Introduction
In men, liver cancer is the second leading cause of
cancer-associated mortality worldwide. The majority of primary
liver cancers are hepatocellular carcinoma (HCC). Furthermore, ~50%
of HCC cases and the associated fatalities occur in China (1). The high rates of recurrence and
metastasis exhibit a major impact on long-term survival, even in
patients who have undergone radical resection or ablation. In fact,
there is a noticeable lack of biomarkers that could effectively
serve as predictive parameters for HCC patients. Several previous
studies have shown that circulating endothelial progenitor cells
(EPCs), a subpopulation of bone marrow-derived cells (BMDCs), could
be used as a prognostic marker in patients with HCC (2–5). Moreover,
it has been found that BM-derived multipotent progenitor cells may
differentiate into endothelial cells and be recruited to partake in
tumor vasculogenesis (6), while the
inhibition of vasculogenesis may markedly inhibit tumor growth
(7,8).
The complexity of BMDCs inspires further interest in the study of
HCC.
Accumulating evidence has suggested that BMDCs play
an important role in promoting tumor progression, including the
incorporation of EPCs into the growing vasculature, and in
subpopulations of BMDCs contributing to tumor neovascularization by
providing growth factors, cytokines or other proangiogenic
molecules (9). A novel mechanism has
been proposed in which BMDCs participate in initiating a
microenvironment that fosters the recruitment of disseminating
tumor cells prior to the arrival of metastatic tumor cells at
distant organ sites (10,11). At these sites, termed as
pre-metastatic niches, clusters of BM-derived hematopoietic
progenitors prime distant tissues for the influx of tumor cells and
the establishment of metastatic lesions (12,13).
However, the controversy over the underlying mechanism never ends.
For example, certain studies have shown that the blockade of
vascular endothelial growth factor receptor-1 (VEGFR1) activity in
BMDCs, which is believed by others to be a requisite in the
regulation of metastasis (10),
neither prevents nor changes the rate of spontaneous metastasis
formation following primary tumor removal (14).
Du et al reported that BM-derived
CD45+ myeloid cells are essential for tumor metastasis,
in which HIF1α contributes to the induction of SDF1α in tumor cells
that in turn promotes tumor progression by recruiting vascular
modulatory BMDCs to stimulate angiogenesis (15). Meanwhile, Ahn and Brown found that
tumors could not grow in matrix metalloproteinase-9-knockout mice,
but that CD11b+ myelomonocytic cells from the
transplanted BM was able to restore tumor growth (16). Due to the diversity of BMDCs, the role
of other subtypes of BMDCs is a research hotspot. For example, data
have shown that the predominant portion of EPCs in growing tumor
vessels derived from the BM are
CD45−VEGFR2+CD133+c-kit+
cells (17), whereas the largest and
most heterogeneous group of BMDCs in tumors consists of
CD45+ myelocytic cells that contribute directly to
neovascularization by expressing a variety of proangiogenic
cytokines, growth factors and proteases (18). It has been demonstrated that the VEGF
family is extremely important for angiogenesis, and that
myeloid-derived VEGF-A is essential to VEGFR2 in inhibiting tumor
progression (19). Sublethal
irradiation does harm to the hematopoiesis of the BM, in which
VEGFR2/3 are the main factors responsible for the recovery of
homeostasis (20). However, a number
