Introduction
Platinum-based drugs, one of the most effective
types of anti-cancer treatment, are widely used in the treatment of
almost all types of solid tumors (1).
Cisplatin (DDP) treatment frequently leads to the acquisition of
chemoresistance (1). Numerous
mechanisms underlying this chemoresistance have been identified;
studies have documented that DDP may trigger the activation of
Jun-N-terminal kinase and p38 mitogen-activated protein kinase
cascades in tumor cells or transformed cell lines (2–4). Protein
kinase B (PKB/AKT) is a cytoplasmic serine/threonine kinase that
positively regulates metabolism, cell cycle progression and cell
survival (5,6). It has been reported that DDP activates
PKB/AKT in several cancer cell lines (4). Inhibition of phosphoinositide 3-kinase
(PI3K) activity decreases the survival of cells exposed to DDP,
suggesting that cisplatin-induced PKB/Akt activation may lead to
cisplatin resistance (4). Activation
of ERK1/2 and AKT has also been reported to be associated with
cisplatin resistance in human lung cancer cells (7). Improvement of our understanding of the
cellular responses to cisplatin remains important to optimize its
use in the clinic.
Period2 (Per2) is a circadian gene (8,9). Mounting
evidence suggests that deregulation of the circadian clock has a
significant role in the development of mammalian cancer (8–11). Studies
have revealed that the expression of Per2 in non-small cell lung
cancer (NSCLC) is decreased (8,9). Negative
expression of Per2 may contribute to development and invasion in
NSCLC (8). To the best of our
knowledge, whether Per2 deregulation is associated with cisplatin
resistance in lung cancer cells remains to be elucidated.
To investigate the role of Per2 in cisplatin
resistance in lung cancer cells, the present study used A549/DDP
cells as a working system. Initially, the expression of Per2 in
A549 and A549/DDP cells was determined. Subsequently, the function
of Per2 in A549/DDP cells was investigated by gene silencing
approaches. In addition, western blot analyses were performed to
identify signaling pathways that mediate the effects of Per2.
Overall, the present study shows that Per2 is downregulated in
A549/DDP cells, and it regulates the biological function of
A549/DDP cells via the AKT/mechanistic target of rapamycin (mTOR)
signaling pathway.
Materials and methods
Antibodies
Anti-pS473AKT (cat. no. 4060; 1:1,000),
anti-pS2448mTOR (cat. no. 2971; 1:2,000), anti-mTOR (cat. no. 2972;
1:2,000) and anti-α-actin (cat. no. 4970; 1:5,000) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA)
and the anti-AKT antibody (cat. no. sc-135829; 1:1,000) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit anti-active caspase-3 (cat. no. ab13585; 1:3,000) and
anti-Per2 (cat. no. ab179813; 1:1,000) antibodies were purchased
from Abcam (Cambridge, UK). The horseradish peroxidase
(HRP)-conjugated secondary antibody (cat. no. ab6721; 1:5,000) was
also purchased from Abcam.
Cell lines
The human lung adenocarcinoma cell line A549 (Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China). was cultured in Dulbecco's modified Eagle's medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA). A549/DDP
cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences). The medium was supplemented with 10% fetal bovine
serum (HyClone; GE Healthcare Life Sciences), and cells were
maintained at 37°C in a humidified atmosphere of 5% CO2.
The cells were subcultured when they reached ~90% confluence using
a 0.25% trypsin solution.
Per2 overexpression
Human Per2 was amplified according to the published
sequence (accession no. AB012614; https://www.ncbi.nlm.nih.gov/nuccore/AB012614) using
specific primers, and cloned into pcDNA 3.1 (Invitrogen; Thermo
Fisher Scientific, Inc.). Following confirmation by sequencing, the
construct was transfected into A549/DDP cells using Lipofectamine™
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions.
