1 than in T2, while
α-hydroxy fatty acids were predominant in T2. Fig. 4B displays the results of the analysis
of the composition of the long-chain base by TLC.
Dihydrosphingosine was observed to be the principal component in
both T1 and T2, while sphingosine or phytosphingosine were not
detected in either of them. These results suggested that the band
detected in T1 but not in T2 was due to differences on the fatty
acid composition.
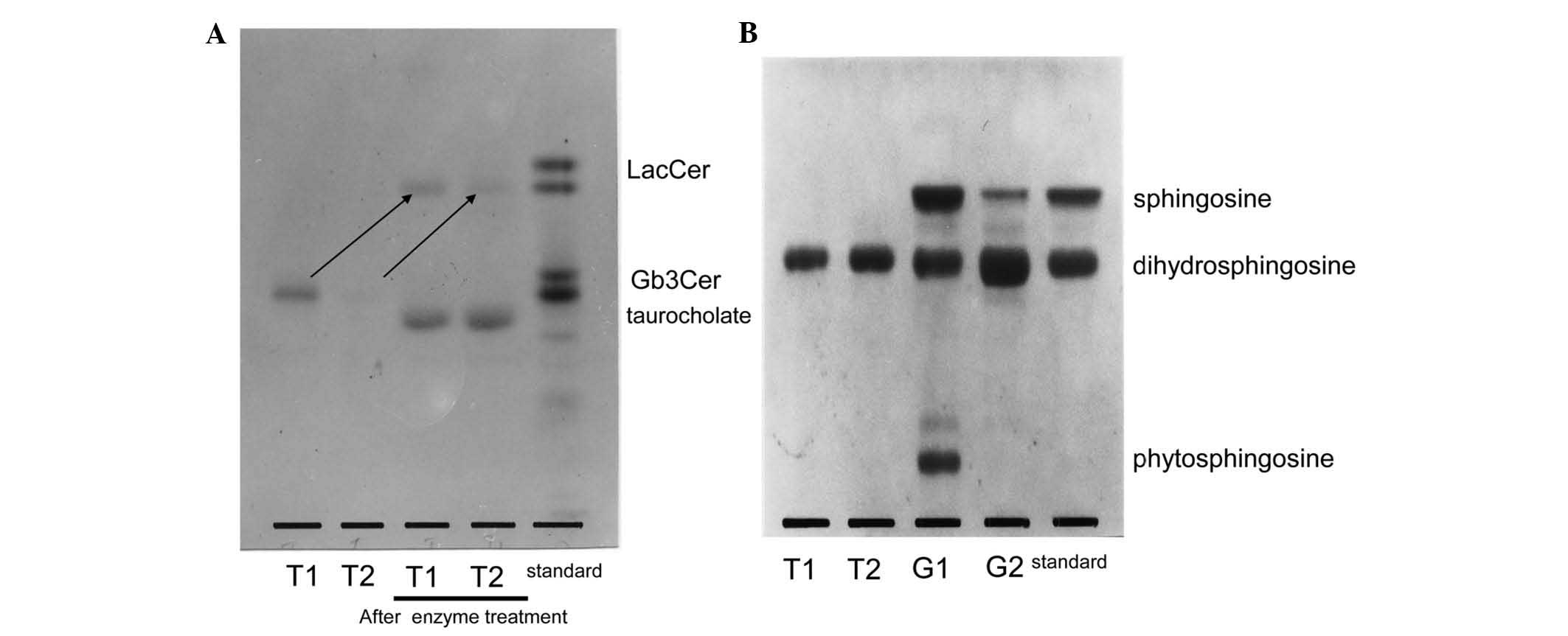 | Figure 4.(A) Structural analysis of the sugar
chains of LacCer, as indicated by arrows, by TLC following enzyme
treatment with α-galactosidase. Upon treatment with the enzyme,
both T1 and T2 changed to LacCer, and both were observed to be
Gb3Cer with a terminal α-galactose. (B) Analysis of the
composition of the long-chain base by TLC. Dihydrosphingosine was
noticed to be the principal component in both T1 and T2, while
sphingosine or phytosphingosine were not detected in either of
them. However, TLC analysis revealed that the ceramide of G1 was
composed of sphingosine, dihydrosphingosine and phytosphingosine,
while G2 contained just sphingosine and dihydrosphingosine. TLC,
thin-layer chromatography; LacCer, lactosylceramide;
Gb3Cer, globotriaosylceramide. |
As shown in Fig. 2A and
B, the migration of the upper band in G1 and G2 on TLC
corresponded to the migration of Gb4Cer, while the band
from G1 was located midway between Gb4Cer and
lactotetraosyl ceramide (Lc4Cer). Therefore, this band
from G1 was likely to be Lc4Cer or neolactotetraosyl
ceramide (nLc4Cer). However, TLC immunostaining using
anti-type I and anti-type II sugar chain antibodies did not detect
either G1 or G2 (data not shown). Fig.
