Introduction
Thymic tumors, including thymomas and thymic
carcinomas, are the most common primary neoplasms of the anterior
mediastinum, with one study quoting an incidence of 2.5–3.2 per
million individuals in the Netherlands (1). Thymic tumors should be considered
malignant sue to their tendency for local invasion, pleural
dissemination and systemic metastases. In total, 30–60% of thymic
tumors show various degrees of invasion into mediastinal fat and
adjacent structures (2–5).
Based on the gross and microscopic invasive
properties of the tumors at surgery, the International Thymic
Malignancy Interest Group (ITMIG) has adopted the Masaoka-Koga
staging system as the official staging system for thymic tumors
(6–8).
Usually, a complete resection can be achieved by surgery in
patients at stage I or II. However, for stage III or IV tumors,
particularly where there is locoregional spread into neighboring
mediastinal organs (stage III tumors), neoadjuvant therapy provides
a survival advantage (9–12). With recent advances in minimally
invasive surgical techniques, certain patients with stage III
thymic tumors have become eligible for surgical evaluation, but
others will require neoadjuvant therapy prior to complete resection
(13). In certain cases, patients who
have undergone invasive procedures have ultimately been found to
have an early-stage thymic tumor; while in others, the
pre-operative stage of the tumor has been underestimated, resulting
in surgical difficulties (14).
Therefore, accurate assessment of tumor invasion into intrathoracic
structures is of great value in deciding upon the appropriate
management of stage III thymic tumors, and precise staging is
important prior to making treatment decisions (15).
At present, computed tomography (CT) is the imaging
modality of choice for the evaluation of mediastinal masses.
Although there have been several studies focusing on the
association between the Masaoka-Koga stage, CT findings and World
Health Organization (WHO) histology (16–26), none
of these studies were dedicated to an evaluation of CT features for
predicting pathological staging. The present study was designed to
evaluate the efficacy of CT features in predicting invasion by
stage III thymic tumors and to assess whether the features can
serve as a guide to selecting the appropriate therapeutic
strategies.
Patients and methods
Approval and consent
This retrospective study was approved by the
Research Committees of the Shanghai Chest Hospital, Shanghai Jiao
Tong University (Shanghai, China) and the Shanghai Jiao Tong
University Affiliated Shanghai Sixth People's Hospital (Shanghai,
China), and a waiver was obtained for informed consent. The study
took place between July 2009 and June 2013.
Patient inclusion and exclusion
criteria
Patients with pre-operative thymic tumors that had
been confirmed by surgical resection and histological examination
were enrolled in the present study. All patients were diagnosed
with stage III thymic tumors on the basis of invasion into
neighboring organs (i.e., the mediastinal pleura, lungs,
pericardium or great vessels), as determined by surgical findings
and histology. CT imaging was performed in all patients within the
2 weeks prior to surgery. All patients underwent complete tumor
resection without pre-operative neoadjuvant therapy. Patients with
incomplete data were excluded.
A total of 66 patients (44 males and 22 females)
ranging in age from 18–77 years (median, 56 years) were enrolled.
Of these, 9 patients presented with myasthenia gravis (MG), 34 with
chest pain and a cough, and 7 with dyspnea, while 16 were
asymptomatic.
Diagnostic criteria
The tumor stage was classified according to the
ITMIG definition of details of the Masaoka-Koga staging system
(27). All histopathological
specimens were reviewed by an experienced pathologist according to
the latest WHO classification (11).
The classification included 7 subtypes of thymomas (types A, AB,
B1, B1+B2, B2, B2+B3 and B3) and thymic carcinoma, which were
determined in accordance with the morphology of epithelial cells
and the lymphocyte-to-epithelial cell ratio (28).
Definition of stage III
According to the ITMIG definition of details of the
Masaoka-Koga staging system (27),
macroscopic or microscopic invasions into neighboring organs (i.e.,
the pericardium, great vessels or lungs) and any involvement
(either partial or penetrating) of the mediastinal pleura were
classified as stage III.
CT imaging
The CT examinations were performed using a Philips
Brilliance 64-slice helical CT (Philips Brilliance, Cleveland,
Ohio, USA). In all patients, the CT examinations used 64×0.625-mm
collimation, a slice thickness of 5.00 mm, a slice increment of
5.00 mm, a pitch of 1.08, 120 kVp and 200 mA, and a 512×512 image
matrix through the thorax prior to and after intravenous injection
of a contrast medium (iohexol; 300 mg iodine/ml; 1.5 ml/kg) at a
rate of 3.0 ml/sec. All CT images were captured at mediastinal
(window width, 400–450 HU; window level, 30–50 HU) and lung (window
width, 1,000–1,500 HU; window level, −650 to −500 HU) windows.