of unknown factors remain with regard to BMDCs.
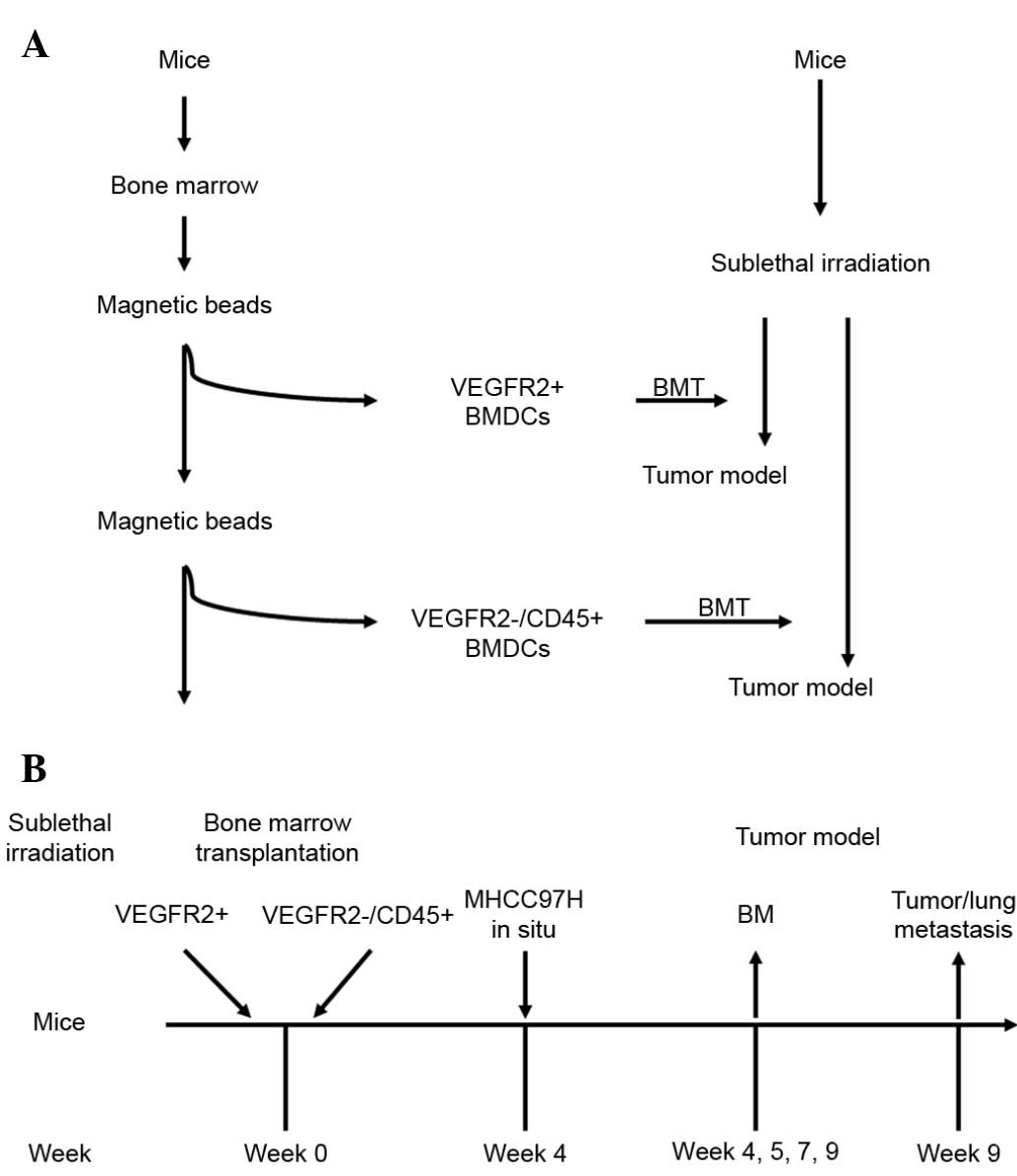
In the present study, HCC models with BM function
deficiency/reconstruction were established by sublethal
irradiation/BM transplantation (BMT). The effects of
VEGFR2+ or VEGFR2−CD45+ BMDCs on
HCC progression were dynamically observed. It was found that
BM-derived VEGFR2+ cells play an extremely important
role during the middle stage of tumor growth, while BM-derived
VEGFR2−CD45+ cells have multipotent
differentiation abilities, which promotes the tumor progression in
the late stage of HCC and is independent of BM-derived
VEGFR2+ cells.
Materials and methods
Animals and tumor models
Male, athymic, BALB/c nu/nu mice (5 week-old; mean
weight, 20 g/mouse; n=6 mice/test) were obtained from the
Experimental Animal Center of the Institute of Hematology, Chinese
Academy of Medical Sciences. All mice were bred in laminar-flow
cabinets under specific pathogen-free conditions. The housing
conditions were as follows: Room temperature, 26–28°C; sterilized
food and water; and artificial lightening with 10 h of light and 14
h of dark. The experimental protocol was approved by the Tianjin
Medical University Experimental Animal Care Committee (Tianjin,
China).
The human HCC MHCC97H cell line with high metastatic
potential was obtained from the Liver Cancer Institute of Fudan
University and cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5% CO2
(21). HCC tumor models were
established in nude mice by subcutaneous or orthotopic inoculation
of 1.0×106 cells in 0.2 ml NaCl solution (0.9%), as
previously described (22). At the
end of 9th week, the mice were sacrificed by cervical dislocation.
The tumors were collected for the subsequent analysis.
Irradiation and BMT
The sensitivity of the athymic BALB/c nu/nu mice to
whole-body irradiation was characterized through longitudinal
studies first, using different doses of radiation (from 5 to 9 Gy).
Next, BMT was performed after use of strain-specific sublethal
doses of radiation (6.5 Gy) to ensure a high degree of chimerism.
BM was harvested from donor BALB/c nu/nu mice. The
VEGFR2+ fraction from the total BMDC population was
magnetically isolated using magnetic beads conjugated with rat
monoclonal anti-mouse VEGFR2 antibody (CD309 MicroBead kit;
catalogue no. 130-097-346; Miltenyi Biotec, Inc., Cambridge, CA,
USA). The VEGFR2+-depleted BMDCs were then used to
harvest VEGFR2−CD45+ fractions with rat
monoclonal anti-mouse CD45 antibody and beads (CD45 MicroBead kit;
catalogue no. 130-052-301; Miltenyi Biotech, Inc.). Mice received
6.5 Gy irradiation 24 h prior to the BMT, and were randomly
assigned to receive one of the following BMTs: VEGFR2+
BMDCs alone, VEGFR2−CD45+ BMDCs alone or
unfractionated BMDCs. A total of 2.00×106 BM cells were
transplanted into the mice via tail injection. Control mice
(n=6/test) without irradiation received a tail vein injection of
saline (100 µl). Mice receiving transplants from
VEGFR2+, VEGFR2−CD45+ or whole
BMDC donors are referred to as VEGFR2-BMT, CD45-BMT or Total-BMT
mice, respectively. After 4 weeks of BM reconstruction, tumor
models were established in the mice (Fig.
1).
Immunohistochemical analysis of CD31
and platelet-derived growth factor receptor α (PDGFRα)
Acetone-fixed, 5-µm thick, frozen sections of HCC
tumors were stained with rat monoclonal anti-mouse CD31 (1 µg/ml;
catalogue no. sc-18916L; Santa Cruz Biotechnology Inc., Dallas, TX,
USA) or monoclonal goat anti-mouse PDGFRα (2 µg/ml; catalogue no.
