Introduction
Hepatocellular carcinoma (HCC) represents more than
95% of primary liver cancers, with more than half a million new
cases annually (1,2), ranking as the fifth most common
malignant tumor worldwide and the third leading cause of
cancer-associated death (1,3). Due to the asymptomatic development at
early stages, deficiencies in early diagnostic accuracy, rapid
progression and metastasis, limited treatment options, and frequent
postoperative recurrence, HCC displays a dismal prognosis, coupled
with high mortality, and has become a major public health problem,
particularly in developing countries (3–5).
Patients who are diagnosed early can often be cured
by surgical resection, liver transplantation or radiofrequency
ablation, leading to an increase in overall survival (4). However, for most HCC patients, therapy
remains difficult due to a high frequency of relapse after
resection or liver transplantation (1). In HCC with extrahepatic spread, only an
anti-angiogenic and mitogen-activated protein kinase inhibitor,
sorafenib, has been shown to improve overall survival of patients
from 8 to 11 months (1,2,5). For
advanced HCC patients, intraarterial chemoembolization or
percutaneous ethanol injection lead to minimal survival benefits
(6,7).
Therefore, it is of distinct importance to discover and develop
novel preventive strategies and therapeutic approaches for HCC.
The human chondrolectin (CHODL) gene, located on
chromosome 21q21, is detected as a 2.6-kb transcript containing six
exons and five introns (8). The open
reading frame of CHODL encodes a type I transmembrane
N-glycosylated protein consisting of 273 amino acids (molecular
weight, ~36 kDa), containing a single carbohydrate recognition
domain (CRD) for C-type lectins in the extracellular region
(8,9).
CHODL shows predominantly perinuclear localization in transiently
transfected COS1 cells, and immunofluorescent staining also reveals
strong punctate signals in the cytosol and cell membrane (8). Reverse transcription-polymerase chain
reaction analysis and immunohistochemistry (IHC) images demonstrate
that the expression of CHODL is mainly limited to vascular muscle
of the testis, smooth muscle of the prostate stroma, heart muscle,
skeletal muscle, crypts of the small intestines and red pulp of the
spleen (8,9).
In general, type I transmembrane proteins containing
a C-type lectin CRD motif display a wide variety of functions,
including cell recognition, complement activation, embryonic
development and immune regulation (10). In mice, CHODL expression is tightly
regulated during early embryonic development, which is of great
importance for interactions between growth cones of motor axons and
the horizontal myoseptum (11,12). CHODL
aberrant expression is demonstrated in spinal muscular atrophy
(SMA) mouse models (11,12). CHODL is highly expressed in motor
neurons and has distinct effects on neurite outgrowth in zebrafish
(11). CHODL messenger (m) RNA
expression in human adults was found predominantly in muscle cells,
suggesting a basic role in muscle formation (8). The CHODL splice isoforms (CHODLf,
CHODLδE and CHODLfδE) of the CHODL family were found to be
differentially expressed in thymocytes and lymphocytes, indicating
an association with human T cell development and maturation
(10,13).
CHODL may be involved in tumor metastasis processes,
including cell recognition, communication, cell-cell adhesion or
interactions with the extracellular matrix (ECM) (14). Recently, evidence has shown that CHODL
is overexpressed in the majority of lung cancers, indicating a
possible correlation between CHODL and invasion activity of lung
cancer cells (14). IHC staining
revealed that short survival of patients with non-small cell lung
cancer (NSCLC) was associated with strong CHODL staining, and
multivariate analysis confirmed this to be an independent
prognostic factor. Induction of exogenous CHODL overexpression
conferred growth and invasive activity to mammalian cells, while
downregulation of CHODL by small interfering RNAs suppressed growth
of lung cancer cells. CHODL is likely to be a prognostic factor and
potential target for the development of anticancer drugs (14).
HCC is prone to rapid progression and metastasis,
leaving cytotoxic chemotherapy ineffective, and making it necessary
to investigate new diagnostic and therapeutic biomarkers. In this
study, we aimed to explore the role of CHODL in HCC migration and
invasion, and to evaluate a possible clinical application.