Image analysis
The CT features of the thymic tumors were reviewed
independently by two radiologists with 15 and 8 years of
experience, respectively. The radiologists were blinded to each
other, and to the clinical and pathological information. The
radiologist with 15 years of experience reassessed the CT findings
1 month after the initial evaluation. The following anatomical and
invasive characteristics were assessed: i) Tumor size: Long
diameters were measured in the maximal cross-section CT images. ii)
Tumor contours: Smooth, lobulated or irregular. iii) Tumor density:
Homogeneous or heterogeneous. The heterogeneous density included
gross unevenness and a CT value difference of 20 HU following
administration of contrast medium. iv) Calcification: Yes or no. v)
Necrosis/cystic components: Yes or no. A non-enhanced region with
water density was regarded as either cystic or necrotic. vi)
Invasion: The grading according to invasion, as assessed by CT
features, is summarized in Table I.
Any one of the findings listed in Table
I suggested invasion into the organ.
 | Table I.Location and grade of invasion. |
Table I.
Location and grade of invasion.
| Location | Grade I | Grade II | Grade III | Grade IV |
|---|
| Mediastinal
pleura | Irregular interface
with absence of space between the tumor and mediastinal pleura
only | Irregular interface
with absence of space between the tumor and mediastinal pleura,
with mediastinal pleural thickening, but without pleural
effusion | Irregular interface
with absence of space between the tumor and mediastinal pleura,
with pleural effusion, but without mediastinal pleural
thickening | Irregular interface
with absence of space between the tumor and mediastinal pleura,
with mediastinal pleural thickening and pleural effusion |
| Lung | Single lobular tumor
convex to the lung, without adjacent lung abnormalities such as
patchy inflammation or fibrosis of the adjacent lung
parenchyma | Single lobular tumor
convex to the lung with adjacent lung abnormalities | Multilobular tumor
convex to the lung, without adjacent lung abnormalities | Multilobular tumor
convex to the lung with adjacent lung abnormalities |
| Pericardia | Irregular interface
with absence of space between the tumor and pericardium only | Irregular interface
with absence of space between the tumor and pericardium, with
pericardial thickening, but without pericardial effusion | Irregular interface
with an absence of space between the tumor and pericardium, with
pericardial effusion, but without pericardial thickening | Irregular interface
with absence of space between the tumor and pericardium, with
pericardial thickening and pericardial effusion |
| Great blood
vessels | Tumor abutting
<50% of the vessel circumference and absence of space between
the tumor and blood vessel, without oppression, deformation and
occlusion of the vessel | Tumor abutting ≥50%
of the vessel circumference and absence of space between the tumor
and blood vessel, without oppression, deformation and occlusion of
the vessel |
|
Statistical analysis
Data were expressed as the mean ± standard deviation
for continuous variables and numbers with percentages for
categorical variables. A P-value of <0.05 was interpreted as a
statistically significant difference. The consistency of the
assessments by the two radiologists and for the same radiologist
prior to and after 1 month was assessed by a weighted κ
coefficient. The diagnostic efficacy of the CT features in
comparison with pathological findings was evaluated by their
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV) and accuracy. For the statistical analyses,
SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) was
used.
Results
Patients and disease
characteristics
Among the 66 patients, all the thymic tumors were
located in the anterior mediastinum; on the left side in 31
patients, on the right side in 27 patients and on the midline in 8
patients. The mean size was 5.6±1.4 cm, ranging from 2.9–9.9 cm. On
the basis of the WHO classification, thymic carcinomas were present
in 25 patients (25/66, 37.9%), type B3 thymomas in 18 (18/66,
27.3%), type B2+B3 thymomas in 8 (8/66, 12.1%), type B2 thymomas in
10 (10/66, 15.2%), type B1+B2 thymomas in 3 (3/66, 4.5%) and type A
thymomas in 2 (2/66, 3.0%). The pathological results for the 66
patients were as follows: Single organ invasion including pleural
invasion in 5 cases and pericardial invasion in 18 cases; pleural
and lung invasion in 9 cases; pleural and pericardial invasion in 6
cases; pericardial and vessel invasion in 2 cases; pleural, lung
and pericardial invasion in 15 cases; pleural, pericardial and
vessel invasion in 3 cases; and pleural, lung, pericardial and
vessel invasion in 8 cases. Overall, there was pleural invasion in
46 cases (46/66, 69.7%), lung invasion in 32 (32/66, 48.5%),
pericardial invasion in 52 (52/66, 78.8%) and vessel invasion in 13
(13/66, 19.7%).
The κ values ranged from 0.74–0.93, suggesting good
agreement between the two radiologists, and prior to and after 1
month. The weighted κ coefficients for the two radiologists, and
for the same radiologist prior to and after 1 month are shown in
Table II.
 | Table II.Consistency estimates of the
radiologist evaluations. |
Table II.
