Introduction
Multidrug resistance (MDR) is a major hurdle for
successful chemotherapy, and accounts for failure in >90% of
patients (1,2). The mechanisms of MDR have been well
documented in the literature (1,2). Of all
these mechanisms, overexpression of P-glycoprotein (P-gp), which is
encoded by the MDR1 gene, is the most typical and commonly
encountered theory. P-gp is an important transporter protein
belonging to the adenosine triphosphate (ATP)-binding cassette
(ABC) family of membrane transporters. Due to its participation in
the efflux of numerous cytotoxic anticancer drugs from tumor cells,
P-gp could directly influence the pharmacokinetics and
pharmacodynamics of these drugs (3).
As a result, it has become a severe challenge to overcome
P-gp-mediated drug resistance.
Curcumin (Cur), a hydrophobic polyphenol derived
from the rhizome of the herb Curcuma longa, has a wide
spectrum of biological and pharmacological activities. Cur has been
shown to exhibit antioxidant, anti-inflammatory, antimicrobial and
anticarcinogenic activities (4). The
anticancer properties of Cur have been primarily attributed by its
ability to block the transcriptional factor nuclear factor (NF)-κB,
which is a master regulator of inflammation, cell proliferation,
apoptosis and resistance in cells (5). NF-κB regulates the expression of genes
involved in the suppression of the apoptotic response, and is
responsible for tumor cell survival (6). Additionally, Cur is also known to
downregulate the intracellular levels of three major ABC drug
transporters, P-gp, MDR-associated protein 1 (MRP1) and ABCG2,
which are important in MDR (7–9). In spite
of these various promising therapeutic applications of Cur, its
therapeutic efficacy is limited due to its markedly poor water
solubility, and consequently, remarkably low systemic
concentrations are achieved when Cur is consumed orally (4). In addition, Cur suffers from chemical
instability in the gastrointestinal tract (4). Cur is also known to be photosensitive,
thus requiring careful handling (4).
Cur is a substrate of P-gp, which implies that Cur can be pumped
out of tumor cells by P-gp, leading to a reduction in drug
accumulation in tumor cells (10). In
spite of numerous formulations challenges, several formulation
strategies, including nanoparticles, liposomes, complexation with
phospholipids and cyclodextrins, and solid dispersions, are being
developed to improve Cur's bioavailability (11–13).
Several absorption enhancers have also been used to
improve Cur's bioavailability (14).
Piperine (Pip) has been reported to enhance the bioavailability of
Cur both in preclinical studies and in studies on human volunteers
(15). Pip inhibits P-gp-mediated
efflux in Caco-2 cells and CYP3A4-mediated drug metabolism
(16). Pip reduces the ATPase
activity of P-gp at high concentrations, while stimulates it at low
concentrations (17). Pip can reverse
MDR in short- and long-term treatments, and may improve the outcome
of chemotherapy by inhibiting P-gp, MRP1 and breast cancer
resistance protein effectively by downregulating the expression of
these transporter genes (18). A
marketed product available in combination with Pip is
BioPerine® capsules (Sabinsa Corporation, East Windsor,
NJ, USA). Despite its pharmacological activity and safety, this
molecule still remains overlooked due to the lack of a suitable
delivery system that can result in adequate therapeutic levels
in vivo (14).
Several non-ionic surfactants are able to reverse
P-gp-mediated MDR. For example, Pluronic sensitizes MDR tumors by
inhibiting the P-gp drug efflux system through ATP depletion
(19,20). Tocopheryl polyethylene glycol
succinate (TPGS), a water-soluble succinate ester of vitamin E, can
inhibit P-gp-mediated drug efflux and increase the oral
bioavailability of anticancer drugs (21). Brij molecules have also shown efflux
pump inhibitory activity. Dong et al prepared doxorubicin-
and paclitaxel-loaded nanoparticles using Brij 78 as an emulsifying
agent to overcome MDR by inhibiting P-gp and depleting ATP
(22). Our laboratory has previously
identified the structures of Brij required for overcoming MDR, and
observed that Brij 78 and Brij 97 could decrease intracellular ATP
levels and inhibit the ATPase activity of P-gp in MDR cells
(23).
It was hypothesized that combining multiple
strategies for overcoming drug resistance could improve the
efficacy of antitumor drugs. The present study investigated a new
co-delivery system of solid lipid nanoparticles (SLNs) with TPGS
and Brij 78 to allow the anticancer drug Cur and the P-gp modulator
Pip to overcome tumor drug resistance. (Cur+Pip)-SLNs were prepared
by the emulsification evaporation-low temperature solidification
method. In addition to formulation design and optimization, the
physicochemical characterization, encapsulation efficiency (EE) and
in vitro release behavior of (Cur+Pip)-SLNs were further
investigated. In order to understand the efficacy of overcoming MDR
in tumor cells, cytotoxicity assay and cell uptake experiments of
(Cur+Pip)-SLNs were conducted on the paclitaxel-resistant human
ovarian carcinoma cell line A2780/Taxol.
Materials and methods
Chemicals and reagents
Cur (purity 95%) and Pip (purity 98%) were obtained
from Shaanxi Huike Botanical Development Co., Ltd. (Xi'an, China).
Standard substances of Cur and Pip were supplied by the National
Institutes for Food and Drug Control (Beijing, China). Glycerol
monostearate was a gift of Gattefossé (Lyon, France). TPGS was
purchased from Xi'an Healthful Biotechnology Co., Ltd. (Xi'an,
China). Brij 78 was purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Oleic acid was purchased from Sinopharm
Chemical Reagent Co., Ltd. (Shanghai, China). Soya lecithin was
purchased from Aobox Biotechnology Co., Ltd. (Beijing, China).
Sephadex G-50 was obtained from Pharmacia Biotech (GE Healthcare
Life Sciences, Uppsala, Sweden). Verapamil hydrochloride was
purchased from Shanghai Harvest Pharmaceutical Co., Ltd. (Shanghai,
China).
RPMI-1640 medium was obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Penicillin-streptomycin, fetal
bovine serum (FBS) and PBS were purchased from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). Trypsin-EDTA
was purchased from Gibco (Thermo Fisher Scientific, Inc.). MTT and
rhodamine (Rh) 123 were obtained from Sigma-Aldrich (Merck
Millipore). All solvents used in the present study were of
high-performance liquid chromatography (HPLC) grade, and were used
without further purification. Water was purified by distillation
and deionization.
