Introduction
Cancer metastasis remains responsible for the vast
majority of cases of cancer-related morbidity and mortality.
Metastasis, by its definition, is the spread of cancer from the
primary site to the distant tissues. The prerequisite for the
establishment of a diagnosis of metastasis is a consistent
pathology between the primary site and the metastasis (1). However, to fully endow metastatic
potential, cancer cells must accumulate a spectrum of alterations.
The pathological consistency may be compromised during this
accumulation.
The genomic landscape has been revealed for the most
common types of cancer, including lung cancer (2,3). Lung
cancer ranks first in both morbidity and mortality worldwide. Its
annual incidence in China is estimated to be ~500 per million
people (4). With the progress of
genomic technology, lung cancer is considered as a ‘disease of the
genome’. The gene mutations that confer a selective growth
advantage to the tumor cell are called ‘driver’ mutations (5). The most prominent driver gene in lung
cancer is the epidermal growth factor receptor (EGFR) gene
(6). Patients haboring EGFR mutation
respond markedly to EGFR tyrosine kinase inhibitors (TKI) such as
erlotinib (6). The capability of
genetic testing expands our armamentarium to recognize occult
cancer metastasis.
The present study describes a notable case of lung
cancer in which bone metastasis was revealed by genetic profiling,
but was not based on pathological analysis.
Case report
A 44-year-old, non-smoking, male was admitted to
West China Hospital (Chengdu, Sichuan, China) on July 16, 2014 with
pain in the neck and right hip, and weight loss of 11 pounds over 3
weeks. The physical examination was normal. The patient underwent
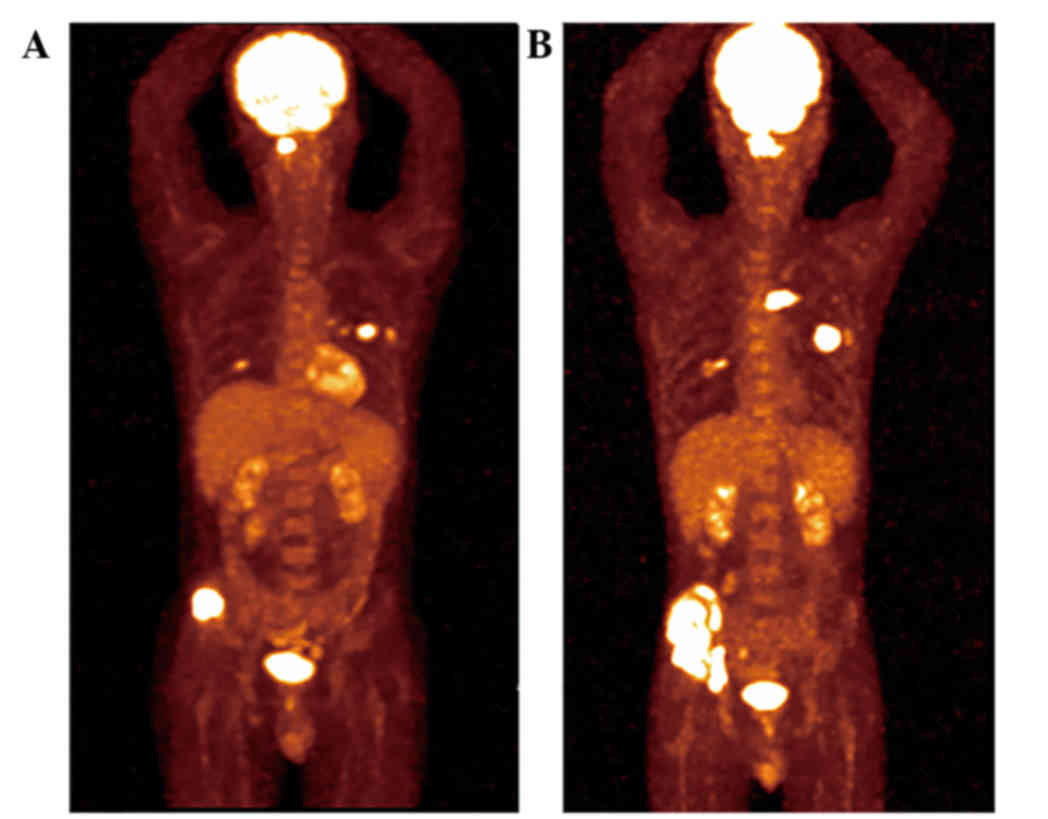
whole-body positron emission tomography-computed tomography, and a
mass in the upper lobe of the left lung and multiple areas of high
metabolism in the bone (ilium and first cervical vertebra) were
revealed. Magnetic resonance imaging of the neck showed destruction
of the Atlas vertebra. The patient then underwent laminoplasty.