Consistency estimates of the
radiologist evaluations.
|
|
| κ value |
|---|
|
|
|
|
|---|
| Mediastinal
invasion | Grade | Of the same
radiologist | Of the two
radiologists |
|---|
| Pleural invasion |
|
|
|
|
| I | 0.78 | 0.78 |
|
| II | 0.89 | 0.81 |
|
| III | 0.78 | 0.78 |
|
| IV | 0.79 | 0.79 |
| Lung invasion |
|
|
|
|
| I | 0.89 | 0.89 |
|
| II | 0.92 | 0.82 |
|
| III | 0.93 | 0.82 |
|
| IV | 0.92 | 0.84 |
| Pericardial
invasion |
|
|
|
|
| I | 0.85 | 0.82 |
|
| II | 0.84 | 0.80 |
|
| III | 0.84 | 0.77 |
|
| IV | 0.88 | 0.83 |
| Vessel
invasion |
|
|
|
|
| I | 0.88 | 0.83 |
|
| II | 0.78 | 0.78 |
|
| III | 0.85 | 0.74 |
CT features
On CT, the thymic tumors presented as oval-shaped in
14 patients (21.2%) and as an lobulated or irregular anterior
mediastinal mass in 52 patients (78.8%). A heterogeneous density
was exhibited in 51 patients (77.3%), 40 of whom (78.4%) exhibited
necrotic and/or cystic components, and 19 of whom (37.3%) showed
calcification.
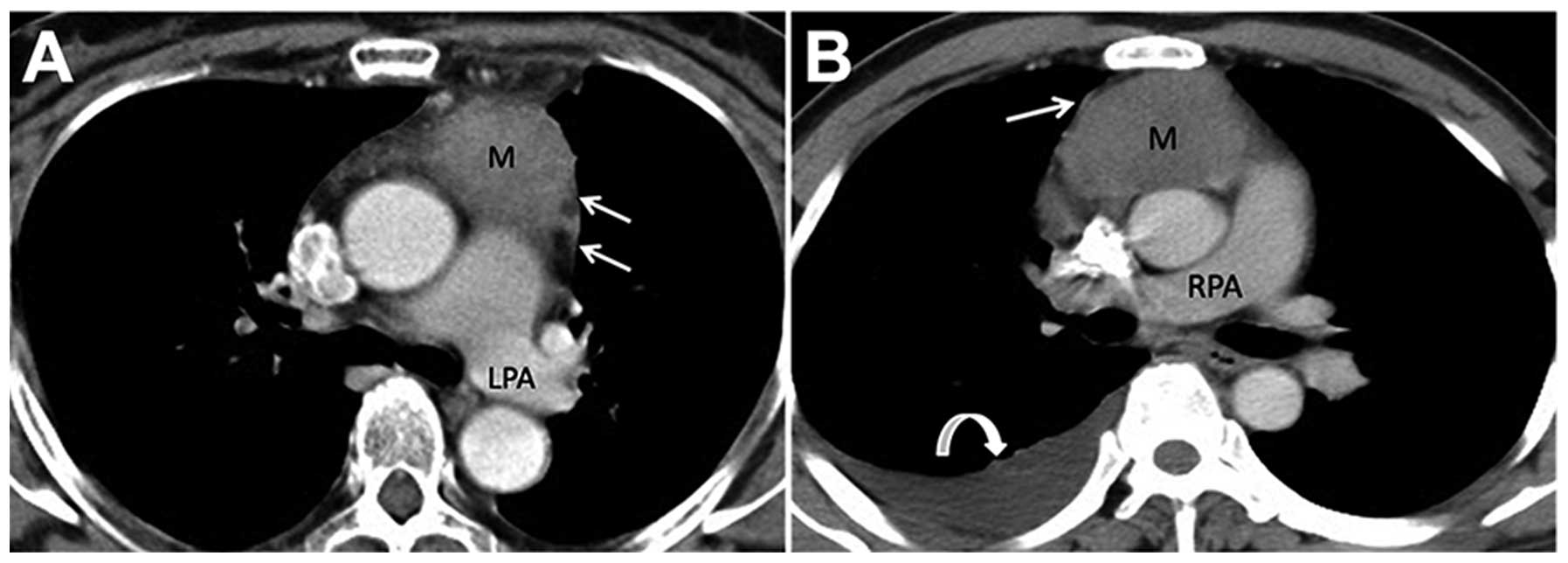
Examples of CT images from patients with mediastinal
pleural invasion are shown in Fig. 1.
The manifestations of the tumor and the mediastinal pleura are
shown in Table II. The majority of
patients (39/66, 59.1%) had an irregular interface with an absence
of space between the tumor and mediastinal pleura.