AF-1062; R&D systems, Minneapolis, MN, USA) antibodies for 18 h
at 4°C. Normal monoclonal rat anti-mouse immunoglobulin (Ig) G2a (1
µg/ml; catalogue no. H106.771; Santa Cruz Biotechnology Inc.) was
used as a negative control. Bound antibodies were detected by
incubation with rhodamine-conjugated monoclonal rabbit anti-goat
IgGR (1 µg/ml; catalogue no. sc-3945; Santa Cruz Biotechnology
Inc.) or fluorescein isothiocyanate (FITC)-conjugated monoclonal
goat anti-rat IgG (catalogue no. sc-2011; Santa Cruz Biotechnology,
Inc.) for 30 min at 37°C. The slides were cross-stained with
4′,6-diamidino-2-phenylindole (1:1,000 dilution; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) for nuclear staining. For
additional negative controls, samples were exposed to secondary
antibodies alone, with the primary antibodies replaced by
phosphate-buffered saline (100 µl). Co-localization of CD31 with
PDGFRα images was counted in five regions of interest.
Antibody staining was observed using a Leica TCS SP2
Microscope Confocal system (Leica, Heidelberg, Germany) or a
Olympus BX40 fluorescence microscope (Olympus, Tokyo, Japan).
Dynamic analysis of
VEGFR2+CD133+ and
CD45+CD133+ BMDCs
On the 4th week post-BMT, BM and peripheral blood
smears were stained by hematoxylin and eosin (HE) staining to
evaluate the efficacy of BM reconstruction. On the 1st, 3rd, 5th
and 7th week after tumor orthotopic inoculation, the BM was
collected and analyzed by flow cytometry. Phycoerythrin (PE)- or
FITC-conjugated anti-CD133, VEGFR2 and CD45 antibodies (Miltenyi
Biotech, Inc.) were used to evaluate the differences between each
group. Non-specific IgG or IgM conjugated with PE or FITC were used
as isotype controls.
Statistical analysis
Data were assessed by an analysis of variance and
Fisher's exact test using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Statistical significance was indicated by P<0.05.
Results
CD45+ and
VEGFR2+ BMDCs facilitate the recovery of BM function and
promote tumor growth
In order to determine the optimal dose of
irradiation, nude mice were pretreated with 9, 8, 7, 6.5 and 6 Gy,
respectively. It was observed that the mice receiving 9, 8 or 7 Gy
irradiation succumbed within 3–7 days of being irradiated. However,
6.5 Gy irradiation caused efficient BM inhibition without mortality
and was chosen as the dose for the further experiments (data not
shown).
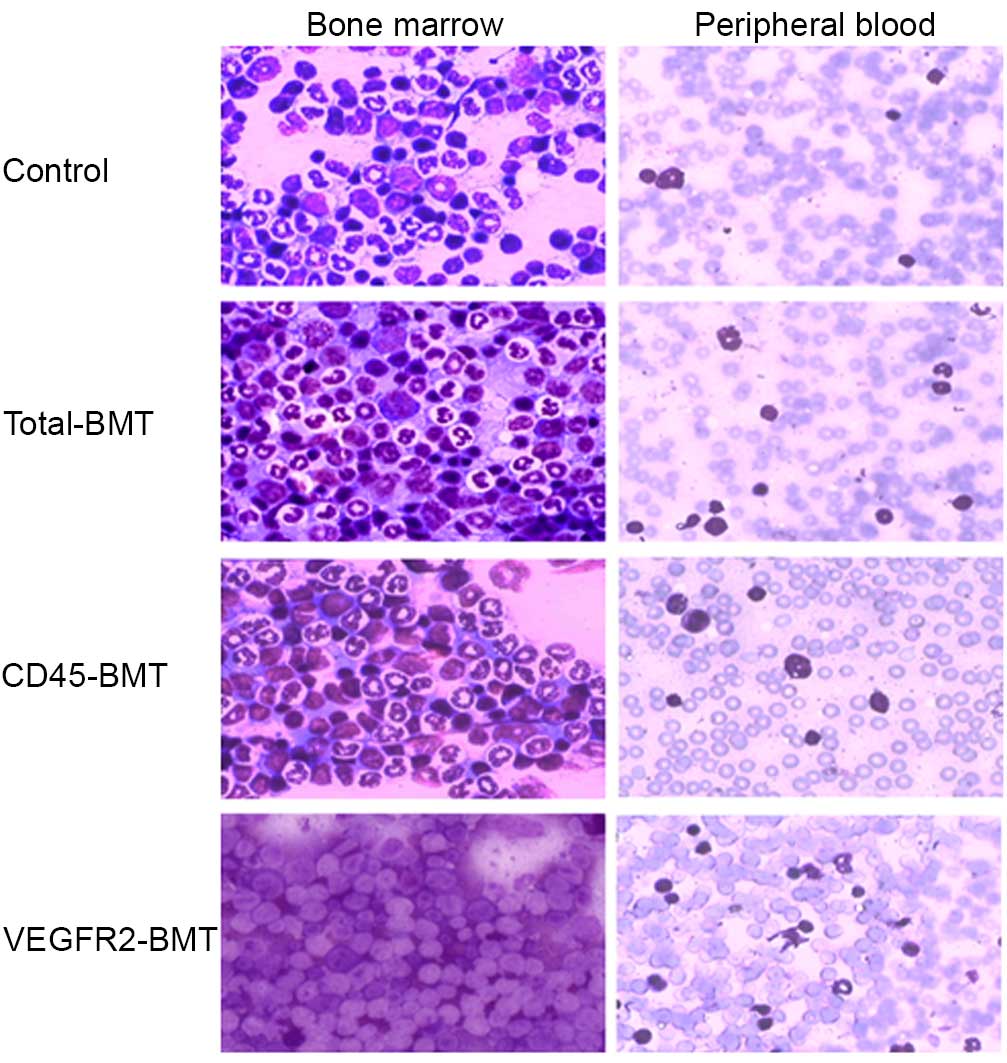
HE staining in the peripheral blood and the BM smear
demonstrated that, to a certain extent, the recovery of BM function
was observed in all the different transplantation groups. The ratio
of naive to mature cells was significantly different among all
groups in the peripheral blood and the BM. The naive cells were
more common in the VEGFR2-BMT group (Fig.
