Introduction
Gastric cancer (GC) is the second leading cause of
cancer-associated mortality worldwide (1). Although recent treatment advances have
improved the clinical outcome of patients with GC (2–5), the
prognosis of those with advanced stage disease is poor due to a
high incidence of metastasis and recurrence. Metastasis contributes
significantly to high cancer mortality rates, and thus the
development of sensitive, specific and convenient diagnostic
methods for the early detection of metastasis is paramount to
reduce these mortality rates (6).
In recent years, attention has been focused on the
proportion of circulating tumor cells (CTCs) as an early detection
marker for metastasis (6). The most
widely studied CTC detection method is based on immunomagnetic
enrichment with epithelial cell adhesion molecule (EpCAM)
antibodies and subsequent immunological identification using
cytokeratin (CK) antibodies (7,8). EpCAM is
a cell-surface molecule involved in cell-to-cell adhesion that is
highly expressed in the majority of epithelial carcinomas (8). CKs form intermediate filaments in
epithelial cells, and are used as specific markers for tumor cells
of epithelial origin (9,10).
In a prospective investigation, quantification of
CTCs using this method revealed that CTCs were an independent
prognostic factor in patients with advanced colorectal (11), breast (12) and prostate (13) cancer.
More recently, it has been hypothesized that
functional heterogeneity may account for the fact that not all
cancer cells in solid tumors have a similar ability to drive
oncogenesis (14). This observation
has led to the cancer stem cell (CSC) hypothesis, which suggests
that CSCs within the tumor can self-renew and proliferate to form
new tumors, and could be associated with cancer metastasis
(14).
A recent study indicated that a portion of CTCs have
characteristics reminiscent of CSCs; these were termed circulating
tumor stem cells (CTSCs) (15).
Compared with CTCs, CTSCs may be a more accurate prognostic factor,
as cancer growth is dependent on cancer stem cells (CSCs), which
are typically resistant to chemotherapy (16).
Cluster of differentiation 44 (CD44) was previously
reported to be a useful CSC marker in MKN45, MKN74 and NCI-N-87 GC
cell lines (17); the
CD44+ cell fraction could generate more spheroid
colonies compared with the CD44− cell fraction.
Furthermore, the CD44+ GC cells showed enhanced
tumorigenicity, chemoresistance and radioresistance in vivo,
compared with the CD44− GC cells (17). In addition, a meta-analysis reported
that CD44 expression in primary tissues was correlated with lymph
node metastasis and venous invasion (18). In particular, the CD44 exon 6 and exon
8–10 variants were correlated with hematogenous metastasis
(19,20).
The primary objective of the present study was to
detect CD44+ CTCs in the peripheral blood of patients
with GC in order to determine the clinical significance of CD44 as
a biomarker of diagnosis and treatment response.
Materials and methods
Patients
The present study included 26 patients with GC who
were admitted to Toyama University Hospital (Toyama, Japan) between
April 2014 and December 2014. The patient population consisted of
17 men and 9 women, with a median age of 72.69 years (range, 48–87
years). A total of 7 patients presented with stage IA disease, 5
with stage IIA, 1 with stage IIB, 3 with stage IIIA, 2 with stage
IIIB, 3 with stage IIIC and 5 with stage IV. With regard to
treatment, 1 patient underwent chemotherapy and 25 patients
underwent gastrectomy (15 distal gastrectomies, 8 total
gastrectomies, 1 partial gastrectomy and 1 remnant gastrectomy).
Clinicopathological classifications were determined by the
International Union Against Cancer Tumor-Node-Metastasis criteria
(7th edition) (21). The response to
chemotherapy was measured using computed tomography (CT) and was
evaluated according to the Response Evaluation Criteria in Solid
Tumors (version 1.1) (22).
Additionally, 10 healthy volunteers, aged 26–81 years (median, 40.0
years), were recruited as negative controls. All subjects provided
informed consent for study inclusion and were enrolled following
Institutional Review Board (Toyama University Hospital) approved
protocols.
Sample preparation
Blood samples (6 ml) were collected in 3-ml
ethylenediaminetetraacetic acid (EDTA) tubes. Peripheral blood
samples were extracted from each patient during general anesthesia
via a median cubital vein or the arterial pressure line prior to
gastrectomy. In the single patient who underwent chemotherapy, the
blood was extracted via a median cubital vein. Peripheral blood
samples were extracted from each healthy volunteer during general
anesthesia via a median cubital vein. Samples were processed and
evaluated as soon as possible following collection.
Elimination of red blood cells from
samples
Blood samples were transferred to 5-ml tubes
containing anticoagulant with EDTA, and were diluted by the
addition of an equal volume (3 ml) of phosphate-buffered saline
(PBS) containing 2% fetal bovine serum (FBS). Next, 6 ml of the
diluted blood sample was subsequently overlaid on a 4-ml
Lymphoprep™ (Cosmo Bio Co, Tokyo, Japan) placed in a 15-ml
centrifuge tube. The mixing of blood and separation fluid was
avoided, and the tube was capped to prevent the formation of
aerosols. The tubes were spun at 800 × g for 20 min at room
temperature in a swing-out rotor centrifuge. After spinning,
mononuclear cells were removed from the distinct band at the
sample/medium interface using a Pasteur pipette without disturbing
the upper layer. Mononuclear cells were diluted in 2 ml PBS
containing 2% FBS, and the cells were subsequently pelleted by
spinning at 250 × g for 5 min at 25°C.
Flow cytometry by
fluorescence-activated cell sorting (FACS) and sample analysis
For staining, human monoclonal EpCAM-allophycocyanin
(APC) (clone HEA125; MACS Miltenyi Biotec, Cologne, Germany) and
CD44-fluorescein isothiocyanate (FITC; clone IM7.8.1; MACS Miltenyi
Biotec) antibodies were used. As negative controls, mouse IgG1-APC
and FITC (clone IS5-21F5; MACS Miltenyi Biotec) isotype control
antibodies were used. All antibodies were diluted 1:100 in 200 µl
PBS containing 2% FBS. At 15 min post-staining, the cells were
diluted in PBS containing 2% FBS and pelleted by spinning at 250 ×
g for 5 min at 4°C. Samples were analyzed on a FACScanto™ II
flow analyzer (BD Biosciences, Franklin Lakes, NJ, USA). A sample
for sorting was analyzed on a FACSAria™ flow sorter (BD
Biosciences), and sorted into a 5-ml tube with 1 ml PBS containing
2% FBS. These materials were processed as follows.