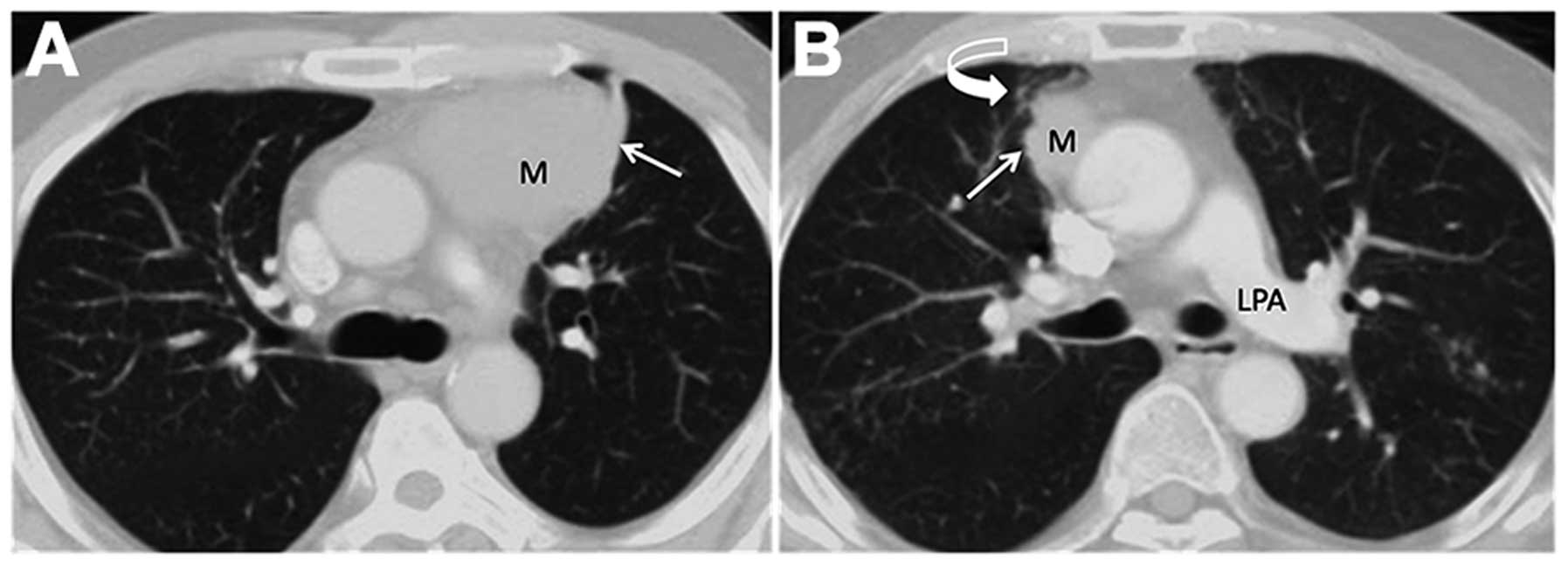
Examples of CT images from patients with lung
invasion are shown in Fig. 2. Thymic
tumors most often (21/66, 31.8%) presented as a single lobular mass
convex to the lung, without adjacent lung abnormalities. There were
20 cases (30.3%) of a multilobular mass convex to the lung, without
adjacent lung abnormalities.
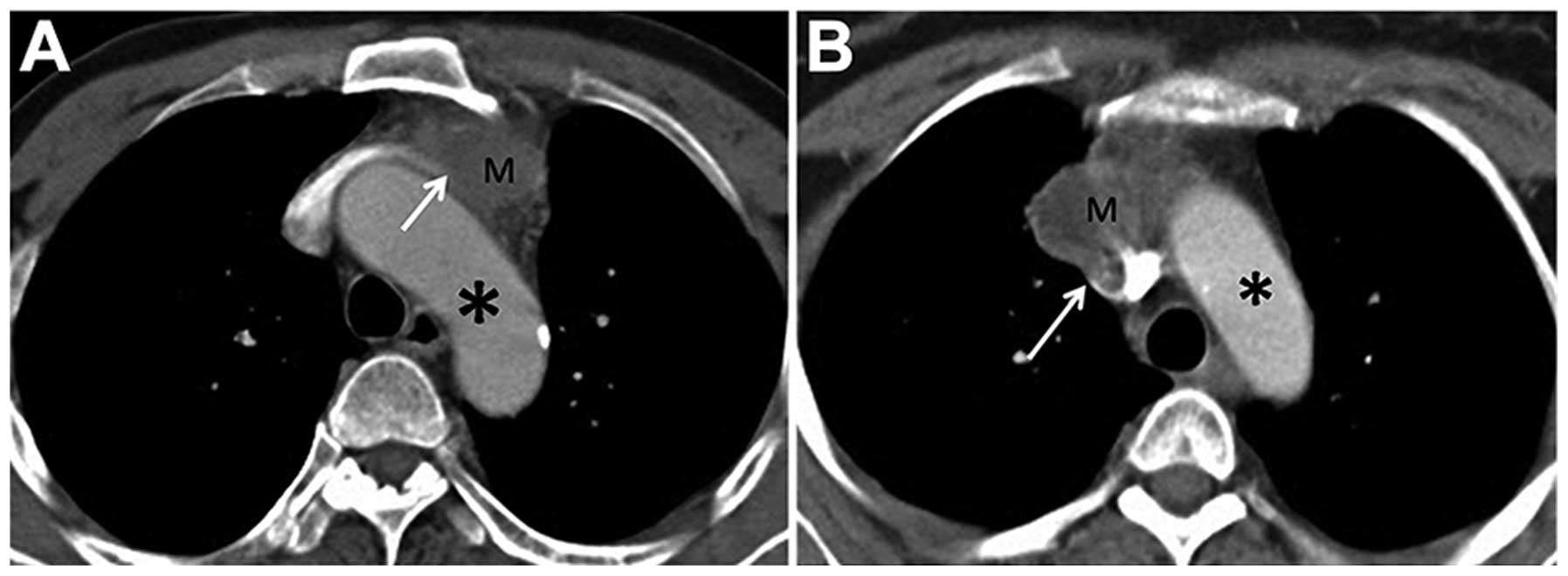
Examples of CT images from patients with pericardial
invasion are shown in Fig. 3. Of the
66 CT images, the most common, in 32 (48.5%) patients, showed an
irregular interface with an absence of space between the tumor and
the pericardium.