Knockdown of Per2 by short hairpin
(sh)RNA
The pSuper vectors (OligoEngine, Seattle, WA, USA)
were used to transcribe functional shRNA. In the vectors,
oligonucleotides targeting Per2 were inserted downstream of the H1
promoter, with their veracity confirmed by double digestion and
sequencing. The target sequences were as follows: shRNA1,
GCGTTACCTCTGAGCACATTG; shRNA2, GCATGGAGGAGAAATCTTTCT; shRNA3:
GGAGTTAGAGATGGTGGAAGA; shRNA4, GCTGCCTTCCCGAAATTTAGA. Transient
transfection of constructs in A549 cells was performed using
Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Transcripts were quantified by RT-qPCR. Briefly,
total RNA was extracted from the cells and reverse transcribed into
cDNA using the First Strand cDNA Synthesis kit (CWBIO, Beijing,
China). Subsequently, qPCR was performed using the UltraSYBR
Mixture (CWBIO) on a thermocycler with the following cycling
conditions: 36 cycles of denaturation at 95°C for 50 sec, annealing
at a temperature dependent on the melting temperature of each
primer set for 30 sec and an extension step at 72°C for 30 sec.
Primer sequences used were as follows: Per2 forward,
5′-GAGACCCAGTCCTGTTTGGT-3′ and reverse, 5′-ATACAGATGCAGTCGCAAGC-3′.
Primers specific for GAPDH were used as a control (forward,
5′-CATCTTCTTTTGCGTCGCCA-3′ and reverse,
5′-TTAAAAGCAGCCCTGGTGACC-3′). The relative expression levels of
Per2 mRNA were calculated using the 2−ΔΔCq method and
normalized to GAPDH (10).
Protein extraction and western
blotting
Whole-cell lysate was extracted with
radioimmunoprecipitation assay buffer in the presence of complete
protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN,
USA). Protein quantification was performed using the performed
using Pierce BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). Protein levels were measured by western blotting. Briefly,
equal amounts of protein (25 µg) were separated by 10% SDS-PAGE,
and transferred to polyvinylidene membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were incubated in blocking
buffer (0.2 mM Tris, 137 mM NaCl, 5% no-fat milk and 0.1% Tween-20)
for 1 h at room temperature and then probed at 4°C overnight with
the primary antibodies against pS473AKT, pS2448mTOR, mTOR, AKT,
caspase-3, Per2 and α-actin. The membranes were rinsed with washing
buffer (0.1% Tween 20, 0.2 mM Tris and 137 mM NaCl) and incubated
with HRP-conjugated secondary antibody (1:5,000) for 1 h at room
temperature, followed by chemiluminescence detection (Luminata
Western HRP Substrate; EMD Millipore, Billerica, MA, USA). The
defined sections of the film were scanned for density measurement
and analyzed using ImageJ v2.1.4.7 software (National Institutes of
Health, Bethesda, MD, USA).
Wound healing assay
A total of 3×105 A549/DDP cells were
plated in a 6-well plate. When cell confluence was <80% at 48 h
subsequent to transfection, wounds were created in confluent cells
using a 200-ml pipette tip. The cells were subsequently rinsed with
medium to remove free-floating cells and debris. Medium was
subsequently added, and culture plates were incubated at 37°C for
48 h. Wound healing within the scrape line was observed at 24 h,
and representative scrape lines for each group were photographed.
Duplicate wells for each condition were examined for each
experiment, and each experiment was repeated 3 times. The sizes of
the wounds were measured using ImageJ v2.1.4.7 software.
MTT and clonogenic assay
Cell proliferation was assessed using conversion of
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
(Sigma-Aldrich; EMD Millipore) to formazan product at 24-h
intervals. For clonogenic assay, A549/DDP cells transfected with
shPer2 vector were plated at equal density (1,000/well) in 35-mm
culture dishes for 14 days. The cells were rinsed with PBS prior to
staining with 0.25% crystal violet/20% ethanol for 5 min and the
number of colonies was counted manually.
Statistical analysis
Experimental data are presented as the mean ±
standard error. All statistical analysis was performed with use of
SPSS version 13.5 (SPPS, Inc., Chicago, IL, USA). Student's t-test
and one-way analysis of variance, followed by Tukey's post hoc
analysis, was applied. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Per2 is downregulated in
A549/DDP cells compared with A549 cells
Western blotting was performed to determine the
expression of Per2 in A549 and A549/DDP cells. As shown in Fig. 1A, DDP treatment caused an increase in
Per2 protein expression, while the A549/DDP cells demonstrated
marked downregulation of Per2 compared with A549 cells. To
determine whether downregulation of Per2 expression was a result of
reduced transcription, Per2 mRNA was also analyzed by RT-qPCR.
Downregulated levels of Per2 mRNA were also detected (Fig. 1B). These results indicated that
expression of Per2 was downregulated in A549/DDP cells compared
with A549 cells.