Examination of sorted cells
Sorted EpCAM+CD44+ cells were
washed twice and diluted in 200 µl cold PBS containing 2% FBS.
Slides and filters were placed into appropriate slots in a cytospin
chamber (Stat Spin; Beckman Coulter, Tokyo, Japan) with the
cardboard filters facing the center. In the event of few cells
being available, 100 µl cold PBS containing 2% FBS was first placed
in each cytospin, which was then spun at 250 × g for 5 min
at 25°C to pre-wet the filter, allowing more cells to reach the
slide. In addition, correct alignment of the filter/slide interface
was ensured. For each sample, 200 µl was added to the appropriate
wells of the cytospin, lids were applied and centrifugation was
performed at 250 × g for 5 min at 25°C. Subsequently, the
filters were removed taking care not to disturb the smears on the
slides.
Each slide was examined under a microscope to check
cell adherence, morphology and monolayer formation. Slides were
dried overnight in a desiccator and evaluated using a transmitted
light microscope (BX61/DP70; Olympus, Tokyo, Japan) equipped with
an ultraviolet light source and filters. A cytotechnologist at the
hospital analyzed the sorted cells with regard to the nuclear to
cytoplasmic ratio, the overall cell size and the size of the
nucleolus.
Immunohistochemical evaluation of
primary tumor tissues
All 25 primary tumors resected during gastrectomy
were evaluated immunohistochemically. Sections (5 µm) from
formalin-fixed paraffin-embedded tissues were mounted on positively
charged slides then dewaxed in xylene and rehydrated. Specimens
were pretreated with KN9 buffer (code KN-09001; Pathology
Institute, Toyama, Japan) for 40 min at 95°C in a water bath,
cooled at room temperature for 20 min and washed with distilled
water (DW). The slides were then blocked for 10 min in 3% peroxide
DW solution, washed with DW and blocked for 5 min in KN buffer
(code KN-09002; Pathology Institute). The slides were stained with
EpCAM (clone VU1D9; Cell Signaling Technology Japan, Tokyo, Japan)
mouse monoclonal antibody (mAb; dilution 1:500; Cell Signaling
Technology), CD44 mouse mAb (clone 156–3C11; dilution 1:400; Cell
Signaling Technology) or CK-Oscar mouse mAb (clone BSB6181;
dilution 1:200; Bio SB, Shiga, Japan) for 30 min. CK-Oscar
identifies cytokeratins 7, 8, 18 and 19, and has been used to
distinguish epithelial carcinoma from non-epithelial tissues
(23–29). Slides were then counterstained using
the peroxidase-conjugated Envision technique (Envision plus Dual
Link Horseradish Peroxidase; DAKO, Glostrup, Denmark). Staining for
EpCAM or CD44 was defined as positive when cells were also positive
for CK-Oscar.
Statistical analyses
Comparisons between groups were evaluated using