Examples of CT images from patients with great
vessel invasion are shown in Fig. 4.
This was observed in 18 of the 66 CT images (27.3%).
Diagnostic efficacy
The diagnostic efficacies of the CT features in
predicting stage III tumors are shown in Table III. Mediastinal pleural invasion was
diagnosed in 65 out of the 66 CT images and was confirmed by the
pathology results in 46 cases. The specificity and PPV were each
100% for grade IV.
 | Table III.Diagnostic efficacy of CT
features. |
Table III.
Diagnostic efficacy of CT
features.
| Site of
invasion | Invasion on CT,
n | Invasion on
pathology, n | Sensitivity,% | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|
| Pleura |
|
|
|
|
|
|
|
| Grade
I | 39 | 26 | 56.5 |
35.0 |
66.7 | 25.9 | 50.0 |
| Grade
II | 19 | 15 | 32.6 |
80.0 |
79.0 | 34.0 | 47.0 |
| Grade
III | 5 | 3 |
6.5 |
90.0 |
60.0 | 29.5 | 31.8 |
| Grade
IV | 2 | 2 |
4.4 | 100.0 | 100.0 | 31.3 | 33.3 |
| Lung |
|
|
|
|
|
|
|
| Grade
I | 21 | 2 |
6.3 |
44.1 |
9.5 | 33.3 | 25.8 |
| Grade
II | 7 | 4 | 12.5 |
91.2 |
57.1 | 52.5 | 50.0 |
| Grade
III | 20 | 13 | 40.0 |
79.4 |
65.0 | 58.7 | 60.6 |
| Grade
IV | 16 | 13 | 40.6 |
91.2 |
81.3 | 62.0 | 66.7 |
| Pericardium |
|
|
|
|
|
|
|
| Grade
I | 32 | 24 | 46.2 |
42.9 |
75.0 | 17.7 | 45.5 |
| Grade
II | 12 | 12 | 23.1 | 100.0 | 100.0 | 25.9 | 39.4 |
| Grade
III | 7 | 6 | 11.5 |
92.9 |
85.7 | 22.0 | 28.8 |
| Grade
IV | 10 | 10 | 19.2 | 100.0 | 100.0 | 25.0 | 36.4 |
| Great vessels |
|
|
|
|
|
|
|
| Grade
I | 10 | 5 | 38.5 |
90.6 |
50.0 | 85.7 | 80.3 |
| Grade
II | 5 | 5 | 38.5 | 100.0 | 100.0 | 86.9 | 87.9 |
| Grade
III | 3 | 3 | 23.1 | 100.0 | 100.0 | 84.1 | 84.9 |
Lung invasion was diagnosed in 64 out of the 66 CT
lung images and was confirmed by the pathology results in 32 cases.
The specificity and PPV were 91.2 and 81.3%, respectively, for
grade IV.
Pericardial invasion was diagnosed in 61 out of the
66 CT images and was confirmed by the pathology results in 52
cases. For grades II and IV, the specificity and PPV were each
100%.
Great vessel invasion was diagnosed in 18 of the 66
CT images and was verified by the pathology results in 13 cases.
For grades II and III, the specificity and PPV were each 100%.
Discussion
The present study has illustrated pictorially the CT
features of stage III thymic tumors. A focus was placed upon the
association between the tumors and adjacent structures, such as the
mediastinal pleura, lung, pericardium and great vessels. These CT
features proved useful for the identification of tumor invasion for
the radiologists and surgeons.