Per2 knockdown in A549/DDP cells by
shRNA protects from apoptosis, but promotes proliferation and
migration
To investigate the potential role of Per2 in lung
cancer, the present study examined various biological functions of
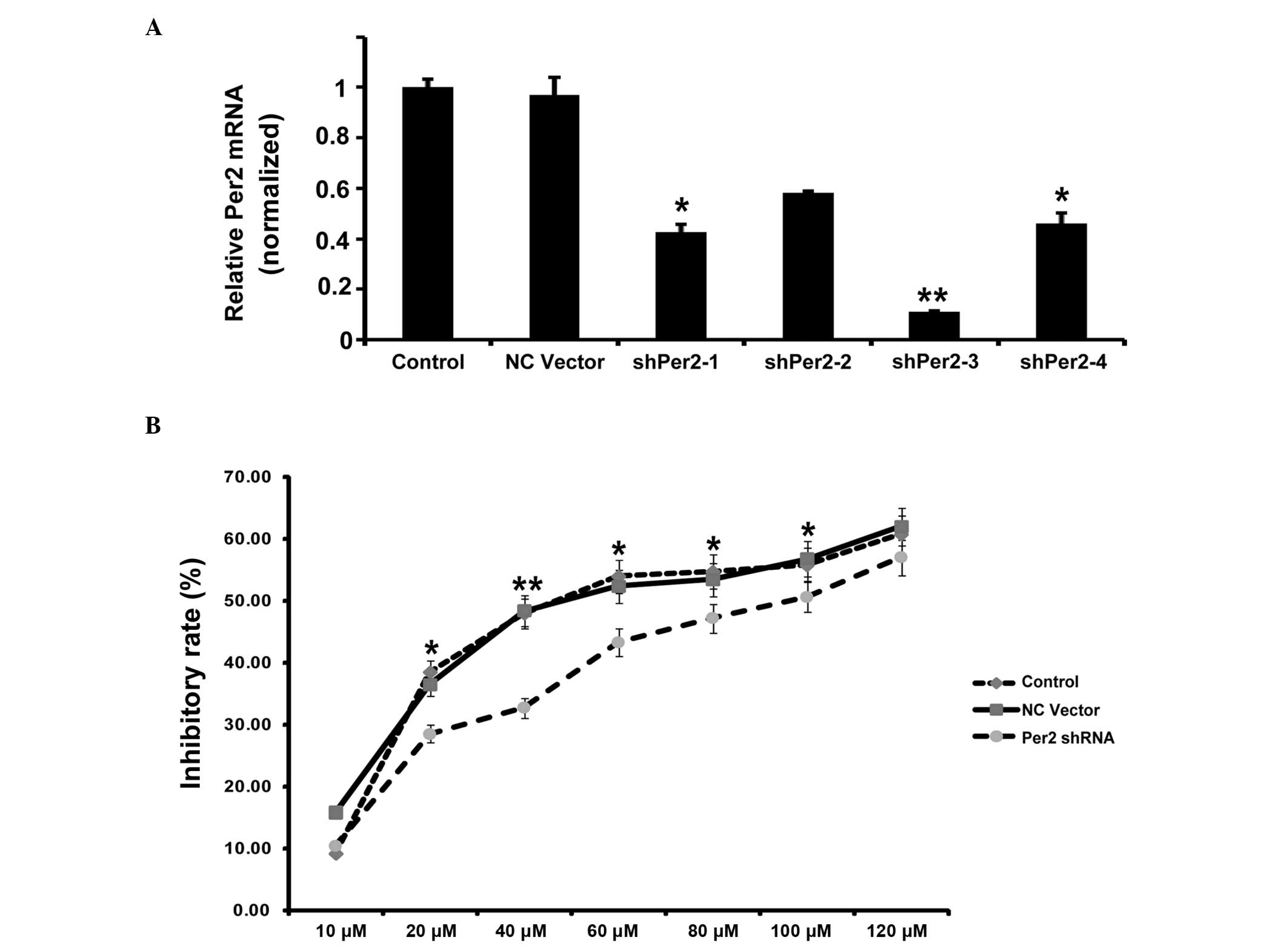
A549/DDP cells upon Per2 knockdown by shRNA. Effective inhibition
of Per2 was demonstrated by semiquantitative RT-PCR (Fig. 2A). RT-qPCR indicated that the shRNA1
and shRNA3 reduced Per2 mRNA levels to 42 and 11%, respectively,
compared with the controls (mean level of the nonspecific RNA
control). Subsequently, the present study used shRNA3 for
additional examination. In order to observe the impact of Per2 on
the proliferation of A549/DDP cells, the inhibition rates of
A549/DDP cells treated with various concentrations of DDP at 24 h
were calculated. As shown in Fig. 2B,
the growth inhibition rate increased along with increasing
concentration of DDP. Per2 knockdown decreases the inhibition rate
of A549/DDP cells compared with controls, independent of the
concentration of DDP (Fig. 2B). The
IC50 values of DDP were 58.1 µmol/l for the nonspecific
RNA control group, and 88.0 µmol/l for the sh-Per2 transfection
group. In clonogenic assays, cells transfected with shPer2-RNA
construct or NC vector construct, together with an untransfected
control, were respectively plated out at low density. Following 2
weeks of culture, the number of colonies that attached to the
substrate reflected the survival efficiency at low cell density.