paired and unpaired Student's t-tests. A P<0.05 was considered
to indicate a statistically significant difference. All data are
shown as the mean ± standard deviation.
Receiver-operating-characteristic (ROC) curves and the
area-under-the-curve (AUC) were used to assess the feasibility of
using EpCAM+CD44+ and
EpCAM+CD44− cell counts as a measure of CTC
number in patients with GC. All statistical analyses were performed
using JMP version 11 software (Statistical Discovery, Tokyo,
Japan).
Results
A comparison of
EpCAM+CD44+ and
EpCAM+CD44− cell proportions in the
peripheral blood between patients with GC and healthy
volunteers
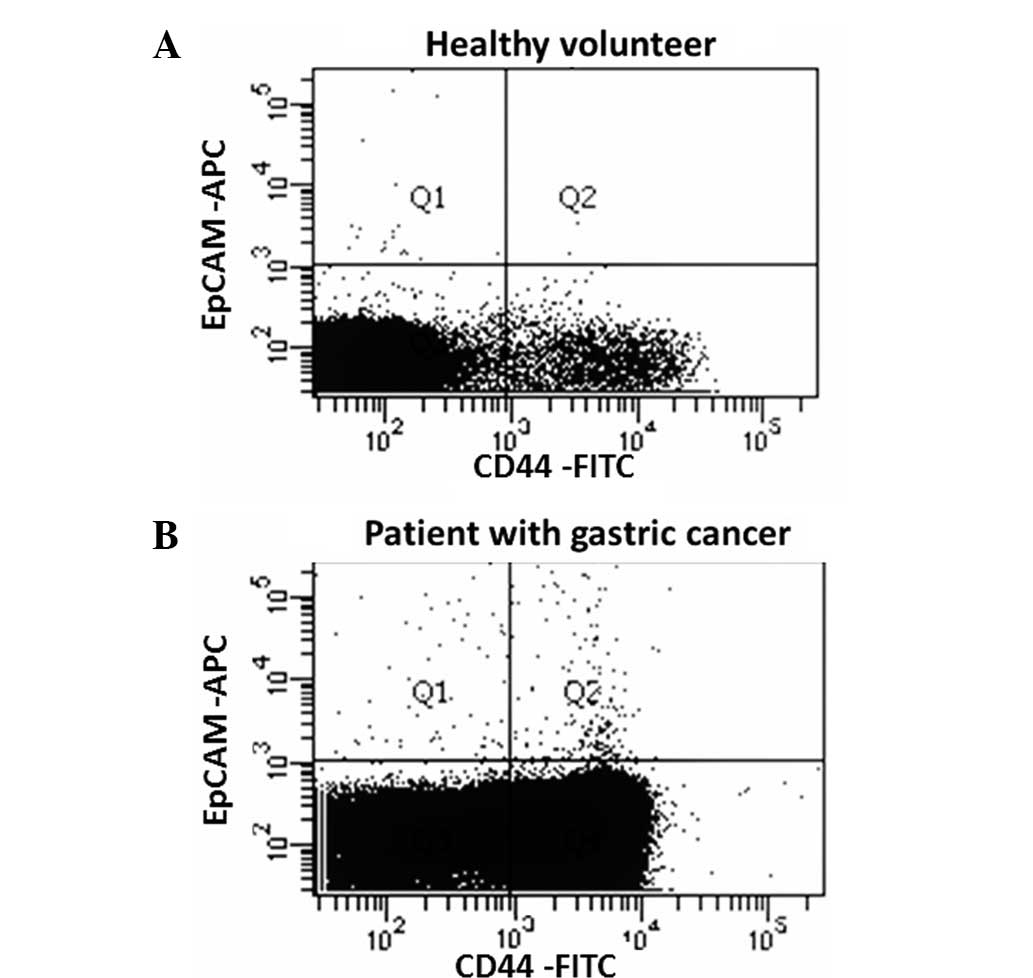
A representative figure of the comparison of the
proportion of EpCAM+CD44+ and
EpCAM+CD44− cells in the peripheral blood
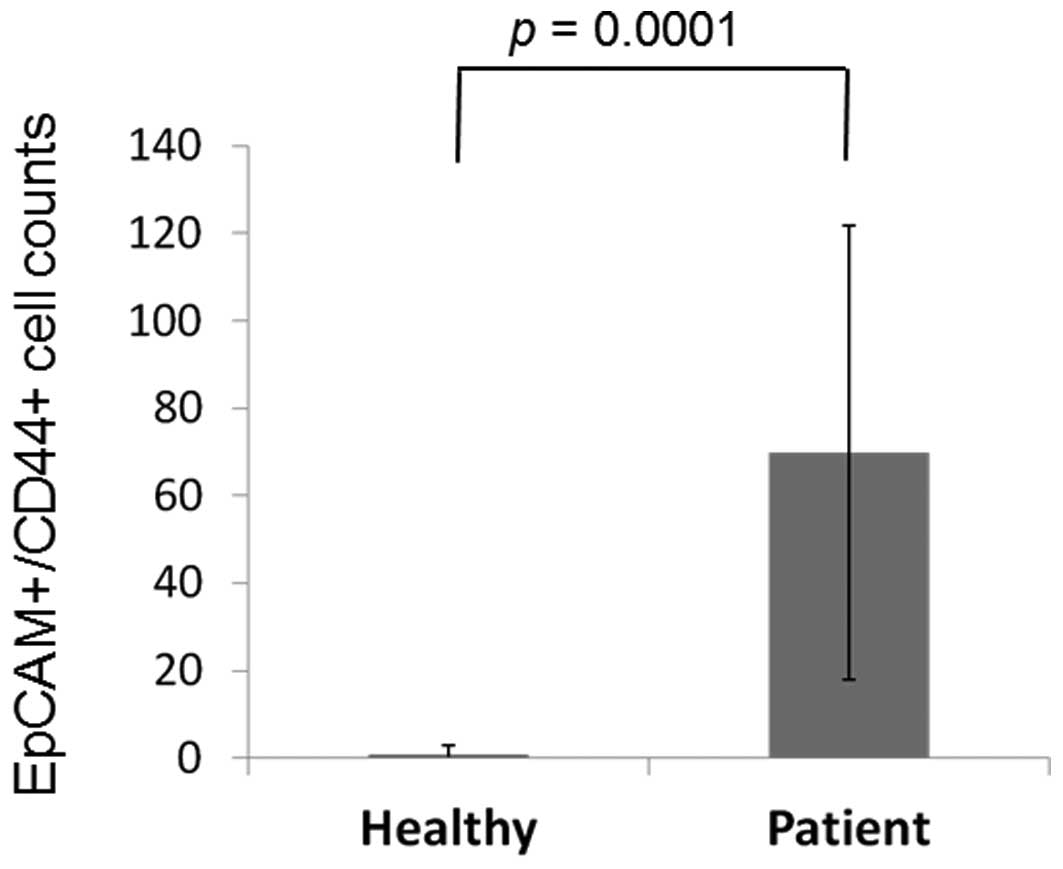
between patients and healthy controls is shown in Fig. 1. EpCAM+CD44+
cells were detected in 3 out of 12 (25.0%) healthy volunteers and
26 out of 26 (100.0%) patients, with mean cell counts of 0.91±2.10
and 69.9±52.0, respectively (P=0.0001; Fig. 2). EpCAM+CD44−
cells were detected in 12 out of 12 (100.0%) healthy volunteers and
26 out of 26 (100.0%) patients, with mean cell counts of 9.83±9.91
and 59.1±88.0, respectively (P=0.0313; Fig. 3).
With ROC curve analysis to diagnose GC, the largest
AUC for EpCAM+CD44+ cell counts was 0.9744,
and the optimal sensitivity and specificity were 92.3 and 100.0%,
respectively (Table I). The largest
AUC for EpCAM+CD44− cell counts was 0.8317,
and the optimal sensitivity and the specificity were 76.9 and
83.3%, respectively (Table I).
 | Table I.Receiver operating characteristic
analysis of the EpCAM+CD44+ and
EpCAM+CD44− circulating tumor cell counts in
the peripheral blood. |
Table I.
Receiver operating characteristic
analysis of the EpCAM+CD44+ and
EpCAM+CD44− circulating tumor cell counts in
the peripheral blood.
| Cell status | Sensitivity, % | Specificity, % | AUC | P-value |
|---|
|
EpCAM+/CD44+ | 97.4 | 100.0 | 0.9744 | <0.0001 |
|
EpCAM+/CD44− | 76.9 | 83.3 | 0.8317 | 0.0005 |
A comparison of the proportions of
EpCAM+CD44+ and EpCAM+CD44- CTCs in the peripheral blood of
patients with GC
Clinicopathological characteristics of the patients
who underwent gastrectomy and their cell counts in the peripheral
blood are shown in Table II. Mean
EpCAM+CD44+ cell counts were correlated with
pathological stage (pStage), pathological wall invasion depth and
venous invasion (v) factors (P=0.0423, 0.0314 and 0.0184,
respectively). By contrast, mean EpCAM+CD44−
cell counts did not show any correlation with the
clinicopathologial factors (Table
II).
 | Table II.Mean
EpCAM+CD44+ and
EpCAM+CD44− CTC counts in the peripheral
blood for each clinicopathological characteristic. |
Table II.