Materials and methods
Specimens and ethics statement
The use of four human HCC specimens was approved by
the Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University
(Guangzhou, China). Four human normal liver tissue specimens
without hepatitis or cirrhosis were obtained by resection from
patients in Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University,
between August 2012 and April 2014. All specimens were collected in
liquid nitrogen and stored at −80°C. Informed written consent was
obtained from all participating subjects. All human experiments
were conducted with the approval of the Ethics Committee of Sun
Yat-Sen Memorial Hospital, Sun Yat-Sen University, and were
performed in accordance with the ethical standards of the Helsinki
Declaration.
Cell culture
HEK293T cells, the human normal liver cell line L02
and the HCC cell lines HepG2, Hun7, SMMC7721, Bel7402, MHCC-97L and
MHCC-97H were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Cells were grown in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% defined
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin, 100 µg/ml streptomycin and 2 mmol/l glutamine
(Thermo Fisher Scientific, Inc.). The cells were cultured in 5%
CO2 humidified at 37°C.
IHC
Formalin-fixed and paraffin-embedded slides from
human HCC specimens and human normal liver tissue specimens were
incubated with the specific primary anti-CHODL antibody (1:400;
ab76710; Abcam, Cambridge, MA, USA) for 30 min at 37°C, and
subsequently incubated in a moist chamber shaking overnight at 4°C.
This was followed by three 5-min washes with PBS, and slides were
then incubated with the biotin-avidin secondary antibody (1:1,000;
ab7235; Abcam) at 37°C for 30 min. Slides then were washed with PBS
for 5 min (three times), and incubated with an
avidin-biotin-peroxidase complex (1:1,000; Vectastain; Vector
Laboratories, Inc., Burlingame, CA, USA) at 37°C for 30 min. The
sections were then stained with 3,3′-diaminobenzidine and
counterstained with hematoxylin for 2 min, dehydrated in gradient
ethanol, cleared in xylene and cover-slipped with neutral balsam.
Negative controls were included in all steps, which were incubated
with PBS instead of the specific primary antibodies.
RNA isolation and RT-quantitative (q)
PCR
Total RNA was extracted using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, and was subsequently subjected to RT
with QuantiTect Reverse Transcription kit (Qiagen, Inc., Valencia,
CA, USA). The CHODL and GAPDH sequences were obtained from PubMed
GenBank (https://www.ncbi.nlm.nih.gov/nuccore/BC009418.1 and
https://www.ncbi.nlm.nih.gov/nuccore/NM_002046). CHODL
expression was analyzed quantitatively using the miScript PCR
System (Qiagen, Inc.) on a Stratagene MX3500P (Agilent
Technologies, Inc., Santa Clara, CA, USA). The system consists of
the miScript Reverse Transcription kit (Qiagen, Inc.) and the
miScript SYBR Green PCR kit (Qiagen, Inc.). The housekeeping gene
GAPDH was used as an internal control. Gene-specific primers were
used: CHODL-forward (F) 5′-GGAAGGAAAGGAACTACGAAATC-3′ and
CHODL-reverse (R) 5′-GTTAAAAGGAGCACAGGGACATA-3′. Primers for GAPDH
were as follows: GAPDH-F: 5′-GAGTCAACGGATTTGGTCGT-3′ and GAPDH-R:
5′-GACAAGCTTCCCGTTCTCAG-3′. All primers were synthesized by Thermo
Fisher Scientific, Inc. Equal amounts of RNA were reverse
transcribed into complementary (c) DNA. The RT reaction was
performed at 42°C for 30 min and then 70°C for 15 sec. qPCR was
performed using the cDNA as template under the following
conditions: PCR initial activation at 95°C for 15 min, followed by
40 cycles at 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec.
The expression level of CHODL was normalized as relative expression
to GAPDH. Relative expression was calculated as 2-ΔΔCq, where Cq is
the quantification cycle and −ΔΔCq=−(Cq CHODL-Cq GADPH). All
experiments were performed in triplicate.
Western blot analysis
Human HCC samples and normal liver specimens (100 mg
each) were lysed in 1 ml lysis buffer containing 1%
phenylmethanesulfonyl fluoride following the manufacturer's
instructions for the radioimmunoprecipitation assay kit (Beyotime
Institute of Biotechnology). The lysates were centrifuged at 12,000
rpm (9,901 × g) for 15 min at 4°C twice to remove cellular debris.