The shPer2-RNA transfectants showed increased colony formation
ability by 136% compared with the vector control (Fig. 3A and B). Subsequently, the present
study detected the apoptosis associated protein active-caspase-3 by
western blotting. Per2 shRNA decreased the expression of
active-caspase-3 in A549/DDP cells induced by serum-starvation for
48 h (Fig. 3C). These results
indicate that Per2 knockdown promotes cell proliferation and
survival.
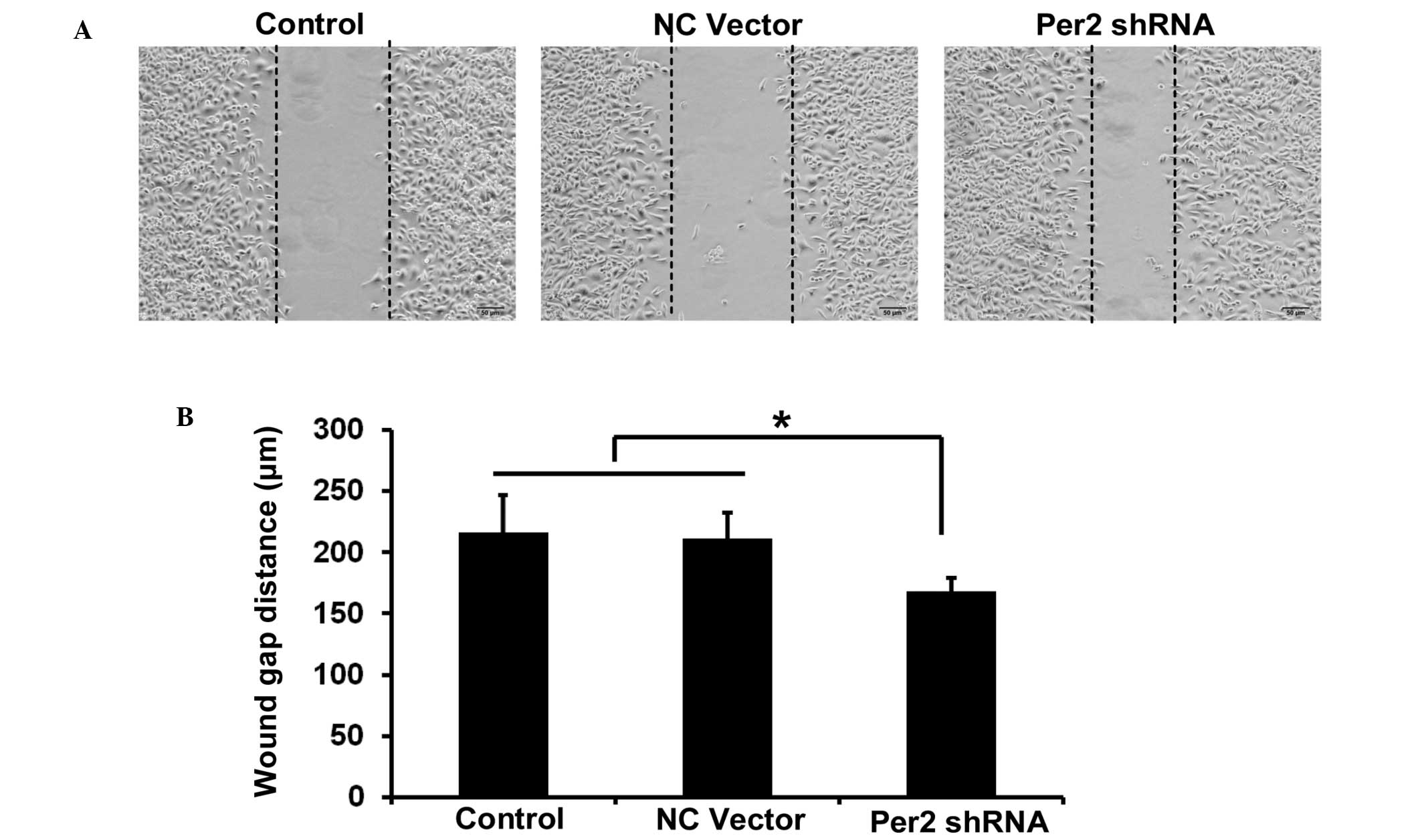
The migration ability of A549/DDP cells was
investigated by wound healing assay. The wound healing assay
results revealed that the distance of migration in A549/DDP cells
was significantly increased following transfection with shPer2
(Fig. 4A and B).