Biopsies were performed in the right ilium and left lung, and the
tissue specimens were fixed in 10% formaldehyde, embedded in
paraffin, and cut into sections (5-µm). For immunohistochemical
analysis, the sections were incubated for 1 h at 37°C with primary
antibodies against cytokeratin (CK) 5/6 (1:100; MAB-0276; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China), CK7 (1:200; ZM-0071;
OriGene Technologies, Inc., Beijing, China), P63 (1:200; CM163C;
Biocare Medical, LLC, Concord, CA, USA), NapsinA (1:200; RAB-0639;
Fuzhou Maixin Biotech Co., Ltd.) and thyroid transcription factor
(1:200; 8G7G3/1; Abcam, Cambridge, MA, USA). Hematoxylin was used
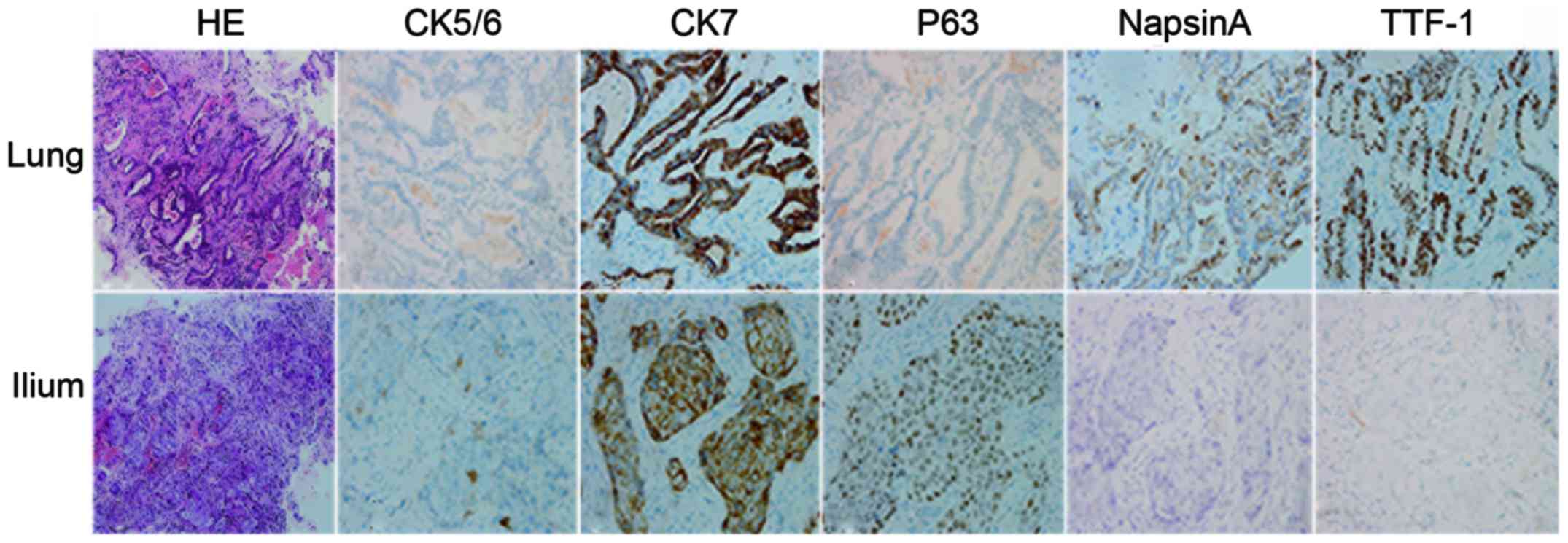
for counterstaining. The pathological examination revealed
different histological types with different immunohistochemical
phenotypes (Fig. 1). However, exactly
the same genetic profile between these two lesions confirmed the
same entity (Table I). A diagnosis of
adenocarcinoma in the left lung with bone metastasis was
established (cT3N3M1b, stage IV) (7).
 | Table I.Results of genetic testing from the
lung and ilium were identical. |
Table I.