Studies have used CT for differentiating early
(stage I and II) from advanced tumors (stage III and IV), according
to the surgical classification of stages proposed by Masaoka-Koga
(23–25). Preoperative CT features, such as large
tumor size, lobulated tumor and infiltration of fat surrounding the
tumor, suggest a high probability of Masaoka stage III or IV
disease. However, it is challenging to accurately identify the
stage of thymoma by the varying degree of fat obliteration between
tumor and mediastinal structures (23,25).
Proper management decisions for thymic tumors are based largely on
the invasion site and the extent to which the tumors are present on
CT imaging. It is key to determine to what extent the CT features
agree with the pathological results. To the best of our knowledge,
this is the first study that has focused on evaluating the efficacy
of CT features in predicting stage III thymoma invasion. In the
study, the most frequent invasive site was the pericardium,
followed by the pleura, lungs and great vessels. The degree of
invasion varied according to the association between the tumor and
adjacent structures, and identifying true and false phenomena in
the CT images proved to be challenging.
A previous study into the use of pleural invasion
features to judge the tumor stage did not demonstrate the
diagnostic accuracy of CT findings (25). In the present study, the signs of
pleural invasion were subdivided into 4 grades to evaluate the
efficacy of CT features in predicting stage III thymoma invasion.
It was found that images suggesting an irregular interface with an
absence of space between the tumor and pleura was the most invasive
sign, and that this exhibited a higher sensitivity (56.52%) than
the other signs evaluated. However, its PPV was just 66.67%. When
the fat space disappeared and mediastinal pleural thickening or
pleural effusion occurred simultaneously, the diagnostic
specificity was as high as 80.00 and 90.00%, respectively. If all 3
signs occurred at the same time (grade IV), the specificity and PPV
each reached 100%. Thus, as long as the absence of a fat space
occurred with pleural thickening and/or pleural effusion,
mediastinal pleural invasion can be confidently predicted. By
contrast, mediastinal pleural invasion does not occur
pathologically without these signs. In the present study, the
sensitivity, NPV and accuracy of CT features for diagnosing
mediastinal pleural invasion ranged from 4.35–56.52%, from
25.93–34.04% and from 31.82–50.00%, respectively. In the study by
Tomiyama et al (24),
infiltration of the pleura was identified by CT in only 5 out of 24
cases with surgically proven pleural infiltration. Although it was
occasionally difficult to define mediastinal pleural invasion in
the present patients, any attempt to focus more consistently on the
mediastinal pleura represents a step forward (29). It has been reported that the results
obtained using analyses of thinner or thicker sections are not
significantly different (24);
therefore, further research, including magnetic resonance imaging
analysis, is required to improve the diagnostic efficacy.
With the exception of a single lobular mass convex
to the lung without adjacent lung abnormalities, the diagnostic
specificities for the other 3 grades of CT lung invasion features
were 91.18% for grade II, 79.41% for grade III and 91.18% for grade
IV. Therefore, no lung invasion can be predicted in the absence of
these CT characteristics. Since the specificity and PPV were 91.18
and 81.25%, respectively, in grade IV, a multilobular thymic tumor
with adjacent lung abnormalities is the most important sign for
diagnosing lung invasion via CT imaging. Lung invasion occurred
more frequently in multilobular thymic tumors than in single
lobular tumors in the present study. The 64 cases of lung
involvement based on CT were verified pathologically in 32
patients. In a study by Zerhouni et al (30), in which the CT findings of 10 cases of
invasive thymoma were reported, lung involvement was correctly
diagnosed by CT in 6 patients. Tomiyama et al (24) reported that infiltration of the
adjacent lung was identified by CT in only 1 out of 9 cases with
histologically proven pulmonary infiltration. This difference in CT
findings compared with the present study was presumably due to the
different disease stages.
The identification of pericardial invasion is also
an essential step prior to surgery, as it may affect the
therapeutic strategy. In grades II and IV in the present study, all
cases of pericardial invasion identified by CT were confirmed by
the pathology results, and the diagnostic specificity and PPV were
each 100%. As long as there was an absence of a fat space, with
pericardial thickening with or without pericardial effusion on CT
imaging, pericardial invasion could be confirmed pre-operatively.
Since the diagnostic specificity and PPV for grade III were 92.86
and 85.71%, respectively, the CT characteristics of an absence of
space between the thymic tumor and pericardium with pericardial
effusion are useful in predicting pericardial invasion.