Per2 knockdown increases the activity
of the PI3K/AKT/mTOR signaling pathway, and overexpression of Per2
may reduce the activity and promote apoptosis of A549 cells
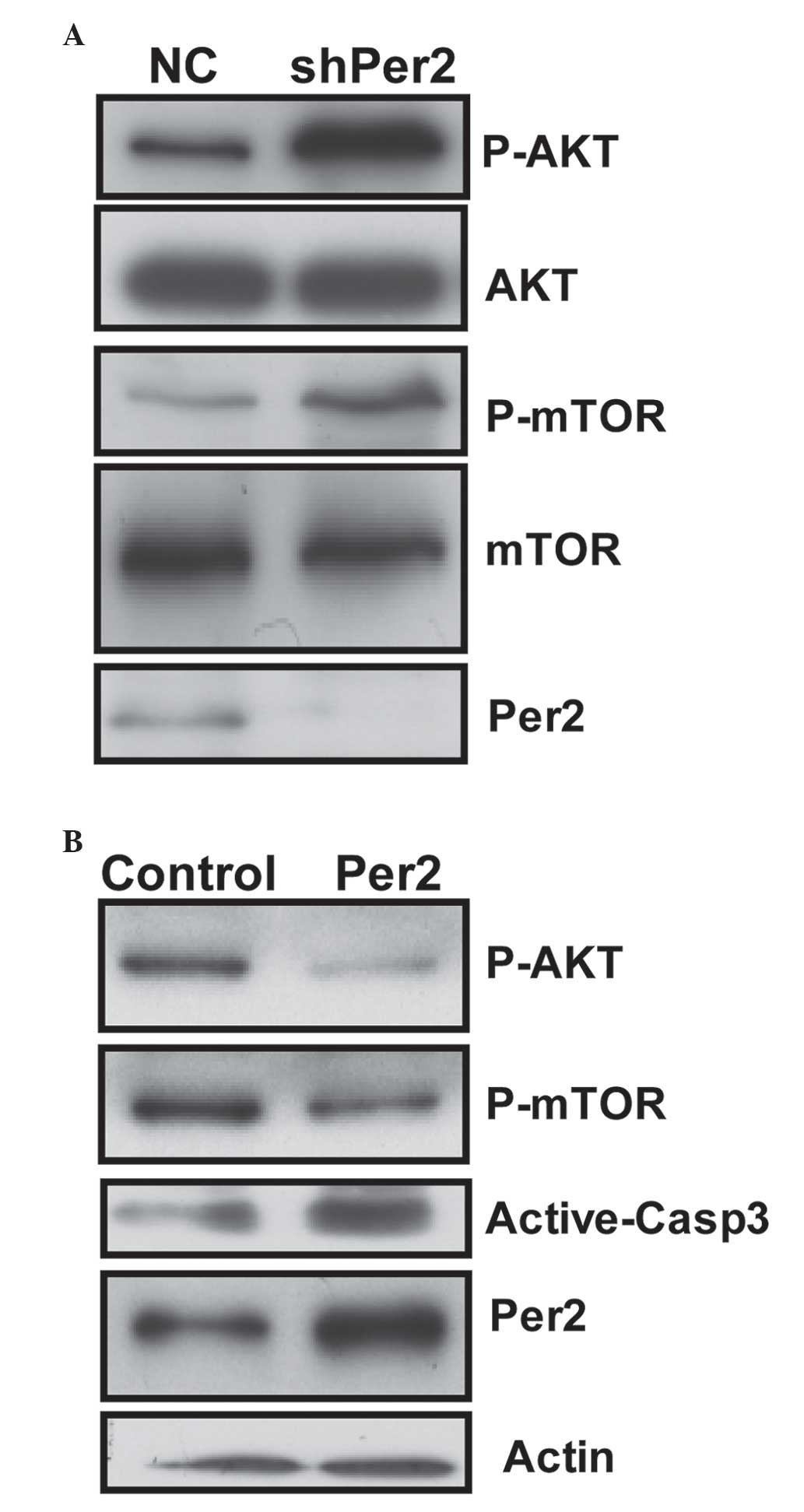
The present study investigated whether
PI3K/AKT/mTOR, which is important in regulating cell proliferation
and apoptosis, is involved in Per2-mediated cell death in A549/DDP
cells. Per2 knockdown by shRNA was observed to cause a significant
increase in the phosphorylation (Ser473) of AKT protein and (S2448)
mTOR in cells (Fig. 5A). However,
shRNA transfection did not cause any change in the protein levels
of total AKT. This transfection resulted in increased levels of the
phosphorylated (activated) form of mTOR (Ser2448), a downstream
target of PI3K/AKT, which may promote cell growth. The results of
the present study revealed a potent effect of Per2 on AKT/mTOR
signaling.
To additionally determine whether the expression
level of Per2 is involved in AKT/mTOR signaling in A549/DDP cells,
expression plasmids for Per2 were constructed. The constructs were
transfected into A549 cells for 48 h. Ectopic expression of Per2
protein resulted in a dramatic reduction in the active form of AKT
and mTOR. Furthermore, ectopic expression of Per2 increased the
quantity of active-caspase-3 protein upon serum starvation stimuli
(Fig. 5B). These results confirm the
effect of Per2 on AKT/mTOR signaling, and indicate Per2 is
functional in apoptosis regulation of A549/DDP cells.
Discussion
Lung cancer remains the leading cause of death among
malignant tumors worldwide. The incidence of NSCLC, the most common
form of lung cancer, is increasing (12). Due to insensitivity to cytotoxic
agents, identifying molecules that drive lung cancer growth,
survival and metastasis is critical.
Deregulation of the circadian clock has been