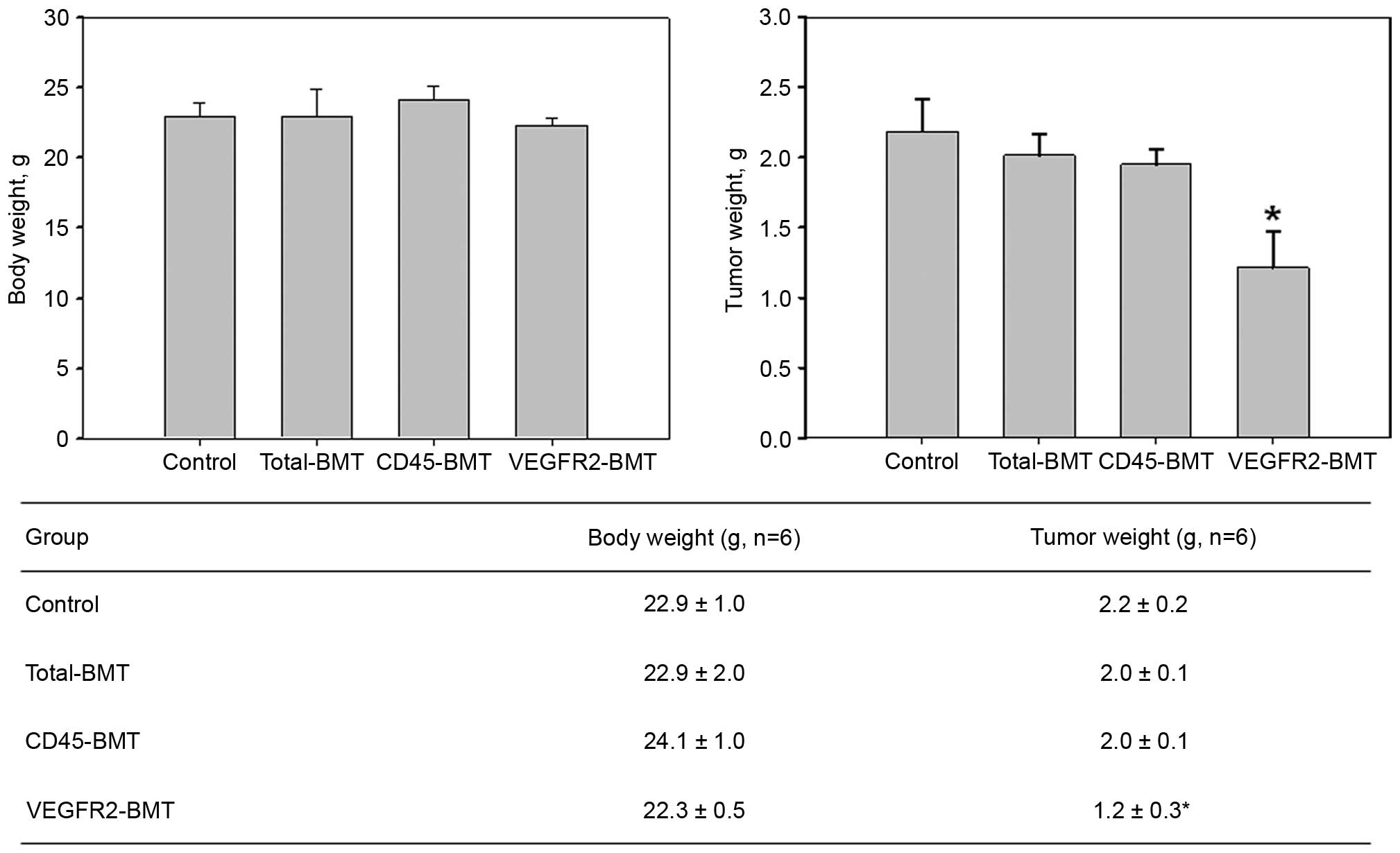
2). The growth of the mice was not significantly affected
(P=0.108). However, the tumor growth was markedly suppressed in the
VEGFR2-BMT group (Fig. 3). These data
suggested that sublethal irradiation inhibited the tumor growth.
However, it was the subpopulation of
VEGFR2−CD45+ cells, but not the
VEGFR2+ BMDCs that facilitated tumor progression.
Enhancement of tumor growth by
VEGFR2−CD45+ BMDCs is independent of
VEGFR2+ BMDCs
Magnetic beads were used to isolate the
VEGFR2+ BMDCs, which was followed by CD45+
BMDC selection. The VEGFR2+ and
VEGFR2−CD45+ BMDCs were used for subtype BMT.