Results of genetic testing from the
lung and ilium were identical.
| Region | EGFR | ALK | ROS-1 |
|---|
| Lung | G719X+S768I | Wild-type | Wild-type |
| Ilium | G719X+S768I | Wild-type | Wild-type |
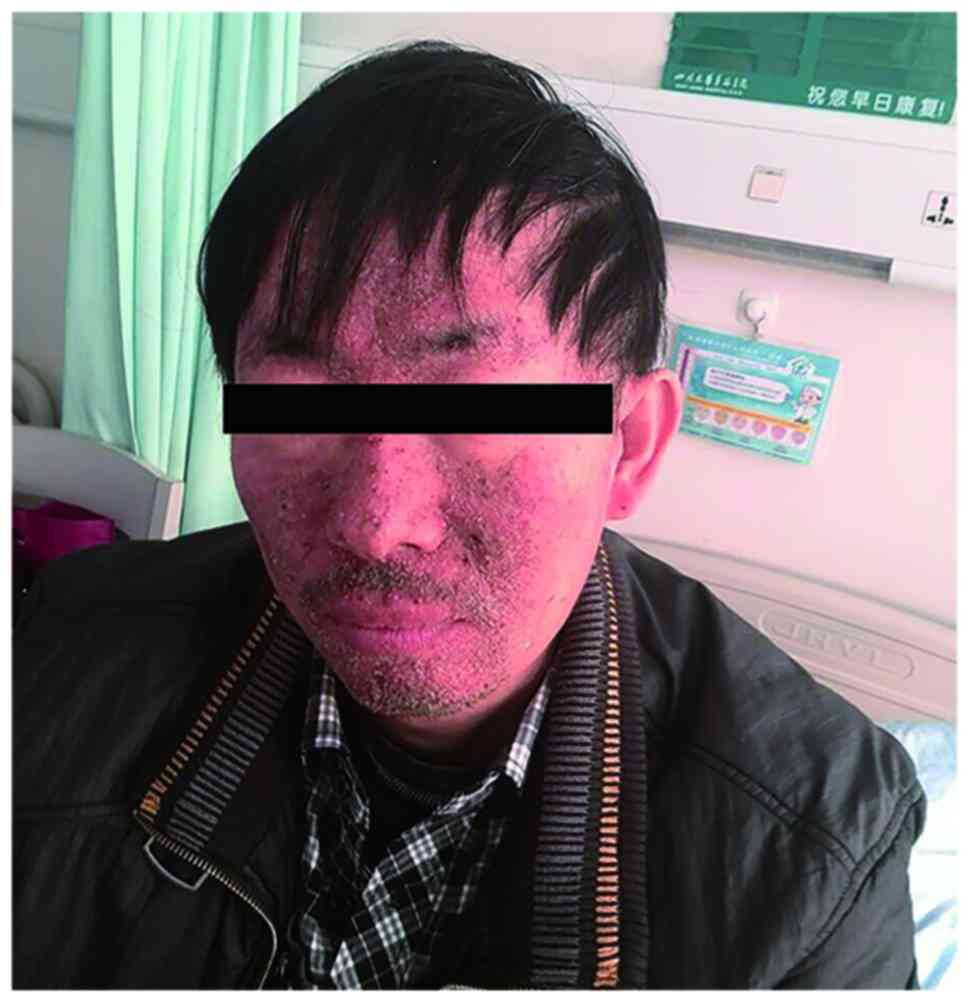
Zoledronic acid (4 mg) was administered each month
to prevent severe bone-related events. The patient was also
prescribed erlotinib (150 mg, once per day). The medication was
well-tolerated, with the exception of a persistent acneform rash on
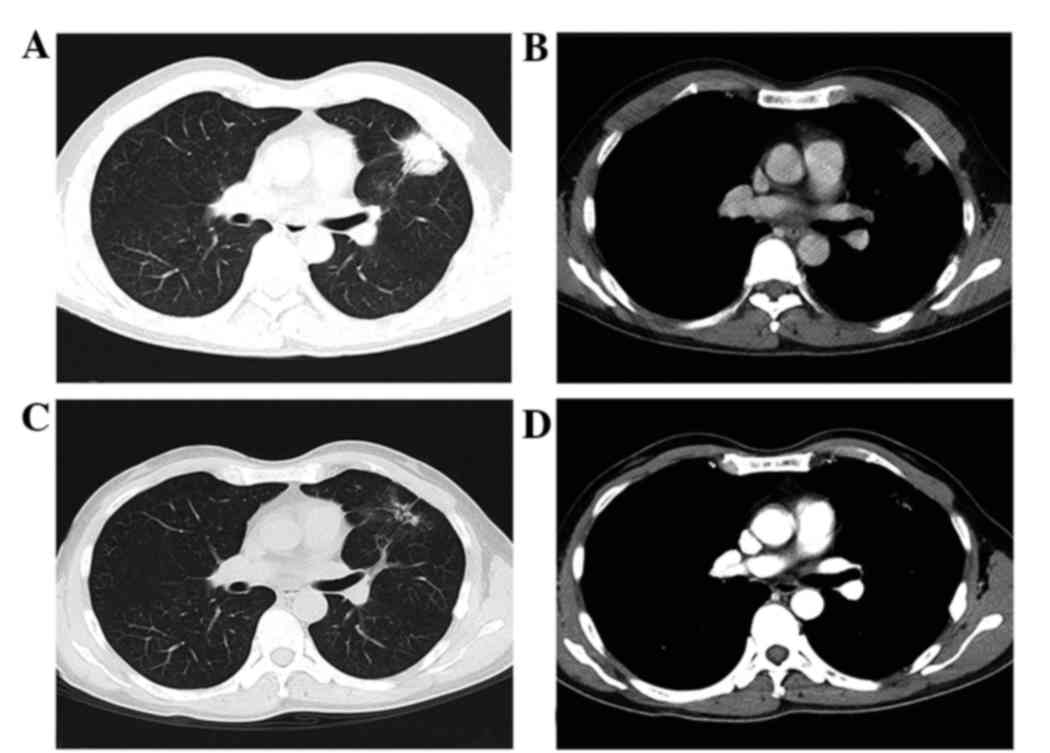
the face (Fig. 2). This TKI treatment
achieved a partial response in the primary lung lesion (Fig. 3) and relief of the pain in the right
ilium. However, progression of the disease occurred after 6 months
of erlotinib treatment (Fig. 4). The
patient is currently being treated with doublet chemotherapy
consisting of cisplatin (75 mg/m2, day 1) and
gemcitabine (1,000 mg/m2, days 1 and 8). Chemotherapy is
administered every 3 weeks. Written informed consent was obtained
from the patient.
Discussion
Lung cancer remains the most common cause of
cancer-related mortality worldwide (6). Non-small cell lung cancer (NSCLC)
constitutes ~80% of all cases. Targeted therapy has proven its
superiority over chemotherapy in patients selected for genetic
testing. Therefore, genetic testing has become a focus in lung
cancer, and consequently, the genomic landscape has been
established by high-throughput sequencing (2,3). The
accumulation of genetic data should therefore pose an impact on
daily clinical practice.
Traditionally, the present patient would have been
diagnosed with different types of cancer in the lung and ilium due
to the different pathological types. However, the perfectly matched