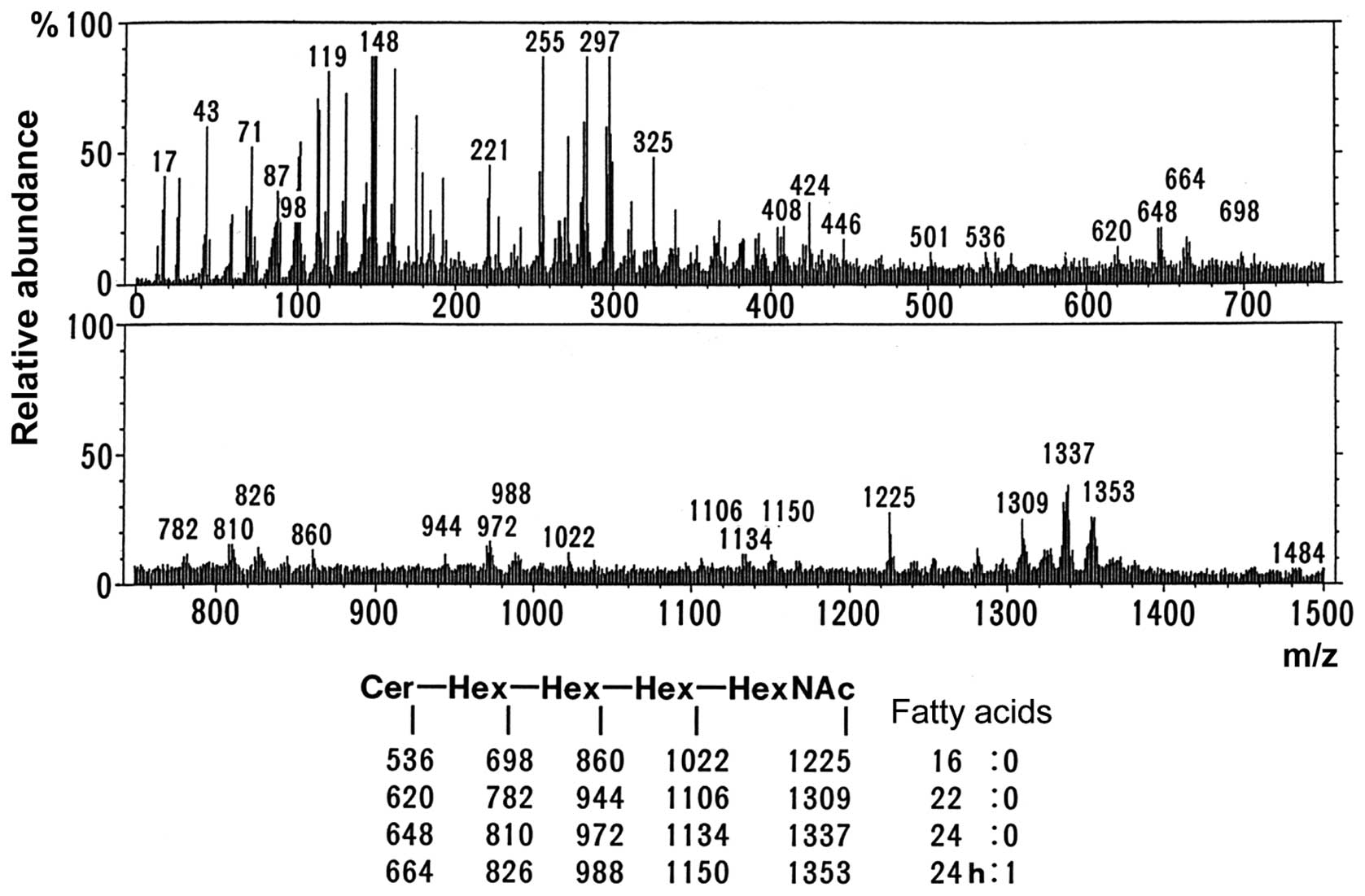
6 displays the mass spectrum obtained by direct analysis of G2
upon purification. The molecular ion peaks shown in the lower part
of Fig. 6 indicated that the
structure was Cer-Hex-Hex-Hex-HexNAc (being Cer, ceramide; Hex,
hexose; and HexNAc, N-acetylhexoseamine). These results suggested
that the sugar chains of the G1 and G2 bands were both similar to
the sugar chain of Gb4Cer. As shown in Fig. 5, the fatty acid composition exhibited
no appreciable differences between G1 and G2. Analysis of the
composition of the long-chain base by TLC (Fig. 4B) revealed that it was composed of
sphingosine, dihydrosphingosine and phytosphingosine in G1, while
it contained sphingosine and dihydrosphingosine in G2. Thus, the
presence of phytosphingosine was characteristic of G1. Based on
these results, the ceramide in the G1 band that was only detected
in well-differentiated endometrial cancer was observed to contain
phytosphingosine.