Mean
EpCAM+CD44+ and
EpCAM+CD44− CTC counts in the peripheral
blood for each clinicopathological characteristic.
| Characteristic | n |
EpCAM+/CD44+ CTC
count | P-value |
EpCAM+/CD44− CTC
count | P-value |
|---|
| Gender
(male/female) | 8/17 | 59.1/89.8 | 0.2001 | 68.2/41.1 | 0.3316 |
| Age (<75/>75
years) | 12/13 | 63.4/74.9 | 0.5995 | 67.8/50.6 | 0.6338 |
| pStage
(I/II–IV) | 6/19 | 43.2/77.1 | 0.0423a | 32.1/68.2 | 0.0846 |
| pT (1/2–4) | 7/18 | 44.1/78.6 | 0.0314a | 30.7/70.8 | 0.0682 |
| pN (−/+) | 14/11 | 68.9/69.0 | 0.4974 | 47.5/74.9 | 0.2445 |
| ly (−/+) | 6/19 | 79.3/65.6 | 0.6708 | 37.8/62.6 | 0.1314 |
| v (−/+) | 8/17 | 43.1/81.1 | 0.0184a | 28.8/74.0 | 0.0538 |
| Her2 (−/+) | 10/9 | 54.2/61.8 | 0.3667 | 35.6/104.0 | 0.0880 |
| CEA (−/+) | 20/5 | 74.0/48.6 | 0.9517 | 39.5/139.8 | 0.1392 |
| CA19-9 (−/+) | 22/3 | 65.4/97.3 | 0.1624 | 51.5/118.3 | 0.2230 |
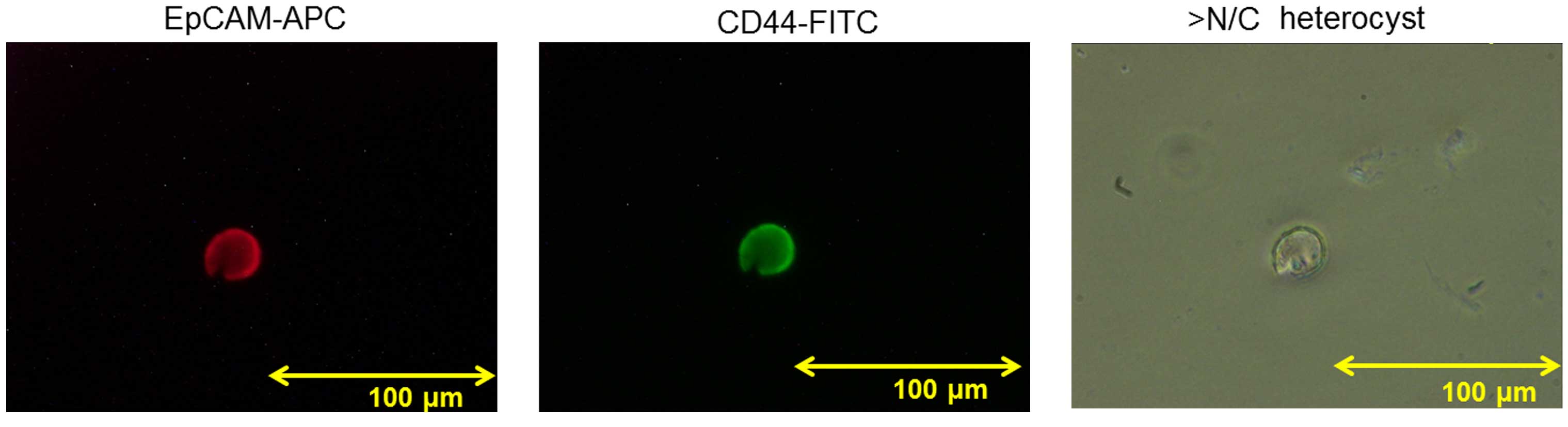
Sorted EpCAM+CD44+ CTCs
EpCAM+CD44+ CTCs were
evaluated in 1 case of GC. A representative figure of EpCAM-APC
(red) and CD44-FITC (green) cell staining is shown in Fig. 4. The overall cell size was ≥20 µm, the
nuclear to cytoplasmic ratio was high and the nucleolus was
enlarged. The cell was identified as a heterocyst rather than a
white blood cell (WBC), indicating malignant cytology.
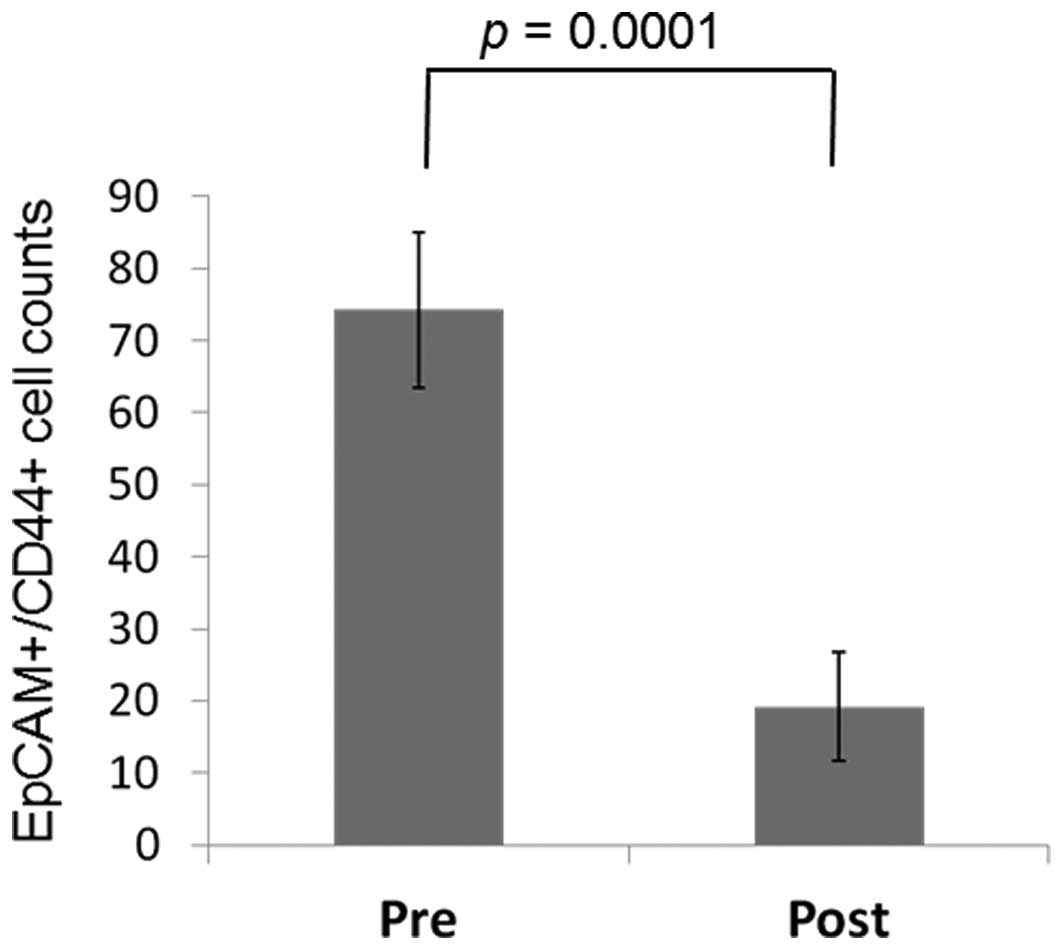
A comparison of EpCAM+CD44+ CTC counts
pre- and post-gastrectomy
In 23 of the 25 (92.0%) patients who underwent a
gastrectomy, EpCAM+CD44+ CTCs were counted
following the gastrectomy, as well as at follow-up, with a mean
follow-up time of 96.26±80.32 days. Post-gastrectomy, the
EpCAM+CD44+ CTC counts were decreased in 21
out of 23 (91.3%) patients to 19.2±7.48 cells compared with a
pregastrectomy count of 74.2±10.8 cells (P=0.0001) (Fig. 5).