implicated in numerous types of cancer (8–11,13). The circadian clock proteins Per1 and
Per2 function in a series of cellular processes that coordinate the
circadian clock in the brain and peripheral tissues (13). Mice that are deficient in the Per2
gene are more cancer-prone and sensitive to γ-irradiation (13). Overexpression of Per2 induces
apoptosis and alters the expression levels of apoptosis-associated
genes in tumor cells (13).
Furthermore, loss of Per2 under hypoxia upregulates organic cation
transporter 1-mediated epithelial-to-mesenchymal (EMT) gene
expression and enhances tumor malignancy in breast cancer (14). In addition, cells with downregulated
Per2 expression have prolonged high levels of AKT T308
phosphorylation following growth factor stimulation or DNA damage
(14). However, whether Per2 has a
role in lung cancer cells remains to be elucidated. In the present
study, it is reported that Per2 participates in AKT-mediated drug
resistance in A549/DDP lung adenocarcinoma cells. It was observed
that the Per2 expression level is decreased in A549/DDP cells
compared with A549 cells. Per2 knockdown by shRNA protects A549/DDP
cells from apoptosis, while promoting its proliferation and
migration. Finally, it was observed that Per2 knockdown results in
increased activation of the PI3K/AKT/mTOR signaling pathway. The
results of the present study provide two insights into the
mechanism of Per2-dependent signaling regulation in A549/DDP
cells.
Initially, it was observed that the DDP-resistant
A549 cells demonstrated altered Per2 expression levels. Per2
expression was demonstrated to be significantly reduced in breast
cancer and breast cancer stem cells (15,16). It
has also been indicated that the expression of Per2 in NSCLC is
decreased (8). The results of the
present study demonstrated that Per2 expression was significantly
reduced in A549/DDP cells compared with A549 cells.