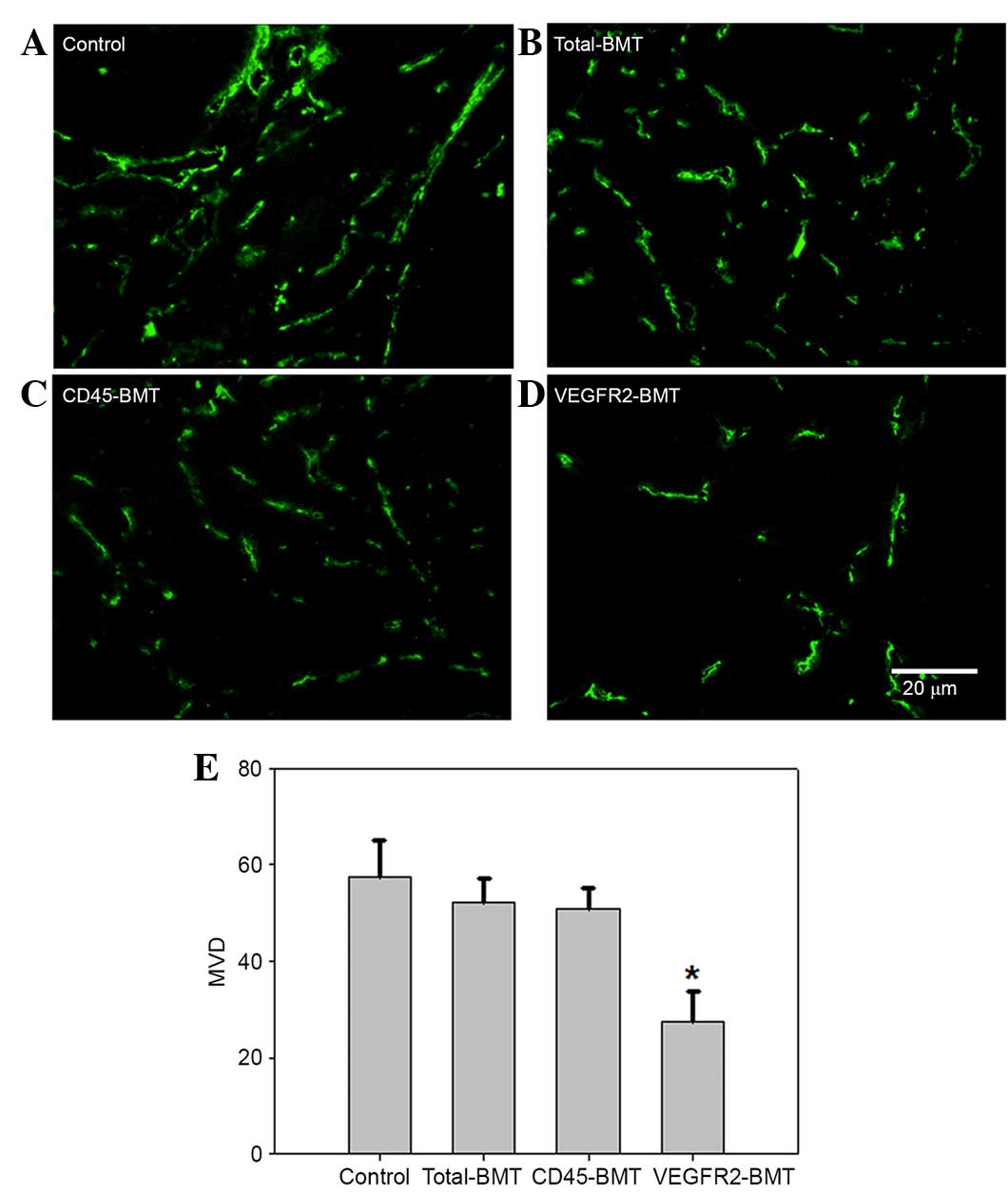
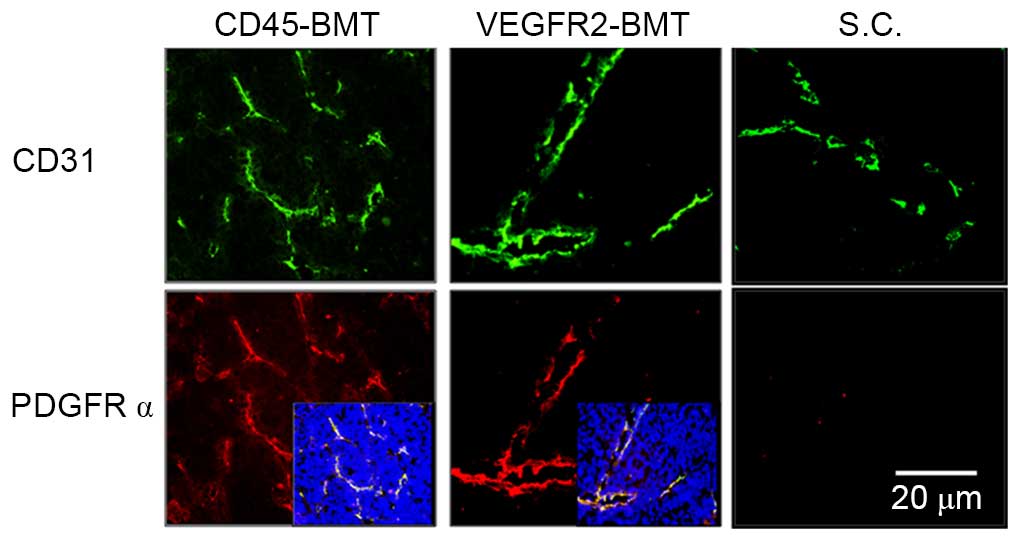
At 5 weeks post-tumor inoculation in situ and
subcutaneously, the expression of CD31 was detected by
immunofluorescence (22). The results
showed that CD31 expression was found in all tumors obtained from
the four groups. Microvessel density (MVD), however, was different
among the groups. The lowest MVD was found in the tumors from the
VEGFR2-BMT mice, whereas the difference was not significant in the
other three groups (Fig. 4). Since
the VEGFR2+ BMDCs had been separated out beforehand in
the CD45-BMT mice, a potential transdifferentiation of
CD45+ BMDCs may have existed, which accounted for the
origin of the VEGFR2+ endothelial precursors and the
subsequent mature endothelial cells in the tumor vasculature.