Collectively, fatty acid and sphingosine in
ceramides from specific neutral glycolipids from
well-differentiated adenocarcinomas were revealed to be
hydroxylated.
Discussion
In the present study, it was observed that
glycolipids with a sugar chain longer than Gb4Cer tended
to be present in well-differentiated endometrial cancer (Fig. 1). In our previous study (3), such a longer chain glycolipid was not
detected in the normal endometrium. Dihydrosphingosine was also
detected as a long-chain base constituent of ceramide in
endometrial cancer, while it was not detected previously in the
normal endometrium (3). These
differences between the normal endometrium and endometrial cancer
suggest that the processes of ceramide and sugar chain synthesis
differ between normal endometrial cells and endometrial cancer
cells. Further studies are warranted to investigate glycolipids
exhibiting slower migration than Gb4Cer on TLC in
well-differentiated endometrial cancer, since there was
considerable individual variation. Analysis by immunostaining may
be necessary for this purpose, as the migration rate on TLC
suggests that blood group substances may be present.
The current study also investigated whether there
were differences in the composition of neutral glycolipids
regarding the degree of differentiation of endometrial cancer. The
Gb3Cer band with a lower migration rate compared with
the upper band of Gb3Cer on TLC had two components in
well-differentiated cancer, while it had only one component in
poorly differentiated cancer. This difference was associated with
the composition of the fatty acids forming the ceramide. Namely,
when the number of carbon atoms was ≥20, non-hydroxy fatty acids
were increased in well-differentiated cancer, while α-hydroxy fatty
acids were increased in poorly differentiated cancer.
Gb4Cer also had two components in well-differentiated
cancer vs. one component in poorly differentiated cancer.
Gb4Cer containing phytosphingosine was specifically
identified in well-differentiated cancer.
Although it is unclear why such changes in
glycolipids with a sugar chain longer than Gb4Cer and
hydroxylated ceramides occur due to tumor differentiation, the
following mechanisms could be proposed: First, it has been
previously reported that the substrate specificity of the
glycolipid-synthesizing glycosyltransferase influences the
structure of ceramide (14); thus,
glycosyltransferase may change with the extent of tumor
differentiation, and this may cause the aforementioned band to
occur specifically in well-differentiated cancer. Second,
Gb3Cer and Gb4Cer both exhibited two bands in
well-differentiated cancer (Fig. 2A and
B), suggesting that certain change may occur prior to ceramide
synthesis that leads to differences in the composition of fatty
acids and the long-chain base employed for ceramide synthesis
between well- and poorly differentiated cancer. Although the
meaning of these changes is unclear, it is possible to suggest that
the amount of hydrophobic ceramide inserted into the cell membrane
varies with the extent of tumor differentiation, resulting in
differences on cell membrane structure between well- and poorly
differentiated cancer that may be involved in determining the
degree of malignancy of endometrial cancer. Long-chain bases have
been reported to regulate the behavior of cancer (15). Therefore, it is necessary to further
analyze such changes in ceramide regarding tumor differentiation at
the level of ceramide synthesis.
In conclusion, the present study analyzed neutral
glycolipids in endometrial cancer and revealed novel findings
concerning ceramide. The results of the current study suggested
that a number of the biological characteristics of the normal
endometrium and endometrial cancer could be associated with the
properties of these glycolipids and their ceramide structures.