Immunohistochemical evaluation of
resected primary tumor tissues
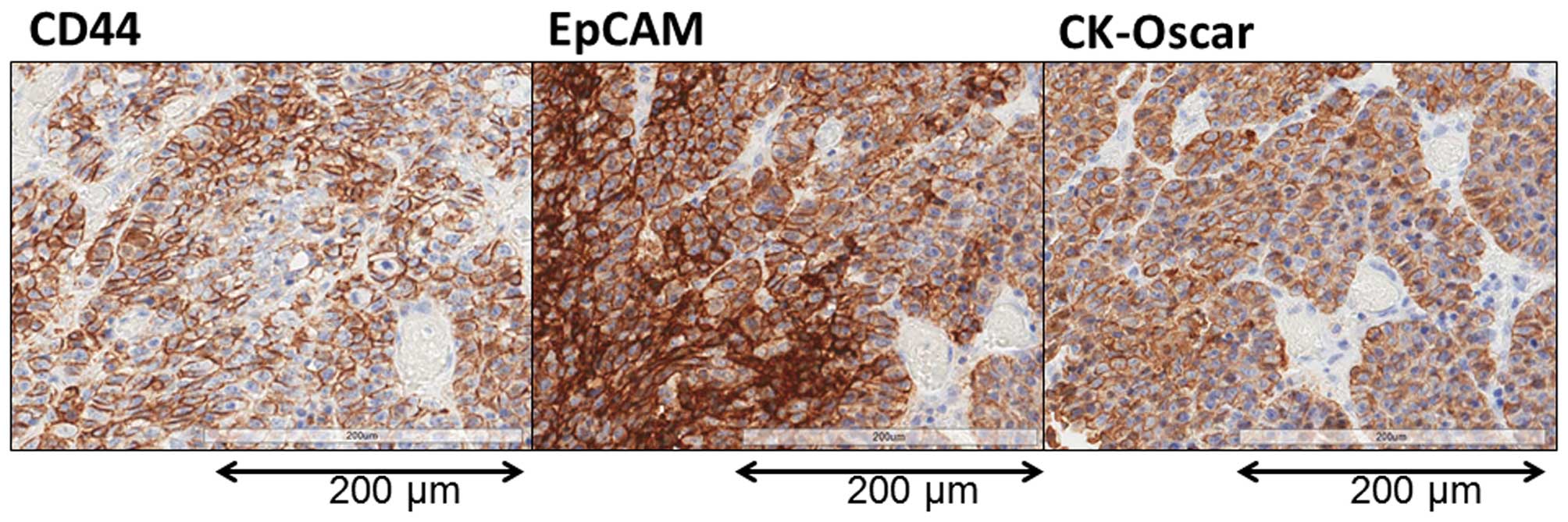
A representative image showing EpCAM and CD44
staining of the primary tumor is shown in Fig. 6. EpCAM and CK-Oscar staining were
detected in all the nucleated tumor cells in all 25 patients. By
contrast, although CD44 was detected in all 25 patients, the
expression was distributed only in a limited region of the tumor
cells.