Protein concentrations in the supernatant were determined by the
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). An equal
amount of protein was separated by 10% SDS-PAGE, then transferred
to polyvinylidene difluoride membranes and blocked with TBS buffer
containing 0.5% Tween 20 (TBST) and 5% non-fat milk for 1 h at room
temperature. Afterwards, the membranes were incubated with the
specific primary antibody against CHODL (1:100; Beyotime Institute
of Biotechnology) overnight at 4°C. After three 7-min washes with
TBST, the blots were incubated with a horseradish
peroxidase-labeled secondary antibody (1:1,000; ab181658; Abcam)
for 1 h at room temperature. The presence of peroxidase was
detected with an enhanced chemiluminescence reagent (Shanghai Long
Island Biotech Co., Ltd.) following three 10-min washes with TBST.
Blots were normalized to GAPDH after background subtraction. The
anti-GAPDH antibody (ab37168) was obtained from Beyotime Institute
of Biotechnology and was diluted 1:100. Each experiment was
repeated in triplicate with similar results.
Construction of lentiviral vectors and
generation of stable cell lines
Total RNA was extracted and subsequently subjected
to RT as mentioned above. The RT reaction was performed at 70°C for
5 min and then 37°C for 30 min. A third generation lentiviral
vector pCDH-CMV-EF1-copGFP (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to overexpress CHODL following the manufacturer's
instructions. The oligonucleotides encoding the designed CHODL
sequence (dihydropyrimidinase like 3) were synthesized by Thermo
Fisher Scientific, Inc. and inserted into the restriction sites
XbaI and EcoRI of the multiple cloning site of the vector.
Infectious lentiviral vectors were harvested at 48 h
post-transfection, and virus titer was calculated by
fluorescence-activated cell sorting analysis of green fluorescent
protein (GFP)-positive HEK293T cells. The titers were then adjusted
to 109 transducing units/ml. Correct CHODL insertions were
confirmed by direct DNA sequencing. HEK293T cells were then
co-transfected with the recombinant replication competent virions
(pCDH-CMV-hCHODL-EF1-copGFP) and three helper plasmids (pGag/Pol,
pRev and pVSV-G)(Shanghai GenePharma Co., Ltd., Shanghai, China)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
SMMC7721 cells were seeded on day 0 and the medium was removed on
day 1. Then, SMMC7721 cells were transduced with the infectious
lentiviruses in fresh transduction medium supplemented with
Polybrene (8 µg/ml; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany). At 24 h post-infection, cells were treated with fresh
medium for 6 h and then cultured in complete medium containing
puromycin (2 µg/ml) for 10–12 days before being used for
experiments. The percentage of GFP-expressing cells and the
fluorescence intensity were observed under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan). CHODL expression
was determined using both RT-qPCR and western blotting
post-transduction, as aforementioned.
Flow cytometry (FCM)
Cell cycle and apoptosis analyses were performed
using propidium iodide (PI) and fluorescein isothiocyanate
(FITC)-Annexin V staining (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). For cell cycle investigation, the human HCC cell line
SMMC7721 and the normal liver cell line L02 were seeded in 96-well
plates at 1×104 cells per well. At 24, 48 and 72 h after
transduction, the cells were lysed with TRIzol for 30 min in an ice
bath, and then trypsinized (Invitrogen; Thermo Fisher Scientific,
Inc.) and washed twice with precooled PBS. Cells were centrifuged
at 3,000 rpm (619 × g) for 5 min at 4°C and fixed in 70% precooled
ethanol for 24 h at 4°C. A total of 1×105 cells were stained with
PI for 10 min. The cell cycle distribution was analyzed by FCM
(FACSCalibur; BD Biosciences, San Jose, CA, USA). For the apoptosis
analysis, 1×105 cells were trypsinized (Invitrogen; Thermo Fisher
Scientific, Inc.) and washed with precooled PBS. Cells were
collected by centrifugation at 3,000 rpm (619 × g) for 5 min at 4°C
and fixed with 75% precooled alcohol. The cells were then incubated
with FITC-Annexin V and PI for 5 min and analyzed by FCM. Each
experiment was repeated in triplicate with similar results.
Cell proliferation assay
A total of 1×104 post-transfection SMMC7721 cells
were seeded into 96-well plates and cultured overnight at 5%
CO2 humidified at 37°C. Once the cells were completely
adherent to the wells, at 20, 44 and 68 h, 20 µl 5 mg/ml MTT
(Sigma-Aldrich; Merck Millipore) was added to the plates, and the
cells were cultured for an additional 4 h. Culture medium was then
removed, and 150 µl dimethyl sulfoxide was added to the plates,
followed by a 15-min incubation. The absorbance at 490 nm was
measured to determine the number of viable cells in each well. All
experiments were performed in triplicate.