genetic profile between these two lesions strongly argued that they
had arisen from the same origin. This conclusion proposes a
challenge to the long-believed metastatic theory of ‘pathological
consistency’.
We argue that the lesions in the lung and bone
belonged to the same disease entity, not only due to their genetic
consistency, but also due to their similar responses to a TKI. TKI
treatment achieved a significant shrinkage in the lung mass,
together with a relief of pain in the bone. The bone lesion was
considered non-measurable according to the Response Evaluation
Criteria in Solid Tumors (8), so it
could not be evaluated precisely.
The same method of deduction has also been proposed
by other studies. For example, Sequist et al tracked the
genotypic and histological evolution of lung cancers acquiring
resistance to EGFR inhibitors (9).
The study found that 14% (5/37) of tumors transformed from NSCLC
into SCLC. Although these patients developed different histological
types of cancer, the same origin of tumor was confirmed by the
maintenance of the EGFR mutation. Bloom et al reported that
by adapting a micro-array platform, the origin of the tumors could
be accurately predicated in >80% of histologically similar
adenocarcinomas (10). In a review of
cancer of unknown primary site, Varadhachary and Raber also
exemplified a notable case, in which immunohistochemistry indicated
a gastrointestinal origin, while radiographic examination found a
solitary lesion in the lung. The microRNA assay finally confirmed a
colon-cancer profile (11). Our
proposal that the lesions in the current patient had arisen from
the same origin was in agreement with these leading-edge
reports.