A comparison of EpCAM+CD44+ CTC counts
pre- and post-chemotherapy
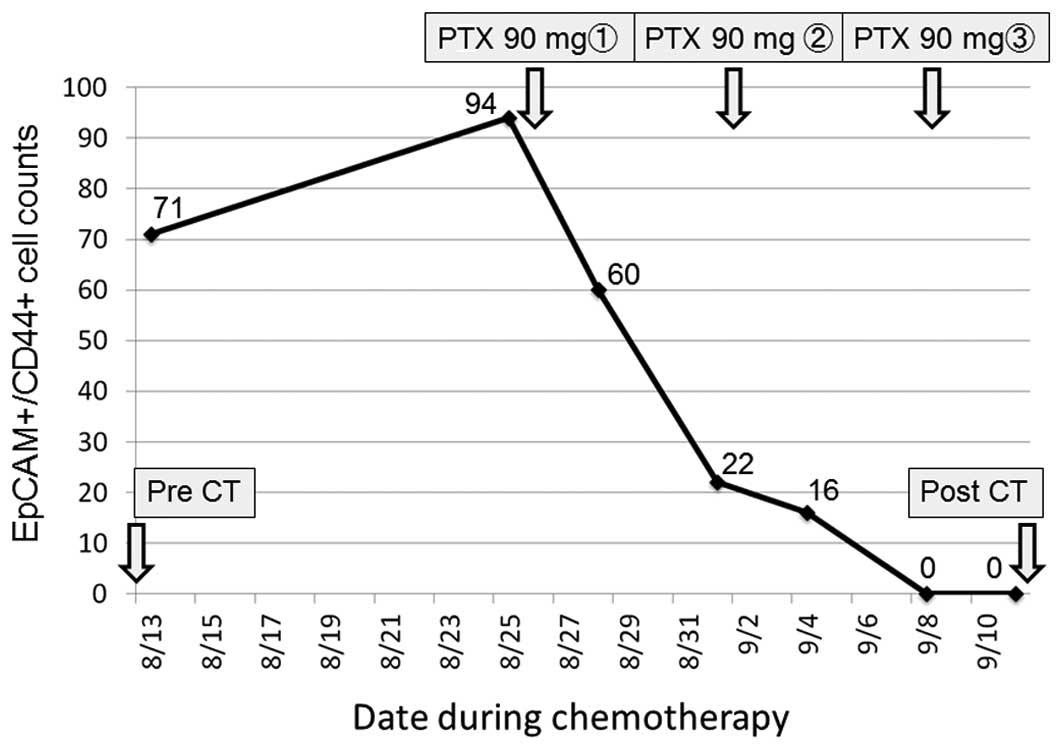
The patient who underwent chemotherapy had been
treated previously with first-line chemotherapy for an inoperable
tumor, but showed disease progression. After the patient was
admitted to Toyama University Hospital, weekly paclitaxel at a dose
of 80 mg/m2/day (90 mg/day) was administered as a
second-line chemotherapy. The peripheral blood
EpCAM+CD44+ CTC count was measured 7 times
during chemotherapy. The proportion of
EpCAM+CD44+ CTCs gradually decreased during
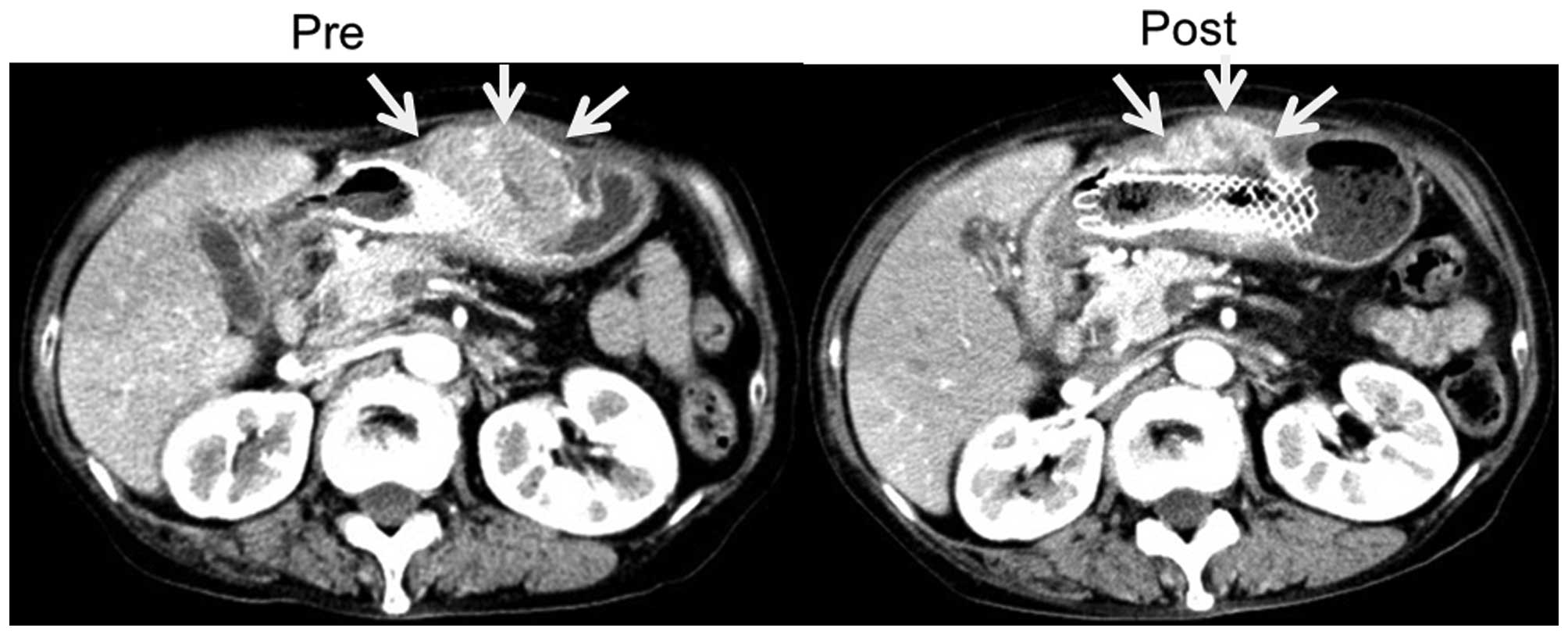
chemotherapy until they could no longer be detected (Fig. 7). After one course of chemotherapy, CT
images showed a partial response in that the size of the main
gastric tumor surrounding the stent was decreased by 30%, and there
was no development of new lesions (Fig.
8).
Discussion
The present study evaluated the association between
the CTCs that express cancer stem cell marker CD44 and
clinicopathological factors in patients with GC. The results
demonstrated that EpCAM+CD44+ CTC counts
detected by FACS correlated with pathological T and v factors. The
number of EpCAM+CD44+ CTCs was significantly
reduced following surgical resection of the primary tumor or
chemotherapy.
In healthy volunteers, the mean number of total
EpCAM+ CTCs (EpCAM+CD44+ plus
EpCAM+CD44− CTCs) in the peripheral blood was
7.6±5.6 (data not shown). The presence of these cells was possibly
caused by a non-specific immunological reaction or contamination
with skin cells (30). In addition, a
previous study in mice demonstrated the presence of
EpCAM+ peripheral blood-derived mesenchymal stem cells
(PBMSCs) with increased expression of gastric epithelial phenotypic
markers (31). In the present study,
in the patients with GC, EpCAM+ CTCs were 114.0±84.5
(data not shown) and were significantly higher than those in
healthy volunteers, reflecting a tumor-bearing state. In patients
with GC, the EpCAM+CD44− CTCs were considered
to be CTCs without CSC potential; they could also be WBCs with a
non-specific immune reaction, contaminated skin cells and/or
transdifferentiated PBMSCs. Further study is required to evaluate
the significance of EpCAM+CD44− CTCs in the
peripheral blood.