Preparation of (Cur+Pip)-SLNs,
Cur-SLNs and blank SLNs
(Cur+Pip)-SLNs were prepared by the emulsification
evaporation-low temperature solidification method (24). In brief, oleic acid, glycerol
monostearate and lecithin were dissolved in ethyl acetate by
ultrasonication at 50°C as the oil phase, and then the drugs in
ethanol were added into the oil phase to obtain the organic phase
containing Cur and Pip. The aqueous phase was prepared by adding
TPGS and Brij 78 to deionized water under stirring for 10 min at
40°C. Subsequently, the organic and aqueous phases were heated
individually to 50–55°C for 2–4 min, and then the organic phase was
injected into the aqueous phase. The suspension was magnetically
stirred at 50°C for 20 min to remove the organic solvent. Finally,
the suspension was cooled in an ice bath to form (Cur+Pip)-SLNs.
The Cur-SLNs and blank SLNs were prepared following the same
procedure, with the exception of adding only Cur or neither drug,
respectively. These formulations were stored in a refrigerator at
4°C for further analysis. The drug content was determined by HPLC
following the disruption of SLNs in acetonitrile.
Characterization of
(Cur+Pip)-SLNs
To measure the EE of Cur and Pip, (Cur+Pip)-SLNs and
Cur-SLNs were analyzed in a Sephadex G-50 column. In total, 0.2 ml
sample of (Cur+Pip)-SLNs was added to the column, and then eluted
with deionized water. The unencapsulated Cur and Pip that remained
in the gel were equilibrated with 30% ethanol. (Cur+Pip)-SLNs
collected from the first 10 ml eluent were destroyed with the
mobile phase by ultrasonication for ~10 min. Another 0.2 ml sample
of (Cur+Pip)-SLNs was diluted with the same volume of mobile phase.
The quantity of Cur and Pip loaded was determined by HPLC. EE was
calculated according to the following formula:
EE=(Wi/Wtotal)x100%, where Wi is
the determined quantity of Cur and Pip in the SLNs suspensions
subsequent to passing over the Sephadex G-50 column, and
Wtotal is the determined quantity of Cur and Pip in the
SLNs suspensions prior to passing over the Sephadex G-50
column.
The contents of Cur and Pip were determined by HPLC
analysis in a system equipped with Millennium 32 software (Waters
Corporation, Milford, MA, USA), a 486 Tunable UV/Visible Absorbance
Detector (Waters Corporation) and a 510 HPLC Pump (Waters
Corporation). The drug was separated using a Diamonsil®
C18 column (200×4.6 mm, 5 µm; Dikma Technologies Inc., Beijing,
China). A 50:35:15 v/v/v methanol:acetonitrile:deionized water
mixture was used as a the mobile phase, at a flow rate of 1.0
ml/min and a temperature of 25°C. The run time was 6 min for each
sample. Detection was monitored at a wavelength of 425 and 343 nm
for Cur and Pip, respectively.
The drug concentrations of Cur and Pip were
calculated from calibration curves. The assay was linear over the
tested concentration range of 0.5–20 µg/ml (r=0.9998) for Cur and
0.2–10 µg/ml (r=0.9998) for Pip. There was no interference from the
excipients of the drugs in the assay. The lower limit of
quantification was 0.025 and 0.030 µg/ml for Cur and Pip,
respectively, which could be measured with acceptable accuracy and
precision. The inter- and intra-day variance of the method was
within the acceptable range of <2%.
Particle size, polydispersity index and zeta
potential of (Cur+Pip)-SLNs were measured by dynamic light
scattering using a Nano ZS90 Zetasizer (Malvern Instruments Inc,
Westborough, MA, USA). The samples were prepared by diluting the
(Cur+Pip)-SLN suspension with deionized water, and the data were
obtained from the average of three measurements. The average was
calculated by dividing the total value by the number of samples.
The size and morphology of (Cur+Pip)-SLNs were observed using
transmission electron microscopy (TEM) (H-7650; Hitachi, Ltd.,
Tokyo, Japan). For TEM analysis, a drop of (Cur+Pip)-SLNs
suspension was placed into a copper grid, air-dried and stained by
adding a drop of 1% uranyl acetate solution for contrast
enhancement. The grid was kept at room temperature to ensure
dryness and then observed under TEM.
The release rate of Cur and Pip from the SLNs in
vitro was measured by dialyzing against release medium (PBS pH
7.4 or 5.5 containing 25% v/v ethanol). In total, 1 ml drug-loaded
SLNs was placed into a dialysis tube (molecular weight cut-off,
14,000 kDa; Viskase Co., Osceola, AR, USA), and the end sealed of
the dialysis tube was immersed fully in 200 ml release medium in a
beaker. The beakers were placed in an incubator at 37±0.5°C and
agitated horizontally at a speed of 90 rpm. At the designated time
intervals, 1 ml release medium was withdrawn and replaced with an
equal volume of fresh release medium to maintain the sink
conditions. In in vitro release experiments, the solubility
of a drug is >5-fold larger than the release of drug
concentration, which is regarded as reaching the sink conditions.
The solution was transferred into an HPLC vial upon filtering
through a 0.45-µm syringe filter. The analysis procedure was
similar to that conducted for the determination of the EE
percentage. The release rate was calculated with the formula:
Release rate=(Wi/Wtotal)x100%, where
Wi is the determined quantity of Cur and Pip in the
release medium at the designated time point, and Wtotal
is the total quantity of Cur and Pip in an equivalent volume of SLN
suspension prior to the release experiment. All the samples were
carefully protected from the light during the experiment. All the
release experiments were repeated in triplicate.
Cell cultures
The paclitaxel-resistant human ovarian carcinoma
cell line A2780/Taxol was kindly provided by Department of
Gynecology and Obstetrics, The First Affiliated Hospital of Harbin
Medical University (Harbin, China). A2780/Taxol cells were grown
using 75-cm3 flasks in a humidified 5%
CO2/95% atmosphere in an incubator at 37°C in RPMI-1640
medium supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin. Cells grown to confluence were subcultured every
other day upon trypsinization with 0.25% trypsin-EDTA.
Cytotoxicity assay in A2780/Taxol
cells
The in vitro antitumor activity of
drug-loaded SLNs, free drugs and excipients was determined by MTT
assay as follows: Briefly, A2780/Taxol cells in logarithmic growth
phase were seeded in 96-well plates at a density of 5,000
cells/well. Following attachment overnight, the culture medium in
each well was carefully replaced with 100 µl fresh medium
containing treatments. The treatments included (Cur+Pip)-SLNs,
Cur-SLNs, free Cur solution, free Pip solution, combination of Cur
and Pip solution, TPGS, Brij 78 and blank SLNs.