Acknowledgements
The present study was partly supported by a
grant-in-aid for scientific research from the Japanese Ministry of
Education, Culture, Sports, Science and Technology (MEXT; Tokyo,
Japan; grant no. 26462538), the MEXT Supported Program for the
Strategic Research Foundation at Private Universities, 2012–2014
(grant no. S1201001) and a grant from Tokai University Research Aid
(Isehara, Japan; grant no. 2014012).
Glossary
Abbreviations
Abbreviations:
|
TLC
|
thin-layer chromatography
|
|
MS
|
mass spectrometry
|
References
|
1
|
Iwamori M: A new turning point in
glycosphingolipid Research. Hum Cell. 18:117–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kubushiro K, Kojima K, Mikami M, Nozawa S,
Iizuka R, Iwamori M and Nagai Y: Menstrual cycle-associated
alteration of sulfogalactosylceramide in human uterine endometrium:
Possible induction of glycolipid sulfation by sex steroid hormones.
Arch Biochem Biophys. 268:129–123. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikami M, Tukazaki K, Nozawa S, Iwamori M
and Nagai Y: Menstrual cycle-associated expression of 2-hydroxy
fatty acyl phytosphingosine-containing GlcCer, LacCer and Gb3Cer in
human uterine endometrium. Biochim Biophys Acta. 1125:104–109.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wegner MS, Wanger RA, Oertel S,
Brachtendorf S, Hartmann D, Schiffmann S, Marschalek R, Schreiber
Y, Ferreirós N, Geisslinger G and Grösch S: Ceramide synthases
CerS4 and CerS5 are upregulated by 17β-estradiol and GPER1 via AP-1
in human breast cancer cells. Biochem Pharmacol. 92:577–589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merrill AH Jr: De novo sphingolipid
biosynthesis: A necessary, but dangerous, pathway. J Biol Chem.
277:25843–25846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omae F, Miyazaki M, Enomoto A, Suzuki M,
Suzuki Y and Suzuki A: DES2 protein is responsible for
phytoceramide biosynthesis in the mouse small intestine. Biochem J.
379:687–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fader AN, Arriba LN, Frasure HE and von
Gruenigen VE: Endometrial cancer and obesity: Epidemiology,
biomarkers, prevention and survivorship. Gynecol Oncol.
114:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soper JT, McCarty KS Jr, Hinshaw W,
Creasman WT, McCarty KS Sr and Clarke-Pearson DL: Cytoplasmic
estrogen and progesterone receptor content of uterine sarcomas. Am
J Obstet Gynecol. 150:342–348. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sherman ME: Theories of endometrial
carcinogenesis: A multidisciplinary approach. Mod Pathol.
13:295–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montalto SA, Hakmi A, Moth P, Raju KS,
Coutts M, Papadopoulos AJ and Devaja O: Well differentiated
endometrioid adenocarcinoma of the uterus: A cancer unit or centre
case? Eur J Gynaecol Oncol. 30:35–39. 2009.PubMed/NCBI
|
|
11
|
Kiguchi K, Iwamori Y, Suzuki N, Kobayashi
Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y and Iwamori M:
Characteristic expression of globotriaosyl ceramide in human
ovarian carcinoma-derived cells with anticancer drug resistance.
Cancer Sci. 97:1321–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takehara K, Kubushiro K, Kiguchi K,
Ishiwata I, Tsukazaki K, Nozawa S and Iwamori M: Expression of
glycolipids bearing Lewis phenotypes in tissues and cultured cells
of human gynecological cancers. Jpn J Cancer Res. 93:1129–1137.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiguchi K, Takamatsu K, Tanaka J, Nozawa
S, Iwamori M and Nagai Y: Glycosphingolipids of various human
ovarian tumors: A significantly high expression of I3SO3GalCer and
Lewis antigen in mucinous cystadenocarcinoma. Cancer Res.
52:416–421. 1992.PubMed/NCBI
|
|
14
|
Yandım M Kartal, Apohan E and Baran Y:
Therapeutic potential of targeting ceramide/glucosylceramide
pathway in cancer. Cancer Chemother Pharmacol. 71:13–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Beckman BS and Foroozesh M: A
review of ceramide analogs as potential anticancer agents. Future
Med Chem. 5:1405–1421. 2013. View Article : Google Scholar : PubMed/NCBI
|