EpCAM has been one of the most used cell surface
markers to detect CTCs in solid tumors, including metastatic
colorectal (32), prostate (33), gastrointestinal (34), and breast (35–38) cancer
tumors. The most widely used method to detect CTCs has been the
CellSearch™ system, which relies on immunomagnetic capture of
EpCAM+ cells in combination with 4′
6-diamidino-2-phenylindole staining, CK immunofluorescence staining
and CD45 immunofluorescence staining to differentiate cancer cells
of epithelial origin from blood cells, which requires fixation of
the cells (17,39).
In the present study, CTCs were detected by flow
cytometry, which enables the analysis of the expression of multiple
cell surface markers in viable cells. Several methods for the flow
cytometric detection of CTCs have been reported previously. In
pancreatic cancer patients who underwent surgical resection, CTCs
were found to be prognostic markers of survival (40), in which a negative depletion procedure
using CD45 and CD34 staining was used to enrich CTCs (41). Other studies demonstrated that
CK+/CD45− CTCs were detected in all examined
patients with metastatic lung cancer (42), while healthy volunteers exhibited
significantly lower counts (43).
However, these previous studies did not describe the gating lines
used for the negative controls. In the present study, mouse
IgG1-APC and FITC isotype control antibodies were used for negative
staining, and the negative gate was defined as follows: In each
immunofluorescence stain (APC and FITC), the criteria of the
negative control and 99.9% of all cell counts were defined. A
direct comparison of the present findings could not be made with
those of other studies due to the different procedures used;
however, the CTC counts detected in previous studies were much
lower than those in the present study. Further investigations are
required to biologically characterize the
EpCAM+CD44+ cells of the present study.
There have previously been studies on the detection
of CTSCs in various solid tumors. In colorectal cancer,
CK+/CD133+ cells were deemed CTSCs (42). In metastatic breast cancer,
CK+/CD44+ cells were markers for peripheral
blood CTCs with a stem-cell phenotype (43). Moreover, CTSCs defined as
CD45−EpCAM+CD44+CD24−
were shown to be useful for the diagnosis, treatment responsiveness
and prognosis of patients with early-stage breast cancer (44). In addition,
CK+/CD44+ CTCs were detected in 70.4% of the
CTC-positive GC patients and CD44+ CTCs were
significantly associated with tumor location, lymph node
metastasis, distant metastasis and recurrence (45). However, there have been no studies on
CTSC detection in GC patients using the combination of cell surface
markers, EpCAM and CD44, which enables evaluation of viable cell
expression. Investigation of the CSC phenotype in sorted
EpCAM+/CD44+ CTCs may provide a biological
basis of CTSCs in GC.
EpCAM+CD44+ CTC counts, but
not EpCAM+CD44− CTC counts, were correlated
with pathological T and v factors in the present study, suggesting
a role of CD44+ CTCs in tumor metastasis. As
pathological progression is generally correlated with prognosis in
cancer patients (46), the flow
cytometric analysis of EpCAM+CD44+ staining
could be a novel prognostic tool in patients with GC. Prospective
studies with long-term follow-up results are awaited.
In summary, the present investigation using flow
cytometry demonstrated that EpCAM+CD44+ CTC
counts significantly increased in patients with GC compared with
healthy volunteers. The number of EpCAM+CD44+
CTCs, but not EpCAM+CD44− CTCs, was
correlated with disease progression and venous invasion in resected
tumor specimens. The number of EpCAM+CD44+
CTCs decreased following surgical resection or chemotherapy.
CD44+ CTCs are suggested to reflect the malignant
potential of the tumor, providing a candidate marker of diagnosis
and treatment response in patients with GC, as well as a candidate
marker to investigate CTSCs.
Acknowledgements
The authors would like to thank M. Kawahara for
providing excellent technical assistance with flow cytometry, and
T. Hori for the cytology evaluation. This study was partly
supported by a Grant-in-Aid for scientific Research (KAKENHI)
(grant no. 23591920).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008:GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siewert JR, Böttcher K, Roder JD, Busch R,
Hermanek P and Meyer HJ: Prognostic relevance of systemic lymph
node dissection in gastric carcinoma. German gastric carcinoma
study group. Br J Surg. 80:1015–1018. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikeda Y, Mori M, Adachi Y, Matsushima T,
Sugimachi K and Saku M: Carcinoembryonic antigen (CEA) in stage IV
gastric cancer as a risk factor for liver metastasis: A univariate
and multivariate analysis. J Surg Oncol. 53:235–238. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furukawa H, Hiratsuka M, Iwanaga T, Imaoka
S, Ishikawa O, Kabuto T, Sasaki Y, Kameyama M, Ohigashi H, Nakamori
S, et al: Adjuvant chemotherapy for advanced gastric cancer. Nippon
Geka Gakkai Zasshi. 97:312–316. 1996.(In Japanese). PubMed/NCBI
|
|
5
|
Palli D: Epidermiology of gastric cancer:
An evaluation of available evidence. J Gastroenterol. 35:(Suppl
12). S84–S89. 2000.
|
|
6
|
Hughes AD and King MR: Manobiotechnology
for the capture and manipulation of circulating tumor cells. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 4:291–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross AH, Herlyn D, Iliopoulos D and
Koprowski H: Isolation and characterization of a
carcinoma-associated antigen. Biochem Biophys Res Commun.
135:297–303. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mostert B, Sleijfer S, Forkens JA and
Gratama JW: Circulating tumor cells (CTCs): Detection methods and
their clinical relevance in breast cancer. Cancer Treat Rev.