After incubation for 24 h, the medium was removed
and the cells were washed with PBS. Then, the viability of the
cells was determined by MTT assay. For that purpose, 50 µl 5 mg/ml
MTT dissolved in PBS was added to each well. The plates were
incubated for an additional 4 h at 37°C, and then the medium was
discarded. Thereafter, 100 µl dimethyl sulfoxide was added to each
well to dissolve the formazan crystals. The absorbance of each well
was assessed on a microplate reader at a wavelength of 490 nm. The
spectrophotometer was calibrated to an absorbance value of 0 using
culture medium without cells, and cells containing culture medium
without nanoparticles or drugs were treated as controls. The
relative cell viability was calculated by
(A)test/(A)control, where (A)test
and (A)control were the average absorbance of the test
and control samples, respectively.
Rh efflux assay
The accumulation of Rh 123 in A2780/Taxol cells was
first detected by confocal laser scanning microscopy (CLSM).
A2780/Taxol cells were seeded onto round glass coverslips in 6-well
plates for 24 h. Once the medium had been removed, the cells were
treated with (Cur+Pip)-SLNs, Cur-SLNs, free Cur solution, free Pip
solution, Cur+Pip solution, TPGS, Brij 78 and blank SLNs separately
for 4 h at 37°C and 5% CO2. Then, Rh 123 (5 µg/ml) was
added, and the cells were incubated for an additional 1 h.
Following this incubation period, the cells were collected and
washed twice with ice-cold PBS buffer (pH 7.4). Coverslips were
placed onto glass microscope slides and inspected by CLSM (BX40;
Olympus Corporation, Tokyo, Japan).
Statistical analysis
The mean and standard deviation (SD) were determined
for each treatment group. Statistical analysis was performed using
a Student's t-test and Microsoft Office 2013 software (Microsoft
Corporation, Redmond, WA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
EE of (Cur+Pip)-SLNs
Compared with polymeric nanoparticles, SLNs
attracted more attention recently, since SLNs could not only avoid
the disadvantages of other colloidal carriers, but also have
excellent inherent properties, including controlled drug release
and drug targeting, high drug load capacity, increased physical
stability and low toxicity (24).
Therefore, the development of (Cur+Pip)-SLNs should be a worthwhile
and promising strategy. In the present study, (Cur+Pip)-SLNs were
prepared by the emulsification evaporation-low temperature
solidification method. The average EE values of Cur and Pip were
87.4±0.6 and 14.7±0.2%, respectively, with a drug load of
19.56±0.18 µg/mg for Cur and 3.26±0.05 µg/mg for Pip. The EE and
drug load of three batches of (Cur+Pip)-SLNs had no significant
difference, with a relative SD of 0.6% for Cur and 0.2% for Pip,
which demonstrated that the preparation process was reproducible
and stable.
Zeta potential and particle size
The zeta potential, particle size and size
distribution of (Cur+Pip)-SLNs were characterized with a Nano ZS90
Zetasizer. Zeta potential is an important parameter used to predict
the physical stability of nanoparticles. A high zeta potential
value indicates increased stability of the system, since it could
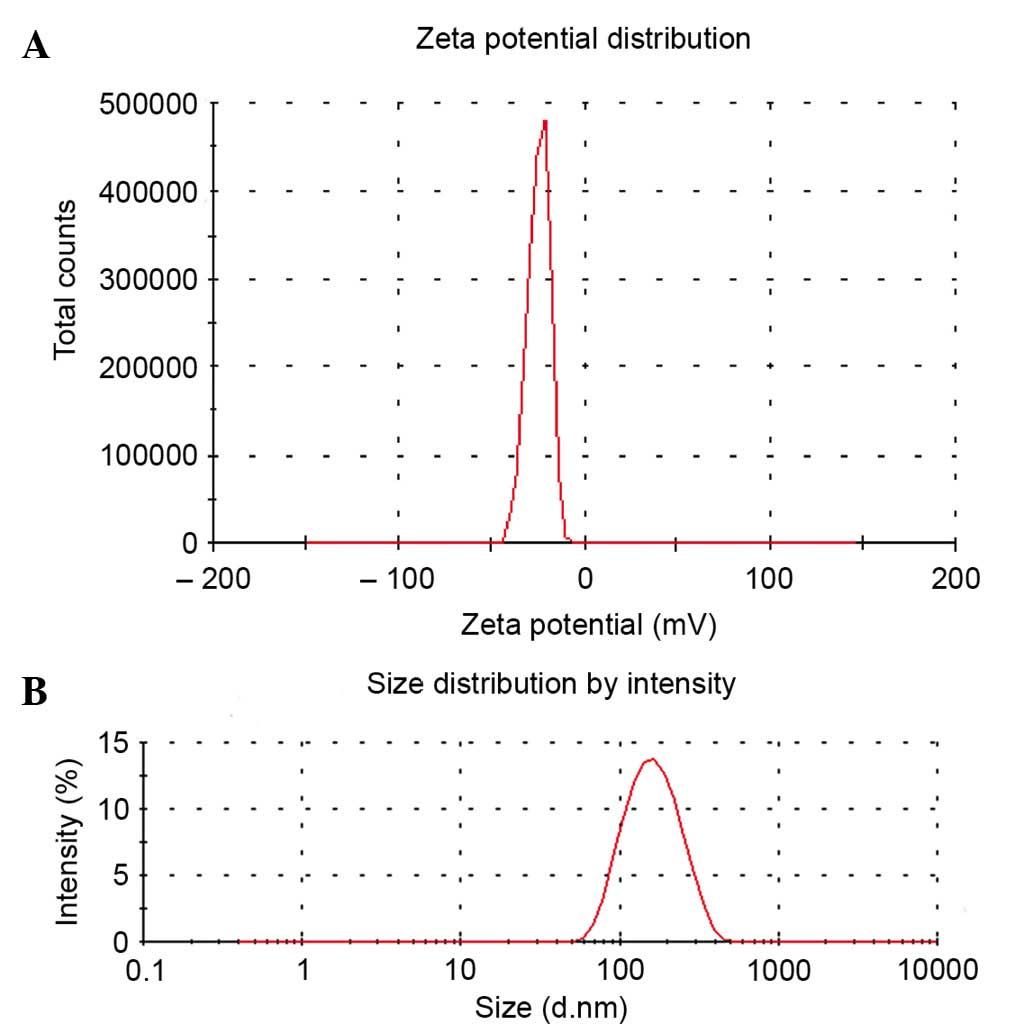
provide a repelling force between the nanoparticles (25). As shown in Fig. 1A, (Cur+Pip)-SLNs had a relatively high
negative zeta potential of ~20 mV. (Cur+Pip)-SLNs exhibited a mean
particle diameter of ~130.8 nm, with a unimodal size distribution
and a polydispersity index of 0.152 (Fig.