There is no consensus as to the number of genes used
in genetic profiling. In the present case, however, there was high
confidence of genetic consistency between the lung and bone
lesions. Over 90% of mutations are located in exon 19 or 21 in the
EGFR gene (exon 19 deletion or L858R point mutation). Other rare
mutations include G719X, L861Q and S768I (12). The EGFR gene in the present patient
contained mutations in two loci: G719X and S768I. Little is known
about the incidence of this type of complicated mutation, but it
was estimated to be ~0.5% (as assessed from 12 cases haboring G719X
and S768I double mutations from 2,544 patients with the EGFR gene
assayed in the past 2 years in West China Hospital). The extreme
paucity of this type of mutation almost ruled out the possibility
of ‘accidental’ consistency.
The next question was why the present patient
apparently had different types of cancer. Previously, a previous
study showed that mixed histological phenotypes were observed in 59
cases from 1,158 lung cancer patients (13). We also reported 21 cases with the
mixed form of neuroendocrine tumors from 2,501 primary lung cancer
cases (14). Therefore, the
heterogeneity in lung cancer was not uncommon as previously
believed. Little is known about the origin of the heterogeneity,
and theories of hierarchy evolution and multi-clonal origin have
been proposed. Notably, the two modes were identified in lung
cancer by deep sequencing (15).
Neither could be verified in the present patient. The observation
of variant histology between primary sites and metastasis was also
observed in other case studies (Table
II) (16,17). These studies challenged the notion of
pathological consistency, but the evidence in support of metastasis
was lacking.
 | Table II.Reported lung cancer patients with
pathologically inconsistent metastases. |
Table II.
Reported lung cancer patients with
pathologically inconsistent metastases.
| First author,
year | Age, years | Gender | Lung cancer
pathology | Location of
metastasis | Pathology of
metastasis | (Ref.) |
|---|
| Glass et al,
2013 | 57 | Male | Squamous | Meninges | Adenocarcinoma | (16) |
| Shelton et al,
2012 | 71 | Male | Squamous | Pleural | Adenocarcinoma | (17) |
| Present study | 44 | Male | Adenocarcinoma | Bone | Squamous |
|
In summary, the current study presented a case of
advanced lung adenocarcinoma with metastatic squamous carcinoma in
the bone. The bone metastasis, although with a different
pathological type, was identified by genetic profiling. Through
this case report, we advocate the importance of using genetic
testing in addition to pathological assessment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 82172684 and 81200640).
References
|
1
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinstein IB: Addiction to oncogenes - the
Achilles heal of cancer. Science. 297:63–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumors. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bloom G, Yang IV, Boulware D, Kwong KY,
Coppola D, Eschrich S, Quackenbush J and Yeatman TJ:
Multi-platform, multi-site, microarray-based human tumor
classification. Am J Pathol. 164:9–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varadhachary GR and Raber MN: Cancer of
unknown primary site. N Engl J Med. 371:757–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riely GJ, Politi KA, Miller VA and Pao W:
Update on epidermal growth factor receptor mutations in non-small
cell lung cancer. Clin Cancer Res. 12:7232–7241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruffini E, Rena O, Oliaro A, Filosso PL,
Bongiovanni M, Arslanian A, Papalia E and Maggi G: Lung tumors with
mixed histologic pattern. Clinico-pathologic characteristics and
prognostic significance. Eur J Cardiothorac Surg. 22:701–707. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li DH, Wang C, Chen HJ, Huang H and Ding
ZY: Clinical characteristics of the mixed form of neuroendocrine
tumor in the lung: A retrospective study in 2501 lung cancer cases.
Thorac Cancer. 6:25–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Bruin EC, McGranahan N, Mitter R, Salm
M, Wedge DC, Yates L, Jamal-Hanjani M, Shafi S, Murugaesu N, Rowan
AJ, et al: Spatial and temporal diversity in genomic instability
processes defines lung cancer evolution. Science. 346:251–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glass R, Hukku SR, Gershenhorn B, Alzate J
and Tan B: Metastasis of lung adenosquamous carcinoma to
meningioma: Case report with literature review. Int J Clin Exp
Pathol. 6:2625–2630. 2013.PubMed/NCBI
|
|
17
|
Shelton DA, Rana DN, Holbrook M, Taylor P
and Bailey S: Adenosquamous carcinoma of the lung diagnosed by
cytology? A diagnostic dilemma. Diagn Cytopathol. 40:830–833. 2012.
View Article : Google Scholar : PubMed/NCBI
|