Furthermore, it was observed that Per2 knockdown
affected the biological function of A549/DDP cells, potentially via
the PI3K-AKT-mTOR signaling pathway. Per2 is thought to be a tumor
suppressor, and is involved in numerous cancer cell functions,
including EMT, invasion and apoptosis (8–10,13,15). The
results of the present study demonstrated that Per2 knockdown by
shRNA protects A549/DDP cells from apoptosis, and promotes
proliferation and migration. Overexpression of Per2 was able to
promote apoptosis of A549/DDP cells. Furthermore, it was observed
that Per2 knockdown by shRNA caused a significant increase in the
phosphorylation of AKT protein and mTOR in cells. By contrast,
overexpression of Per2 could reduce the activity of PI3K-AKT-mTOR
signaling. The results of the present study suggest that the
activity of the PI3K/AKT/mTOR signaling pathway may have roles in
Per2 functioning in A549/DDP cells.
In conclusion, using A549/DDP cells as a working
system, the present study investigated the role of Per2 in DDP
resistance in lung cancer cells. The present study demonstrates
that Per2 is downregulated in A549/DDP cells, and that it regulates
the biological function of A549/DDP cells via the AKT signaling
pathway.
Acknowledgements
The present study was funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (Jiangsu, China) and was supported by grants from the
National Natural Science Foundation of China (Beijing, China; grant
nos., 81572259, 81272602 and 81302011). The abstract was presented
at the 2015 Chinese Congress on Gerontology and Health Industry.
September 11 - September 13, 2015, Suzhou, Jiangsu, China.
References
|
1
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deschesnes RG, Huot J, Valerie K and
Landry J: Involvement of p38 in apoptosis-associated membrane
blebbing and nuclear condensation. Mol Biol Cell. 12:1569–1582.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey P, Raingeaud J, Kaneki M,
Weichselbaum R, Davis RJ, Kufe D and Kharbanda S: Activation of p38
mitogen-activated protein kinase by c-Abl-dependent and
-independent mechanisms. J Biol Chem. 271:23775–23779. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winograd-Katz SE and Levitzki A: Cisplatin
induces PKB/Akt activation and p38(MAPK) phosphorylation of the EGF
receptor. Oncogene. 25:7381–7390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Liu ZM, Li XC, Yao YT and Yin ZX:
Activation of ERK1/2 and Akt is associated with cisplatin
resistance in human lung cancer cells. J Chemother. 25:162–169.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chi C, He ZF, Liu Y, Lin XM and Sun CC:
Expression and clinical significance of circadian gene Per2 in
non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 35:129–131.
2013.(In Chinese). PubMed/NCBI
|
|
9
|
Liu B, Xu K, Jiang Y and Li X: Aberrant
expression of Per1, Per2 and Per3 and their prognostic relevance in
non-small cell lung cancer. Int J Clin Exp Pathol. 7:7863–7871.
2014.PubMed/NCBI
|
|
10
|
Okabe T, Kumagai M, Nakajima Y, Shirotake
S, Kodaira K, Oyama M, Ueno M and Ikeda M: The impact of HIF1α on
the Per2 circadian rhythm in renal cancer cell lines. PLoS One.
9:e1096932014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Zeng ZL, Yang J, Jin Y, Qiu MZ, Hu
XY, Han J, Liu KY, Liao JW, Xu RH and Zou QF: Prognostic relevance
of Period1 (Per1) and Period2 (Per2) expression in human gastric
cancer. Int J Clin Exp Pathol. 7:619–630. 2014.PubMed/NCBI
|
|
12
|
Esposito L, Conti D, Ailavajhala R, Khalil
N and Giordano A: Lung Cancer: Are we up to the Challenge? Curr
Genomics. 11:513–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu L and Lee CC: The circadian clock:
Pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, He X, Yang Z and Jabbari E:
Mammalian PER2 regulates AKT activation and DNA damage response.
Biochem Cell Biol. 90:675–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ
and Chang JG: Deregulated expression of the PER1, PER2 and PER3
genes in breast cancers. Carcinogenesis. 26:1241–1246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sjöblom T, Jones S, Wood LD, Parsons DW,
Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al:
The consensus coding sequences of human breast and colorectal
cancers. Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|