1B). The polydispersity index is an parameter used to represent
the distribution of nanoparticles, and indicates a low aggregation
of particles when its value is <0.5 (26).
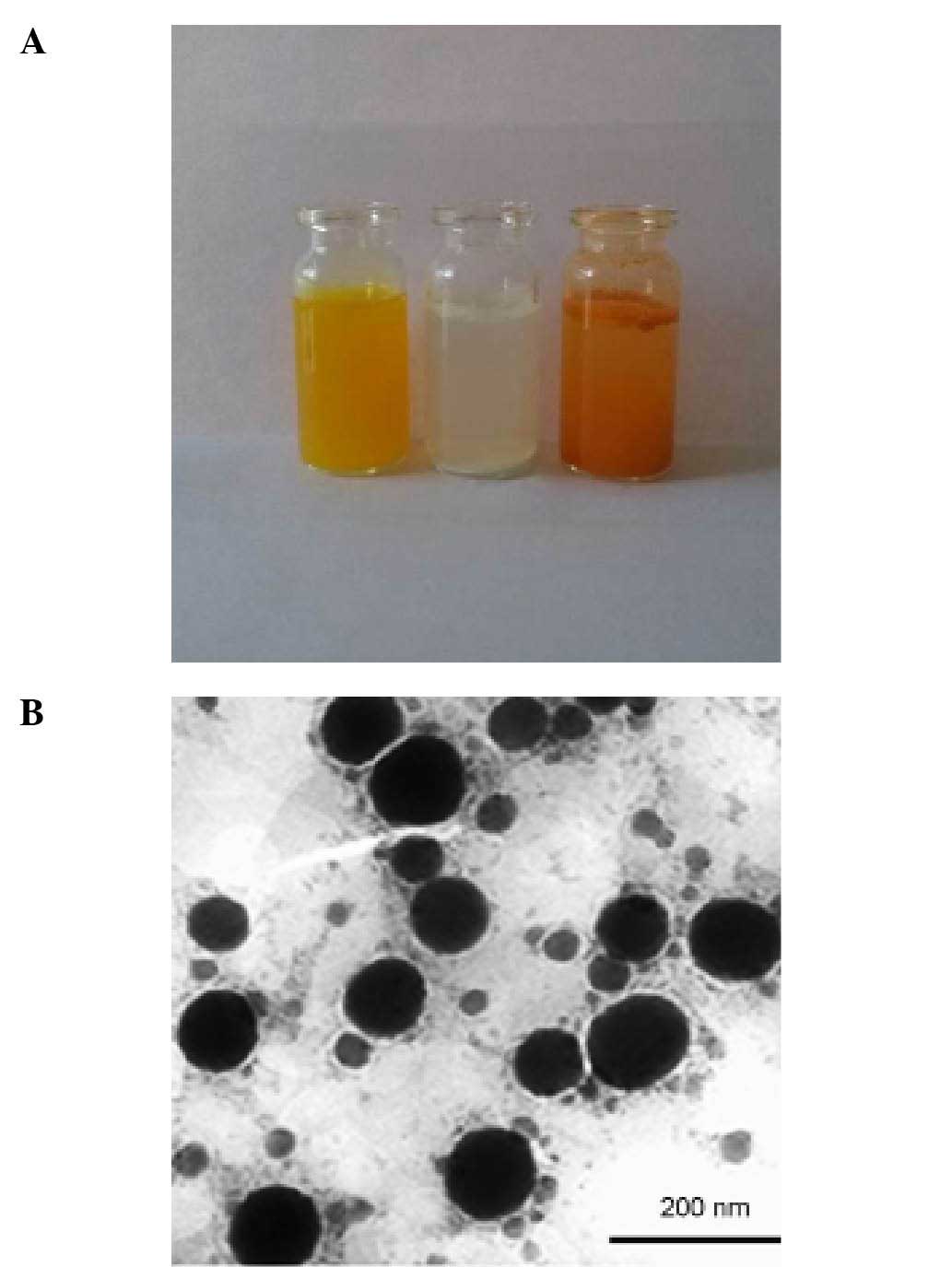
Compared to free Pip solution (Fig. 2A, middle) or free Cur solution
(Fig. 2A, right), (Cur+Pip)-SLNs
(Fig. 2A, left) could be dispersed
homogeneously into water without apparent drug sediments. In
addition, a smooth sphere morphology and uniform size distribution
of (Cur+Pip)-SLNs were observed by TEM (Fig. 2B).
In vitro drug release studies
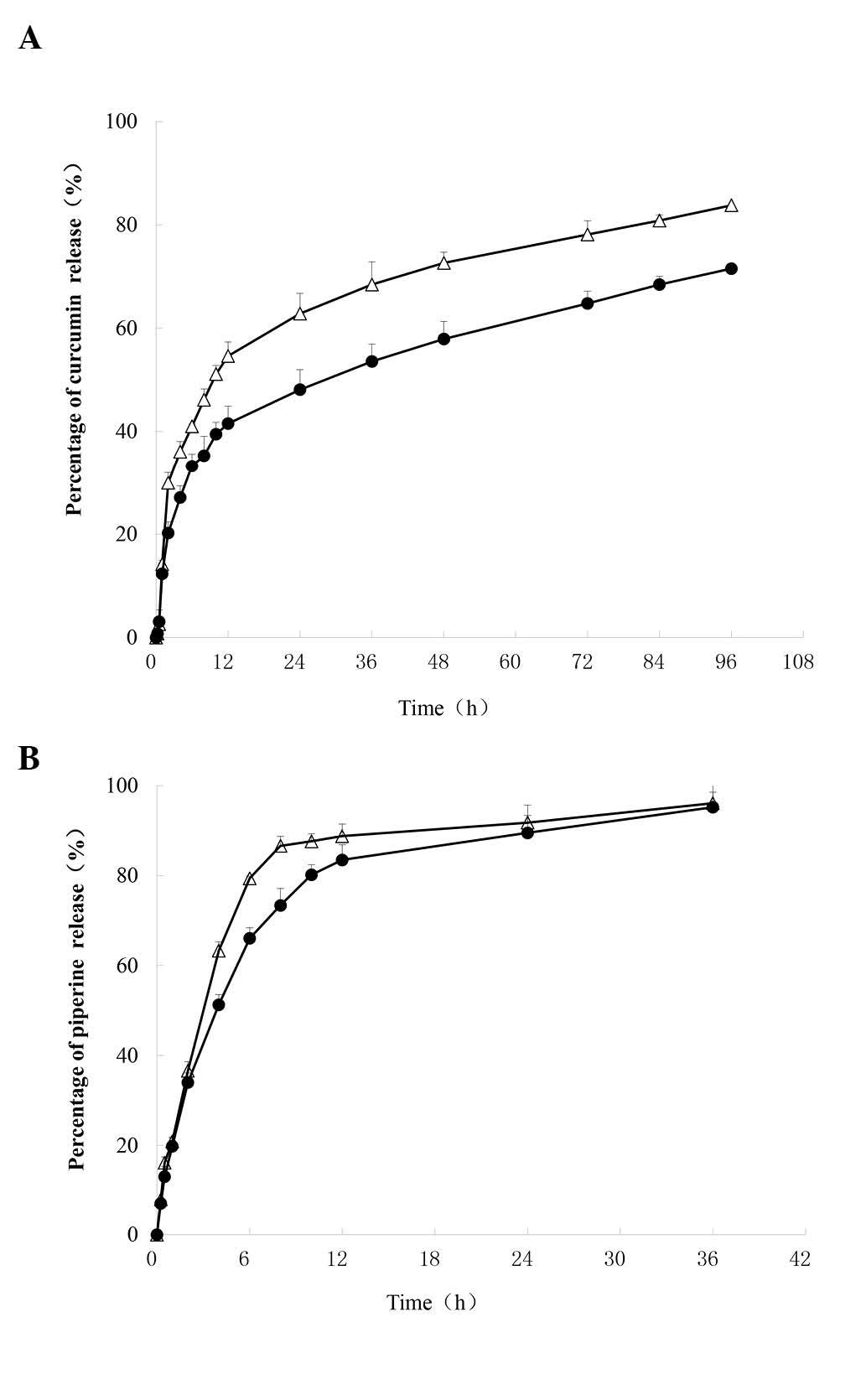
Fig. 3 represents the
in vitro cumulative release profiles of Cur and Pip from
(Cur+Pip)-SLNs in PBS. PBS (pH 5.5) was used in the present study
to simulate the internal environment of tumor cells, since the pH
in tumors is lower than that in normal tissue (27). PBS (pH 7.4) was selected to simulate
the environment of blood (27). Due
to the poor water solubility of Cur and Pip, it is difficult to
maintain a good sink condition when carrying out the in
vitro release experiments. In the present study, 25% v/v
ethanol was added to the release medium to maintain sink
conditions, in which the solubility of Cur and Pip was 0.265 and
0.195 mg/ml, respectively. Approximately 71.5% of Cur was released
at 96 h, and ~89% of Pip was released in 24 h in PBS pH 7.4. In
addition, these profiles exhibited a burst release of ~36% during
the first 2 h of release, which probably is due to the
non-encapsulation of the drugs. In addition, the drug cumulative