35:463–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: Patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osborn M, van Lessen G, Weber K, Klöppel G
and Altmannsberger M: Differential diagnosis of gastrointestinal
carcinomas by using monoclonal antibodies specific for individual
keratin polypeptides. Lab Invest. 55:497–504. 1986.PubMed/NCBI
|
|
11
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grover PK, Cummins AG, Price TJ,
Roberts-Thomson IC and Hardingham JE: Circulating tumor cells: The
evolving concept and the inadequacy of their enrichment by
EpCAM-based methodology for basic and clinical cancer research. Ann
Oncol. 25:1506–1516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wicha MS and Hayes DF: Circulating tumor
cells: Not all detected cells are bad and not all bad cells are
detected. J Clin Oncol. 29:1508–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Dong LP, Zhang N and Zhao CH: Role
of cancer stem cell marker CD44 in gastric cancer: A meta-analysis.
Int J Exp Med. 7:5059–5066. 2014.
|
|
19
|
Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida
M, Iida A, Kimura T, Takeuchi K, Katayama K and Hirose K:
Expression of CD44v6 in advanced gastric cancer and its
relationship to hematogenous metastasis and long-term prognosis. J
Surg Oncol. 79:230–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi A, Saito M, Goi T, Iida A,
Takeuchi K, Hirose K, Nakagawara G, Urano T, Furukawa K and Shiku
H: Exrpession of CD44 variant exons 8–10 in gastric cancer. Jpn J
Cancer Res. 86:1166–1171. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
2009
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–47.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Battifora H: Clinical applications of the
immunohistochemistry of filamentous proteins. Am J Surg Pathol 12
Suppl. 1:24–42. 1988.
|
|
24
|
Gown AM and Vogel AM: Monoclonal
antibodies to human intermediate filament proteins. III. Analysis
of tumors. Am J Clin Pathol. 84:413–424. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knapp AC and Franke WW: Spontaneous losses
of control of cytokeratin gene expression in transformed,
non-epithelial human cells occurring at different levels of
regulation. Cell. 59:67–79. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis JE, Olsen KD and Sebo TJ: Spindle
cell carcinoma of the larynx: review of 26 cases including DNA
content and immunohistochemistry. Hum Pathol. 28:664–673. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mueller JD, Stein HJ, Oyang T, Natsugoe S,
Feith M, Werner M and Rüdiger Siewert J: Frequency and clinical
impact of lymph node micrometastasis and tumor cell
microinvolvement in patients with adenocarcinoma of the
esophagogastric junction. Cancer. 89:1874–1882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato F, Shimada Y, Li Z, Watanabe G, Maeda
M and Imamura M: Lymph node micrometastasis and prognosis in
patients with oesophageal squamous cell carcinoma. Br J Surg.
88:426–432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miller JM, Astles R, Baszler T, Chapin K,
Carey R, Garcia L, Gray L, Larone D, Pentella M, Pollock A, et al:
Guidelines for safe work practices in human and animal medical
diagnostic laboratories. Recommendations of a CDC-convened,
Biosafety Blue Ribbon Panel. MMWR Suppl. 61:1–102. 2012.PubMed/NCBI
|
|
30
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: Clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okumura T, Wang SSW, Takashi S, Tu SP, Ng
V, Ericksen RE, Rustgi AK and Wang TC: Identification of a bone
marrow-derived mesenchymal progenitor cell subset that can
contribute to the gastric epithelium. Lab Invest. 89:1410–1422.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsusaka S, Suenaga M, Mishima Y,
Kuniyoshi R, Takagi K, Terui Y, Mizunuma N and Hatake K:
Circulating tumor cell as a surrogate marker for determining
response to chemotherapy in Japanese patients with metastatic
colorectal cancer. Cancer Sci. 102:1188–1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreno JG, Miller MC, Gross S, Allard WJ,
Gomella LG and Terstappen LW: Circulating tumor cells predict
survival in patients with metastatic prostate cancer. Urology.
65:713–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa
Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S, et
al: Clinical significance of circulating tumor cells in blood from
patients with gastrointestinal cancers. Ann Surg Oncol.
15:3092–3100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lianidou ES and Markou A: Circulating
tumor cells in breast cancer: Detection system, molecular
characterization, and future challenges. Clin Chem. 57:1242–1255.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakamura S, Yagata H, Ohno S, Yamaguchi H,
Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K and Suzuki E:
Multi-center study evaluating circutating tumor cells as a
surrogate for response to treatment and overall survival in
metastatic breast cancer. Breast Cancer. 17:199–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diehn M, Cho RW and Clarke MF: Therapeutic
implications of the cancer stem cell hypothesis. Semin Radiat
Oncol. 19:78–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng S, Tripathy D, Frenkel EP, Shete S,
Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et
al: Circulating tumor cells in patients with breast cancer
dormancy. Clin Cancer Res. 10:8152–8162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayes DF and Smerage J: Is there a role
for circulating tumor cells in the management of breast cancer?
Clin Cancer Res. 14:3646–3650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sergeant G, van Eijsden R, Roskams T, Van
Duppen V and Topal B: Pancreatic cancer circulating tumor cells
express a cell motility gene signature that predicts survival after
surgery. BMC Cancer. 12:5272012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Swennenhuis JF, Reumers J, Thys K,
Aerssens J and Terstappen LW: Efficiency of whole genome
amplification of single circulating tumor cells enriched by
CellSearch and sorted by FACS. Genome Med. 5:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang NF, Shi L, Li H, Hu Y, Du W, Liu W,
Zheng J, Huang S and Qu X: Detection of circulating tumor cells and
tumor stem cells in patients with breast cancer by flow cytometry:
A valuable tool for diagnosis and prognosis evaluation. Tumor Biol.
33:561–569. 2012. View Article : Google Scholar
|
|
44
|
Theodoropoulos PA, Polioudaki H, Agelaki
S, Kallergi G, Saridaki Z, Mavroudis D and Georgoulias V:
Circulating tumor cells with a putative stem cell phenotype in
peripheral blood of patients with breast cancer. Cancer Lett.
288:99–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao
J, Jiang Y, Zhai H, Ji Y and Luo D: Stem cell-like circulating
tumor cells indicate poor prognosis in gastric cancer. Biomed Res
Int. 2014:9812612014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu J, Huang CM, Zheng CH, Li P, Xie JW,
Wang JB and Lin JX: Consideration of tumor size improves the
accuracy of TNM predictions in patients with gastric cancer after
curative gastrectomy. Surg Oncol. 22:167–171. 2013. View Article : Google Scholar : PubMed/NCBI
|