Tumor cells and the liver
microenvironment are indispensable to the expression of PDGFRα in
the tumor endothelium
Immunofluorescence staining was performed in in
situ and subcutaneous tumors. The positive expression of PDGFRα
was only observed in tumors in situ. Meanwhile, the MVD
decreased in the subcutaneous tumors. Although the MVD was
dramatically different in each of the groups with a different BMT,
the expression of PDGFRα was universal (Fig. 5). We have previously shown that the
overexpression of PDGFRα in tumor endothelial cells is closely
associated with the metastatic potential of HCC (22). The present data suggested that not
only the tumor cells, but also the liver microenvironment is
indispensable to the expression of metastasis-related endothelial
markers.
Synergistic effects of BM-derived
CD45+CD133+ and
VEGFR2+CD133+ cells on the progression of
HCC
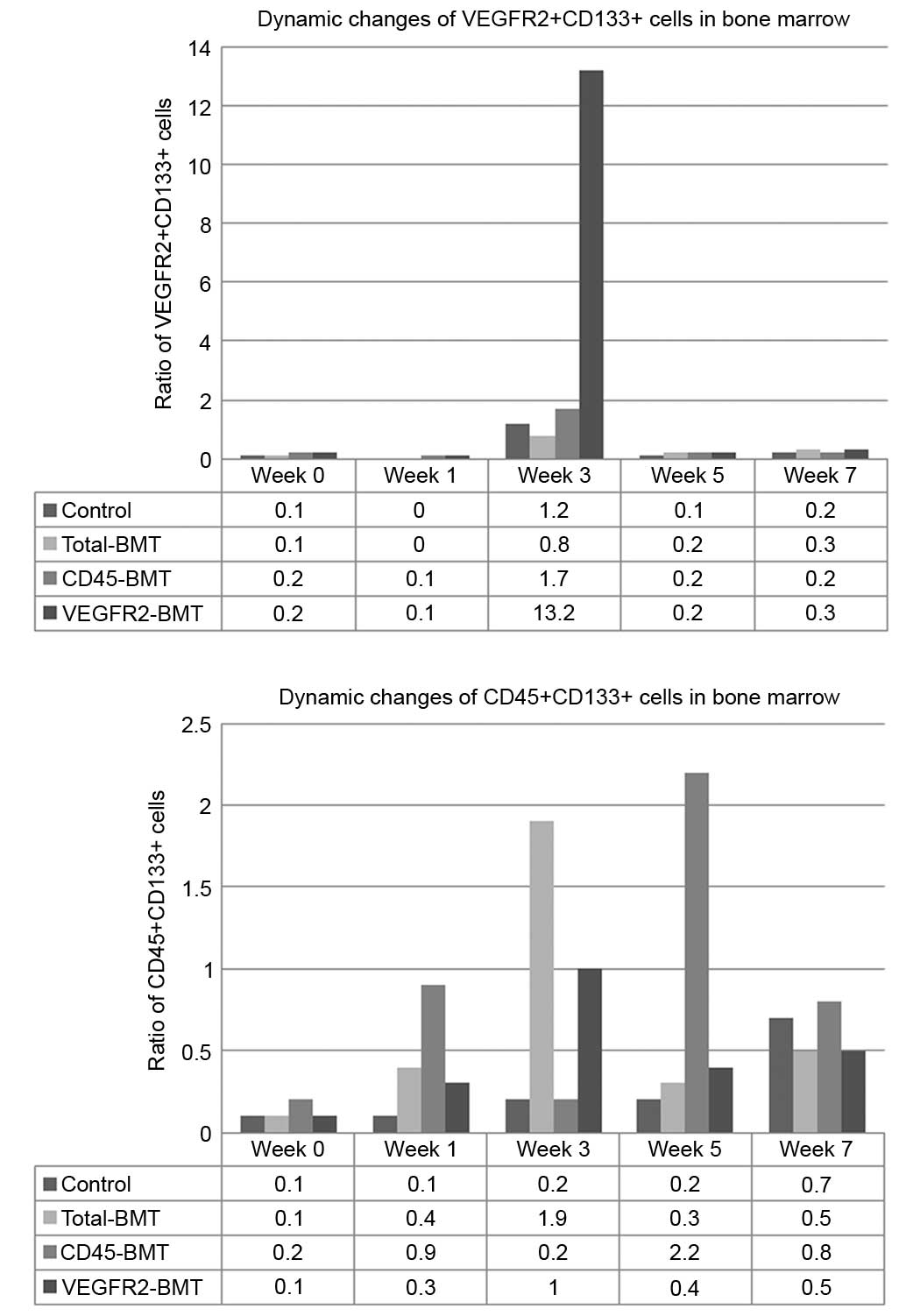
To determine the effects of endothelial progenitor
cells and myeloid progenitor cells on the progression of HCC, the
dynamic changes of BM-derived CD45+CD133+
cells and VEGFR2+CD133+ cells were detected
by flow cytometry. The results showed that the ratio of
VEGFR2+CD133+ cells in this model system
markedly increased in the middle phase of tumor progression (week
3), which reflected the mobilization of EPCs in the BM. With tumor
progression and metastasis over the next few weeks (weeks 5–7), the
ratio of VEGFR2+CD133+ cells rapidly fell
back to the base level. However, rapid tumor enlargement and lung
metastasis occurred in the late phase, consistent with the
increased ratio of CD45+CD133+ cells
(Fig. 6). These data suggested that
vasculogenesis played an important role during the early and middle
phase of tumor growth, while angiogenesis was the major determinant
of HCC progression.