release percentage displayed a slight pH dependence. For instance,
the Cur cumulative release percentage at 96 h was 71.5% at pH 7.4,
while it was 83.7% at pH 5.5, which indicated that the SLNs
released drug more rapidly in the acidic environment of pH 5.5 than
in a pH 7.4 environment.
Cytotoxicity assay in A2780/Taxol
cells
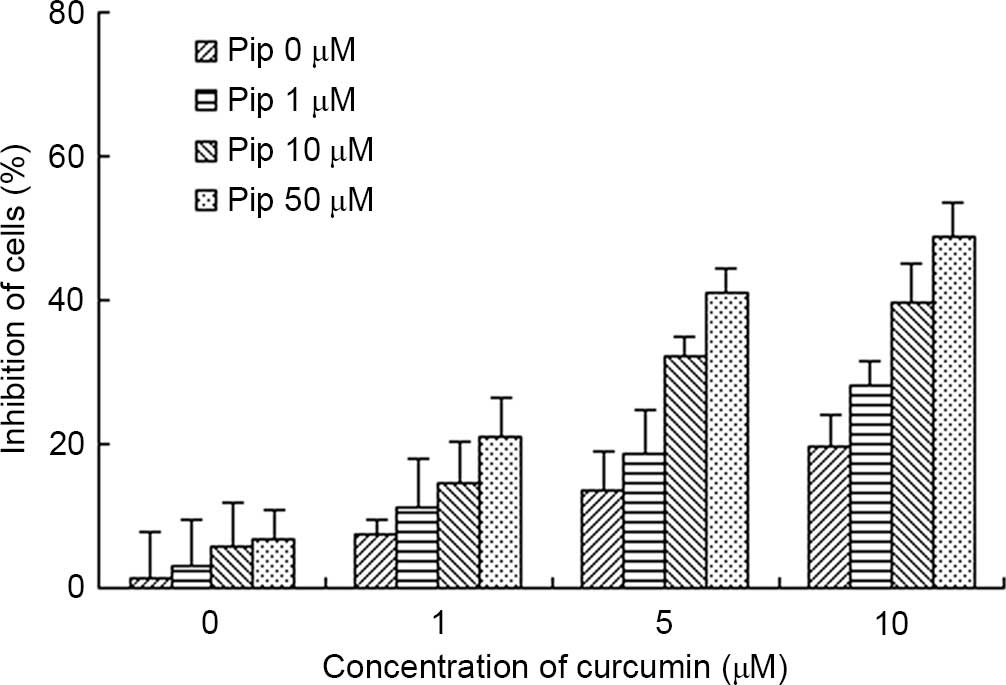
Firstly, the cytotoxicity/cell viability effect of
Pip was investigated in A2780/Taxol cells. When treated with Pip at
concentrations of 1–50 µM, the cytotoxicity on A2780/Taxol cells
was negligible (Fig. 4). Since the
half maximal inhibitory concentration of Pip (318 µM) in
A2780/Taxol cells did not change significantly following the
addition of verapamil [an inhibitor of P-gp (28)], it was confirmed that Pip was not the
substrate of P-gp. Singh et al had studied the cytotoxicity
of Pip in the MCF-7 (a breast cancer cell line) and MDCK cell lines
(28). Their results revealed that
Pip did not exert any undesirable effect at concentrations ≤100
µM.
Secondly, the effect of Pip on Cur-treated cells
viability was investigated in A2780/Taxol cells. With increased
concentration of Pip, the anti-proliferative effect of Cur was
significantly enhanced. Upon treatment of the cells with Cur and
Pip (10 and 50 µM, respectively), the inhibition rate of the cells
was 59.90±8.40%, which was significantly higher than that observed
with Cur (25.85±5.74%) and Pip (6.87±4.52%) treatment alone
(Fig. 4). These results indicate that
Pip, an inhibitor of P-gp, can enhance the anti-proliferative
effect of Cur, which is a substrate of P-gp (10).