It has been reported that thymic carcinomas have a
higher prevalence of great vessel invasion than thymomas (13,17). By
contrast, the present study found that invasive thymomas (61.5%;
8/13) had a higher prevalence of great vessel invasion than thymic
carcinomas (30.8%, 4/13). The exclusion of inoperative cases in the
present study may have contributed to this finding. The diagnostic
specificities and PPVs for CT findings in grades II and III were
100%. The CT manifestations of tumors abutting ≥50% of the vessel
circumference or oppression, deformation and occlusion of the
vessels definitely suggested vessel invasion. Since the specificity
for just an absent fat space (grade I) was 90.57%, it can be
confidently predicted that no vessel invasion is present if there
is space between the tumor and blood vessels. The NPVs and
diagnostic accuracies for the other 2 grades ranged from
84.13–86.89% and from 84.85–87.88%, respectively. Therefore, a lack
of invasion can be confidently diagnosed in the absence of these CT
features. However, the diagnostic sensitivities for all 3 grades
were as low as 23.08–38.46%. Possible reasons for this were patient
selection bias and the incomplete resection of certain invaded
vessels, which would have resulted in negative pathology
findings.
The present study has certain limitations that
should be considered alongside the results. The study was
retrospective; therefore, there was no way of comparing the
predictive value of CT features with any other method of evaluating
stage III thymic tumors prior to treatment. Also, the study sample
size was quite small due to the limited number of patients;
multi-center studies including more patients would provide more
evidence for these results, as well as providing evidence of the
reproducibility of the CT grading of the thymic tumor features.
In summary, in the first study to focus on
evaluating the efficacy of CT features in predicting stage III
thymoma, CT features were found to be effective. Consequently,
familiarity with CT features for predicting stage III thymic tumors
facilitates diagnostic accuracy, and thus may be useful in
optimizing patient treatment.
Acknowledgements
The authors are grateful to Dr Li Zhu, Dr Qun-Hui
Chen, Dr Zhi-Chun Zheng, Dr Yi-Feng Jiang (radiologists at the
Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai,
China) and Dr Zhi-Tao Gu (thoracic surgeon at the Shanghai Chest
Hospital) for collecting the majority of the imaging data. The
authors would like to thank Dr Lei Zhu (pathologist at the Shanghai
Chest Hospital) for performing the pathological examinations. This
study was partially sponsored by the National Natural Science
Foundation of China (grant no. 81271609) and the Shanghai Municipal
Commission of Health and Family Planning Scientific Research Task
(grant no. 201440442).
References
|
1
|
de Jong WK, Blaauwgeers JL, Schaapveld M,
Timens W, Klinkenberg TJ and Groen HJ: Thymic epithelial tumours: A
population-based study of the incidence, diagnostic procedures and
therapy. Eur J Cancer. 44:123–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batata MA, Martini N, Huvos AG, Aguilar RI
and Beattie EJ Jr: Thymomas: Clinicopathologic features, therapy,
and prognosis. Cancer. 34:389–396. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofmann W, Möller P, Manke HG and Otto HF:
Thymoma. A clinicopathologic study of 98 cases with special
reference to three unusual cases. Pathol Res Pract. 179:337–353.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis JE, Wick MR, Scheithauer BW, Bernatz
PE and Taylor WF: Thymoma. A clinicopathologic review. Cancer.
60:2727–2743. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salyer WR and Eggleston JC: Thymoma: A
clinical and pathological study of 65 cases. Cancer. 37:229–249.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koga K, Matsuno Y, Noguchi M, Mukai K,
Asamura H, Goya T and Shimosato Y: A review of 79 thymomas:
Modification of staging system and reappraisal of conventional
division into invasive and non-invasive thymoma. Pathol Int.
44:359–367. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falkson CB, Bezjak A, Darling G, Gregg R,
Malthaner R, Maziak DE, Yu E, Smith CA, McNair S, Ung YC, et al:
The management of thymoma: A systematic review and practice
guideline. J Thorac Oncol. 4:911–919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Detterbeck FC, Wang Z and Loehrer
PJ Sr: Standard outcome measures for thymic malignancies. J Thorac
Oncol. 5:2017–2023. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim ES, Putnam JB, Komaki R, Walsh GL, Ro
JY, Shin HJ, Truong M, Moon H, Swisher SG, Fossella FV, et al:
Phase II study of a multidisciplinary approach with induction
chemotherapy, followed by surgical resection, radiation therapy,
and consolidation chemotherapy for unresectable malignant thymomas:
Final report. Lung Cancer. 44:369–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rea F, Sartori F, Loy M, Calabrò F,
Fornasiero A, Daniele O and Altavilla G: Chemotherapy and operation
for invasive thymoma. J Thorac Cardiovasc Surg. 106:543–549.