Discussion
In tumors, new blood vessels develop not only from
pre-existing vessels (angiogenesis), but can also be formed from
circulating vascular progenitor cells originating from the BM
(vasculogenesis). There is a large amount of evidence demonstrating
that EPCs and proangiogenic hematopoietic cells are able to support
the vascularization of tumors and play a synergistic role with
angiogenesis (23). Moreover,
BM-derived myeloid progenitor cells could differentiate into
mononuclear cells (granulocytes, macrophage or dendritic cells)
involved in the regulation of angiogenesis and tumor endothelial
cells directly forming the tumor vasculature (24,25).
However, the synergistic effect of different subpopulations of
BMDCs on tumor progression in HCC has not yet been reported.
In the present study, a well established HCC model
with a highly metastatic MHCC97H cell line was utilized to
investigate tumor growth and metastasis following sublethal
irradiation and the subsequent transplantation of BMDC
subpopulations. It was found that BM-derived
VEGFR2+CD133+ cells promoted tumor
progression at the middle stage of HCC, while BM-derived
CD45+CD133+ cells were extremely important
for the metastasis of HCC at the middle and late stages.
It has been demonstrated that EPCs exhibit their
effects in the early stage of tumor formation (26,27). A
recent study showed that EPCs were incorporated into the tumor
vasculature with a 20–35% efficiency on days 4–6 post-tumor
implantation, but then became diluted by local non-BM-derived
endothelial cells, thus resulting in only 1% of EPCs being detected
in the tumor vasculature following 4 weeks of growth (28). The present data indicated that
VEGFR2+CD133+ cells played an important role
in the middle stage of HCC tumor growth. This is consistent with
the current notion that the BMDCs confer a promotional role on the
existing blood vessels rather than de novo
neovascularization in tumors, which is hypothesized to be a result
of the highly proangiogenic features of these cells (23).
In addition to EPCs, other types of BMDCs, such as
myeloid cells, also contribute essentially to tumor progression.
Myeloid cells are hematopoietic cells that are positive for VEGFR1
and eventually give rise to macrophages, monocytes and
granulocytes/neutrophils. The cells share similar features with
EPCs in that they may express endothelial specific markers,
including CD31, CD34 and VEGFR2 under certain circumstances. A
recent study illustrated that in breast and lung carcinoma models,
the recruitment of myeloid cells, but not EPCs, facilitates tumor
regrowth after local irradiation (29), while the inhibition of vasculogenesis,
instead of angiogenesis by blocking BMDC influx into the tumors,
prevents the recurrence of glioblastoma following irradiation
(8). The present data showed that the
transplantation of VEGFR2−CD45+ BMDCs not
only guaranteed the growth of the mice but also promoted the growth
of the tumors. Even the expression of PDGFRα, a specific
endothelial marker of HCC metastasis (22) was observed in the tumor endothelium.
Due to the negative transplantation of VEGFR2+ BMDCs, in
the present study, we speculated that BM-derived
VEGFR2−CD45+ cells may have the ability of
transverse differentiation to endothelial progenitors, which
promised that tumor growth would proceed in the early and middle
stages of HCC progression. In addition, it is likely that
VEGFR2+CD133+ cells and
CD45+CD133+ cells coordinately exhibit
effects on tumor progression, and that
CD45+CD133+ cells take over the control of
tumor progression after the initiation of
VEGFR2+CD133+ cells. Therefore,
CD45+CD133+ cells may be the target for the
inhibition of metastasis for late-stage HCC, while
VEGFR2+CD133+ cells may be a good choice for
early- and middle-stage intervention. Finally, the present study
found that sublethal irradiation, which attenuates the growth of
the tumor, may offer a novel strategy for cancer treatment.
In conclusion, BMDCs play an important role in HCC
progression. Different types of BMDCs may synergistically exhibit
effects on HCC tumor growth and metastasis. The stage of tumor
should be considered when targeting BMDCs.
Acknowledgements
The authors would like to thank Professor Ning Zhang
(Tianjin Medical University) for providing experimental support.