TPGS and Brij 78 can inhibit P-gp, thus sensitizing
MDR cells (23). In the present
study, the effect of SLNs with TPGS and Brij 78 on sensitizing MDR
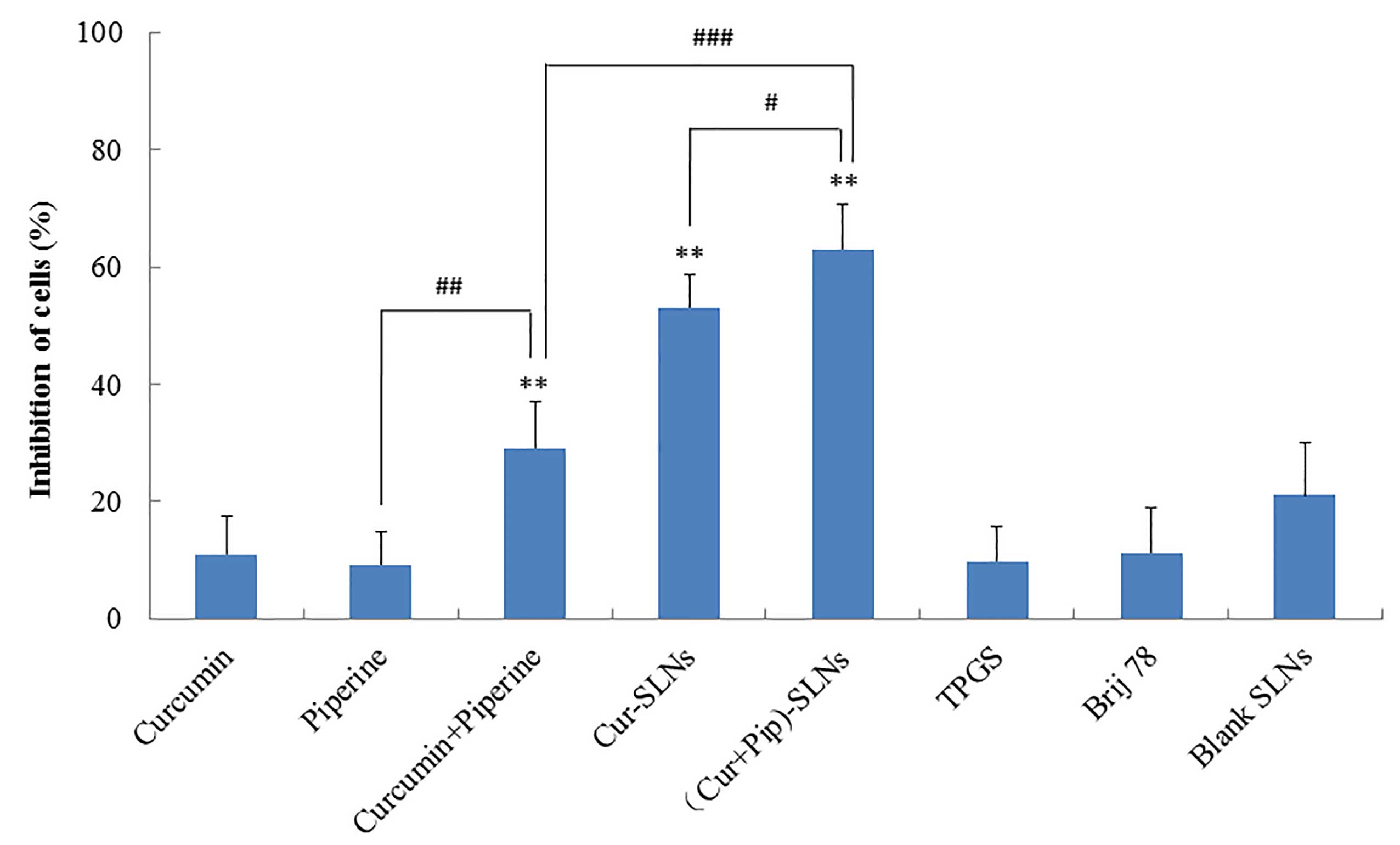
cells was investigated in A2780/Taxol cells. As shown in Fig. 5, cell inhibition following treatment
with Cur-SLNs or (Cur+Pip)-SLNs was higher than that observed
following treatment with free Cur or Cur-Pip solutions (P<0.01).
By contrast, blank SLNs, free Brij or TPGS did not apparently
inhibit cell growth. It was demonstrated that SLNs with TPGS and
Brij 78 could sensitize MDR cells (21). SLNs serve as potential anticancer drug
delivery nanocarriers, since they exhibit great superiority to
modulate drug release, improve anticancer activity and overcome
MDR. SLNs have also been shown to increase the cellular
accumulation of drugs, since they could decrease the resistance of
P-gp-expressing cells.
When treated with (Cur+Pip)-SLNs, MDR cells
exhibited the highest response to its cytotoxic action (P<0.01),
which presumably resulted from the synergistic effect of Cur, Pip
and Pip-mediated P-gp inhibition in A2780/Taxol cells, thus
amplifying the Cur-induced cytotoxicity and overcoming MDR in tumor
cells. The effect of Pip on increasing the intracellular Cur
concentration was the key to the enhancement of the cytotoxicity in
resistant cells.
P-gp serves a significant role in the
bioavailability of several drugs, mainly cytotoxic hydrophobic and
anticancerous drugs (2). In the
present study, although the drug load of Pip in (Cur+Pip)-SLNs
(3.26±0.05 µg/mg) was not very high, the enhancement of
cytotoxicity exerted by Cur was remarkable, since Pip is known to
be a strong inhibitor of P-gp (28).
Rh efflux assay
MDR is a major clinical problem that reduces the
efficacy of a large number of chemotherapeutic agents. The most
classical mechanism of MDR is the overexpression of P-gp, which can
successfully pump out multiple antineoplastic agents from cells,
thereby decreasing their intracellular accumulation and leading to
drug resistance (2). To investigate
whether (Cur+Pip)-SLNs inhibit the function of P-gp, the
intracellular accumulation of Rh 123, which is a substrate of P-gp,
in the presence or absence of SLNs, was examined using A2780/Taxol
cells. It was reported that P-gp function was significantly
correlated with Rh 123 efflux, and that inhibition of P-gp could
result in increased intracellular accumulation of Rh 123 (29). Thus, Rh 123 could be a fluorescent dye
used to monitor P-gp function (30).
As shown in Fig. 6A, Rh 123
fluorescence was scarcely detected in A2780/Taxol cells treated
with free Rh 123, which indicated a strong efflux phenomenon of Rh
123 in resistant cells. The inhibited Rh 123 efflux in A2780/Taxol
cells treated with free Cur was hardly observed. However, treatment
with Pip could cause apparently accumulation of the fluorescent dye
Rh 123 in the cells (Fig. 6C). It has
been reported that Pip could be used for the design and development
of safe non-toxic P-gp inhibitors, since the structural features of
Pip could bind in the vicinity of the ATP binding site (28). This suggests that Pip could be used
for the development of the next generation of P-gp inhibitors. In
addition, treatment with the surfactants TPGS and Brij 78 could
also cause accumulation of Rh 123 (Fig.