1993.PubMed/NCBI
|
|
11
|
Ströbel P, Marx A, Zettl A and
Müller-Hermelink HK: Thymoma and thymic carcinoma: An update of the
WHO classification 2004. Surg Today. 35:805–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venuta F, Rendina EA, Pescarmona EO, De
Giacomo T, Vegna ML, Fazi P, Flaishman I, Guarino E and Ricci C:
Multimodality treatment of thymoma: A prospective study. Ann Thorac
Surg. 64:1585–1592. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu ZT, Mao T, Chen WH and Fang W:
Comparison of video-assisted thoracoscopic surgery and median
sternotomy approaches for thymic tumor resections at a single
institution. Surg Laparosc Endosc Percutan Tech. 25:47–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toker A, Sonett J, Zielinski M, Rea F,
Tomulescu V and Detterbeck FC: Standard terms, definitions, and
policies for manimally invasive resection of thymoma. J Thorac
Oncol. 6:(7 Suppl 3). S1739–S1742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marulli G, Lucchi M, Margaritora S,
Cardillo G, Mussi A, Cusumano G, Carleo F and Rea F: Surgical
treatment of stage III thymic tumors: A multi-institutional review
from four Italian centers. Eur J Cardiothorac Surg. 39:e1–e7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong YJ, Lee KS, Kim J, Shim YM, Han J
and Kwon OJ: Does CT of thymic epithelial tumors enable us to
differentiate histologic subtypes and predict prognosis? AJR Am J
Roentgenol. 183:283–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JL, Weisbrod GL and Herman SJ:
Computed tomography and pathologic correlations of thymic lesions.
J Thorac Imaging. 3:61–65. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fon GT, Bein ME, Mancuso AA, Keesey JC,
Lupetin AR and Wong WS: Computed tomography of the anterior
mediastinum in myasthenia gravis. A radiologic-pathologic
correlative study. Radiology. 142:135–141. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomiyama N, Johkoh T, Mihara N, Honda O,
Kozuka T, Koyama M, Hamada S, Okumura M, Ohta M, Eimoto T, et al:
Using the world health organization classification of thymic
epithelial neoplasms to describe CT findings. AJR Am J Roentgenol.
179:881–886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadohara J, Fujimoto K, Müller NL, Kato S,
Takamori S, Ohkuma K, Terasaki H and Hayabuchi N: Thymic epithelial
tumors: Comparison of CT and MR imaging findings of low-risk
thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol.
60:70–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yakushiji S, Tateishi U, Nagai S, Matsuno
Y, Nakagawa K, Asamura H and Kusumoto M: Computed tomographic
findings and prognosis in thymic epithelial tumor patients. J
Comput Assist Tomogr. 32:799–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marom EM, Milito MA, Moran CA, Liu P,
Correa AM, Kim ES, Komaki R, Erasmus JJ, Hofstetter WL, Rice DC and
Swisher SG: Computed tomography findings predicting invasiveness of
thymoma. J Thorac Oncol. 6:1274–1281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Priola AM, Priola SM, Di Franco M, Cataldi
A, Durando S and Fava C: Computed tomography and thymoma:
Distinctive findings in invasive and noninvasive thymoma and
predictive features of recurrence. Radiol Med. 115:1–21. 2010.(In
Italian). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomiyama N, Müller NL, Ellis SJ, Cleverley
JR, Okumura M, Miyoshi S, Kusumoto M, Johkoh T, Yoshida S, Mihara
N, et al: Invasive and noninvasive thymoma: Distinctive CT
features. J Comput Assist Tomogr. 25:388–393. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qu YJ, Liu GB, Shi HS, Liao MY, Yang GF
and Tian ZX: Preoperative CT findings of thymoma are correlated
with postoperative Masaoka clinical stage. Acad Radiol. 20:66–72.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruffini E, Filosso PL, Mossetti C, Bruna
MC, Novero D, Lista P, Casadio C and Oliaro A: Thymoma:
Inter-relationships among world health organization histology,
Masaoka staging and myasthenia gravis and their independent
prognostic significance: A single-centre experience. Eur J
Cardiothorac Surg. 40:146–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Detterbeck FC, Nicholson AG, Kondo K, Van
Schil P and Moran C: The Masaoka-Koga stage classification for
thymic malignancies: Clarification and definition of terms. J
Thorac Oncol. 6:(7 Suppl 3). S1710–S1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tagawa T, Kometani T, Yamazaki K, Okamoto
T, Wataya H, Seto T, Fukuyama S, Osoegawa A, Hirai F, Sugio K and
Ichinose Y: Prognosis and therapeutic response according to the
World Health Organization histological classification in advanced
thymoma. Surg Today. 41:1599–1604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Detterbeck FC, Moran C, Huang J, Suster S,
Walsh G, Kaiser L and Wick M: Which way is up? Policies and
procedures for surgeons and pathologists regarding resection
specimens of thymic malignancy. J Thorac Oncol. 6:(7 Suppl 3).
S1730–S1738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zerhouni EA, Scott WW Jr, Baker RR, Wharam
MD and Siegelman SS: Invasive thymomas: Diagnosis and evaluation by
computed tomography. J Comput Assist Tomogr. 6:92–100. 1982.
View Article : Google Scholar : PubMed/NCBI
|