This study was supported by the Natural Science Foundation of China
(grant nos. 30600723, 81372635, 81101871 and 81201644) and the
Major Program of National Natural Science Foundation of Tianjin
(grant no. 11JCZDJC18800).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan
ST and Poon RT: Significance of circulating endothelial progenitor
cells in hepatocellular carcinoma. Hepatology. 44:836–843. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu AX, Sahani DV, Duda DG, di Tomaso E,
Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS,
Lahdenranta J, et al: Efficacy, safety, and potential biomarkers of
sunitinib monotherapy in advanced hepatocellular carcinoma: A phase
II study. J Clin Oncol. 27:3027–3035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bayo J, Marrodán M, Aquino JB, Silva M,
García MG and Mazzolini G: The therapeutic potential of bone
marrow-derived mesenchymal stromal cells on hepatocellular
carcinoma. Liver Int. 34:330–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shih YT, Wang MC, Zhou J, Peng HH, Lee DY
and Chiu JJ: Endothelial progenitors promote hepatocarcinoma
intrahepatic metastasis through monocyte chemotactic protein-1
induction of microRNA-21. Gut. 64:1132–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barajas M, Franchi F, Clavel C, Aranguren
XL, Kramer MG, Abizanda G, Merino J, Moreno C, Gárate L, Guitart A,
et al: Multipotent adult progenitor cells (MAPC) contribute to
hepatocarcinoma neovasculature. Biochem Biophys Res Commun.
364:92–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathieu S, Gerolami R, Luis J, Carmona S,
Kol O, Crescence L, Garcia S, Borentain P and El-Battari A:
Introducing alpha(1,2)-linked fucose into hepatocarcinoma cells
inhibits vasculogenesis and tumor growth. Int J Cancer.
121:1680–1689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kioi M, Vogel H, Schultz G, Hoffman RM,
Harsh GR and Brown JM: Inhibition of vasculogenesis, but not
angiogenesis, prevents the recurrence of glioblastoma after
irradiation in mice. J Clin Invest. 120:694–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiratsuka S, Watanabe A, Aburatani H and
Maru Y: Tumour-mediated upregulation of chemoattractants and
recruitment of myeloid cells predetermines lung metastasis. Nat
Cell Biol. 8:1369–1375. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dawson MR, Duda DG, Chae SS, Fukumura D
and Jain RK: VEGFR1 activity modulates myeloid cell infiltration in
growing lung metastases but is not required for spontaneous
metastasis formation. PLoS One. 4:e65252009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du R, Lu KV, Petritsch C, Liu P, Ganss R,
Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z and Bergers G:
HIF1alpha induces the recruitment of bone marrow-derived vascular
modulatory cells to regulate tumor angiogenesis and invasion.
Cancer Cell. 13:206–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn GO and Brown JM: Matrix
metalloproteinase-9 is required for tumor vasculogenesis but not
for angiogenesis: Role of bone marrow-derived myelomonocytic cells.
Cancer Cell. 13:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rafii S, Meeus S, Dias S, Hattori K,
Heissig B, Shmelkov S, Rafii D and Lyden D: Contribution of
marrow-derived progenitors to vascular and cardiac regeneration.
Semin Cell Dev Biol. 13:61–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grunewald M, Avraham I, Dor Y,
Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L,
Abramovitch R and Keshet E: VEGF-induced adult neovascularization:
Recruitment, retention, and role of accessory cells. Cell.
124:175–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stockmann C, Doedens A, Weidemann A, Zhang
N, Takeda N, Greenberg JI, Cheresh DA and Johnson RS: Deletion of
vascular endothelial growth factor in myeloid cells accelerates
tumorigenesis. Nature. 456:814–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hooper AT, Butler JM, Nolan DJ, Kranz A,
Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al:
Engraftment and reconstitution of hematopoiesis is dependent on
VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell
Stem Cell. 4:263–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue
Q, Chen J, Gao DM and Bao WH: Establishment of cell clones with
different metastatic potential from the metastatic hepatocellular
carcinoma cell line MHCC97. World J Gastroenterol. 7:630–636.
2001.PubMed/NCBI
|
|
22
|
Zhang T, Sun HC, Xu Y, Zhang KZ, Wang L,
Qin LX, Wu WZ, Liu YK, Ye SL and Tang ZY: Overexpression of
platelet-derived growth factor receptor alpha in endothelial cells
of hepatocellular carcinoma associated with high metastatic
potential. Clin Cancer Res. 11:8557–8563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahn GO and Brown JM: Role of endothelial
progenitors and other bone marrow-derived cells in the development
of the tumor vasculature. Angiogenesis. 12:159–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Pozzi A and Young PP: TNFalpha
accelerates monocyte to endothelial transdifferentiation in tumors
by the induction of integrin alpha5 expression and adhesion to
fibronectin. Mol Cancer Res. 9:702–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wara AK, Foo S, Croce K, Sun X, Icli B,
Tesmenitsky Y, Esen F, Lee JS, Subramaniam M, Spelsberg TC, et al:
TGF-β1 signaling and Krüppel-like factor 10 regulate bone
marrow-derived proangiogenic cell differentiation, function, and
neovascularization. Blood. 118:6450–6460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Palma M, Venneri MA and Naldini L: In
vivo targeting of tumor endothelial cells by systemic delivery of
lentiviral vectors. Hum Gene Ther. 14:1193–1206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Palma M, Venneri MA, Roca C and Naldini
L: Targeting exogenous genes to tumor angiogenesis by
transplantation of genetically modified hematopoietic stem cells.
Nat Med. 9:789–795. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi
JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA,
Benezra R and Mittal V: Bone marrow-derived endothelial progenitor
cells are a major determinant of nascent tumor neovascularization.
Genes Dev. 21:1546–1558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kozin SV, Kamoun WS, Huang Y, Dawson MR,
Jain RK and Duda DG: Recruitment of myeloid but not endothelial
precursor cells facilitates tumor regrowth after local irradiation.
Cancer Res. 70:5679–5685. 2010. View Article : Google Scholar : PubMed/NCBI
|