6D and E). Recently, surfactants such as TPGS have been noticed
to modulate efflux pump activity (21,31). These
surfactants are used as excipients in the preparation of
nanoparticles, and are aimed at specifically targeting the
therapeutic drug to tumors and overcoming MDR.
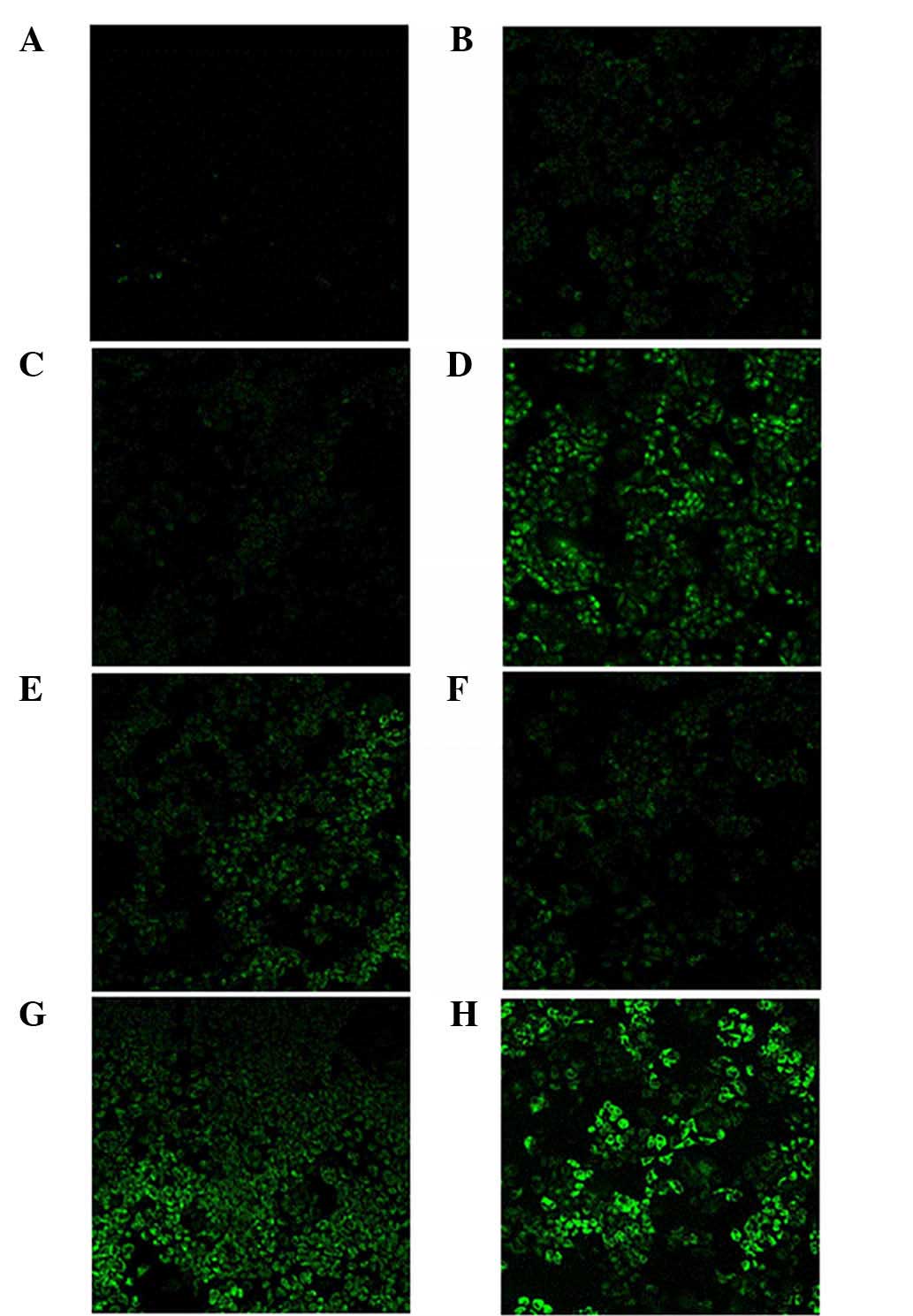 | Figure 6.Confocal microscopic images of
A2780/Taxol cells incubated with (A) medium (control), (B) free Pip
solution, (C) free Cur solution, (D) tocopheryl polyethylene glycol
succinate, (E) Brij 78, (F) blank SLNs, (G) Cur-SLNs and (H)
(Cur+Pip)-SLNs. The green color represents rhodamine 123
fluorescence. Magnification, ×20. Cur, curcumin; Pip, piperine;
SLN, solid lipid nanoparticle. |
In the present study, A2780/Taxol cells treated with
(Cur+Pip)-SLNs exhibited a higher uptake than those treated with
free Cur, free Pip, excipients and control nanoparticles. The
increased accumulation of Rh 123 within A2780/Taxol cells may be a
result of decreased expression of P-gp or inhibited function of
P-gp. In our previous studies (31,32),
western blot analyses were performed to assess the effect of Brij
78 and TPGS on the protein expression levels of P-gp. Upon
pretreatment with Brij 78 or TPGS, the expression level of P-gp
protein was not significantly altered compared with that in control
H460/TaxR cells. These results indicate that inhibiting the
expression of P-gp is not involved in the reversal of P-gp MDR by
Brij 78 or TPGS.
The present study suggests that there are at least
two major reasons for the enhanced uptake of Rh 123 in
P-gp-mediated resistant cells: i) Increased Rh 123 uptake by
endocytosis of SLNs, which helps to partially bypass P-gp; and ii)
decreased efflux of Rh 123 through inhibition of P-gp function
caused by Pip, TPGS or Brij 78.
In conclusion, SLNs with TPGS and Brij 78
co-delivering Cur and Pip were designed and studied to overcome MDR
in A2780/Taxol cells in the present study. (Cur+Pip)-SLNs were
successfully prepared and optimized by the emulsification
evaporation-low temperature solidification method. (Cur+Pip)-SLNs
exhibited high cytotoxicity and allowed efficient intracellular
drug delivery. TPGS and Brij 78 also serve an important role in the
inhibition of P-gp. The combination of Cur and Pip, when
administered in SLNs formulations, resulted in a significant
enhancement in cytotoxicity in drug-resistant A2780/Taxol cells.
This dual inhibitory strategy can have a significant potential in
the clinical management of MDR in cancer. Future in vivo
studies in human tumor xenograft models will further validate this
hypothesis.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (Beijing, China; grant
no. 81302705) and the Returned Overseas Foundation of Heilongjiang
Province of China (Harbin, China; grant no. LC201432).
The authors would like to acknowledge Dr Xiaohan
Tang (Department of Gynecology and Obstetrics, The First Affiliated
Hospital of Harbin Medical University, Harbin, China) for kindly
providing the A2780/Taxol cells.
References
|
1
|
Fodale V, Pierobon M, Liotta L and
Petricoin E: Mechanism of cell adaptation: When and how do cancer
cells develop chemoresistance? Cancer J. 17:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kathawala RJ, Gupta P, Ashby CR Jr and
Chen ZS: The modulation of ABC transporter-mediated multidrug
resistance in cancer: A review of the past decade. Drug Resist
Updat. 18:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shapira A, Livney YD, Broxterman HJ and
Assaraf YG: Nanomedicine for targeted cancer therapy: Towards the
overcoming of drug resistance. Drug Resist Updat. 14:150–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Limtrakul P, Chearwae W, Shukla S,
Phisalphong C and Ambudkar SV: Modulation of function of three ABC
drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance
protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by
tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell
Biochem. 296:85–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chearwae W, Wu CP, Chu HY, Lee TR,
Ambudkar SV and Limtrakul P: Curcuminoids purified from turmeric
powder modulate the function of human multidrug resistance protein
1 (ABCC1). Cancer Chemother Pharmacol. 57:376–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ganta S and Amiji M: Coadministration of
Paclitaxel and curcumin in nanoemulsion formulations to overcome
multidrug resistance in tumor cells. Mol Pharm. 6:928–939. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romiti N, Tongiani R, Cervelli F and
Chieli E: Effects of curcumin on P-glycoprotein in primary cultures
of rat hepatocytes. Life Sci. 62:2349–2358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bisht S, Feldmann G, Soni S, Ravi R,
Karikar C and Maitra A and Maitra A: Polymeric
nanoparticle-encapsulated curcumin (‘nanocurcumin’): A novel
strategy for human cancer therapy. J Nanobiotechnology. 5:32007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maiti K, Mukherjee K, Gantait A, Saha BP
and Mukherjee PK: Curcumin-phospholipid complex: Preparation,
therapeutic evaluation and pharmacokinetic study in rats. Int J
Pharm. 330:155–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tiyaboonchai W, Tungpradit W and
Plianbangchang P: Formulation and characterization of curcuminoids
loaded solid lipid nanoparticles. Int J Pharm. 337:299–306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaikh J, Ankola DD, Beniwal V, Singh D
and Kumar MN: Nanoparticle encapsulation improves oral
bioavailability of curcumin by at least 9-fold when compared to
curcumin administered with piperine as absorption enhancer. Eur J
Pharm Sci. 37:223–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shoba G, Joy D, Joseph T, Majeed M,
Rajendran R and Srinivas PS: Influence of piperine on the
pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. 64:353–356. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhardwaj RK, Glaeser H, Becquemont L,
Klotz U, Gupta SK and Fromm MF: Piperine, a major constituent of
black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol
Exp Ther. 302:645–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Najar IA, Sachin BS, Sharma SC, Satti NK,
Suri KA and Johri RK: Modulation of P-glycoprotein ATPase activity
by some phytoconstituents. Phytother Res. 24:454–458. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Lei Y, Jia Y, Li N, Wink M and Ma Y:
Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes
P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells.
Phytomedicine. 19:83–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabanov AV, Batrakova EV and Alakhov VY:
An essential relationship between ATP depletion and
chemosensitizing activity of Pluronic block copolymers. J Control
Release. 91:75–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batrakova EV, Li S, Brynskikh AM, Sharma
AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE and
Kabanov AV: Effects of pluronic and doxorubicin on drug uptake,
cellular metabolism, apoptosis and tumor inhibition in animal
models of MDR cancers. J Control Release. 143:290–301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Y, Luo J, Tan S, Otieno BO and Zhang
Z: The applications of Vitamin E TPGS in drug delivery. Eur J Pharm
Sci. 49:175–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong X, Mattingly CA, Tseng MT, Cho MJ,
Liu Y, Adams VR and Mumper RJ: Doxorubicin and paclitaxel-loaded
lipid-based nanoparticles overcome multidrug resistance by
inhibiting P-glycoprotein and depleting ATP. Cancer Res.
69:3918–3926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J, Wang Y, Wang D, Wang Y, Xu Z,
Racette K and Liu F: Key structure of brij for overcoming multidrug
resistance in cancer. Biomacromolecules. 14:424–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel BD, Modi RV, Thakkar NA, Patel AA
and Thakkar PH: Development and characterization of solid lipid
nanoparticles for enhancement of oral bioavailability of
Raloxifene. J Pharm Bioallied Sci. 4:(Suppl 1). S14–S16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loveless VS, Surdock CP and Bhattacharjee
H: Evaluation of zeta-potential and particle size of technetium
99mTc-sulfur colloid subsequent to the addition of lidocaine and
sodium bicarbonate. J Nucl Med Technol. 38:49–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yusuf M, Khan RA, Khan M and Ahmed B:
Plausible antioxidant biomechanics and anticonvulsant
pharmacological activity of brain-targeted β-carotene
nanoparticles. Int J Nanomedicine. 7:4311–4321. 2012.PubMed/NCBI
|
|
27
|
Raghunand N, He X, Van Sluis R, Mahoney B,
Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM and
Gillies RJ: Enhancement of chemotherapy by manipulation of tumour
pH. Br J Cancer. 80:1005–1011. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh DV, Godbole MM and Misra K: A
plausible explanation for enhanced bioavailability of P-gp
substrates in presence of piperine: Simulation for next generation
of P-gp inhibitors. J Mol Model. 19:227–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eytan GD, Regev R, Oren G, Hurwitz CD and
Assaraf YG: Efficiency of P-glycoprotein-mediated exclusion of
rhodamine dyes from multidrug-resistant cells is determined by
their passive transmembrane movement rate. Eur J Biochem.
248:104–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang J, Wang Y, Wang D, Xu Z, Racette K
and Liu F: Key structure of brij for overcoming multidrug
resistance in cancer. Biomacromolecules. 14:424–430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang J, Fu Q, Wang Y, Racette K, Wang D
and Liu F: Vitamin E reverses multidrug resistance in vitro and in
vivo. Cancer Lett. 336:149–157. 2013. View Article : Google Scholar : PubMed/NCBI
|