Cell Matrigel migration and invasion
assays
For the invasion assays, Transwell upper chambers
(Costar; Corning Incorporated, Corning, NY, USA) (8 µm) were
incubated with Matrigel (BD Biosciences) (2.5 mg/ml, diluted in
serum-deprived medium) for 5 h at 37°C. In total, 1.0×105 SMMC772
cells were seeded in the upper Transwell chambers, and 500 µl
substrate containing 10% FBS was added into the lower chamber.
Cells were incubated for 24 h, washed twice with PBS and fixed with
4% paraformaldehyde. Finally, the insert membranes were stained
with crystal violet. Ten fields were randomly chosen, and the
permeating cells were counted for each membrane under an inverted
microscope (Leica Microsystems GmbH, Wetzlar, Germany). For the
migration assay, the insert membranes were not coated with
Matrigel, and the cells were cultured under the same conditions.
Each experiment was repeated in triplicate with similar
results.
Statistical analysis
Data are expressed as means ± standard deviation,
and the Student's t test was used to compare the two groups.
One-way analysis of variance was used when comparing multiple
groups, followed by the least significant difference t test to look
for differences amongst groups. Statistical analyses were performed
using SPSS v19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
CHODL is significantly downregulated
in HCC clinical samples and cell lines
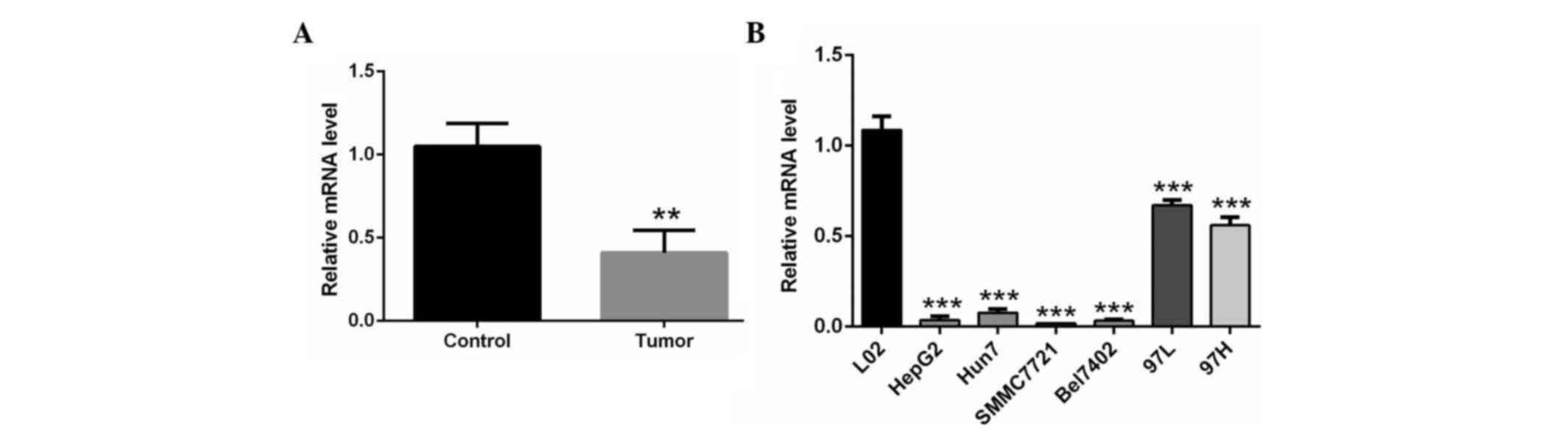
RT-qPCR analyis showed that CHODL mRNA was
significantly lower in the HCC clinical samples than in the normal
liver tissues (P<0.01) (Fig. 1A).
Similar results were obtained in HCC cell lines. CHODL mRNA
expression was downregulated in the HCC cell lines HepG2, Hun7,
SMMC7721, Bel7402, MHCC-97L and MHCC-97H compared with the human
normal liver cell line L02 (P<0.001) (Fig. 1B). The expression of CHODL was lowest
in SMMC7721 cells (Fig. 1B).
Therefore, SMMC7721 cells were selected for subsequent analysis to
elucidate the possible association between CHODL and liver
metastasis.
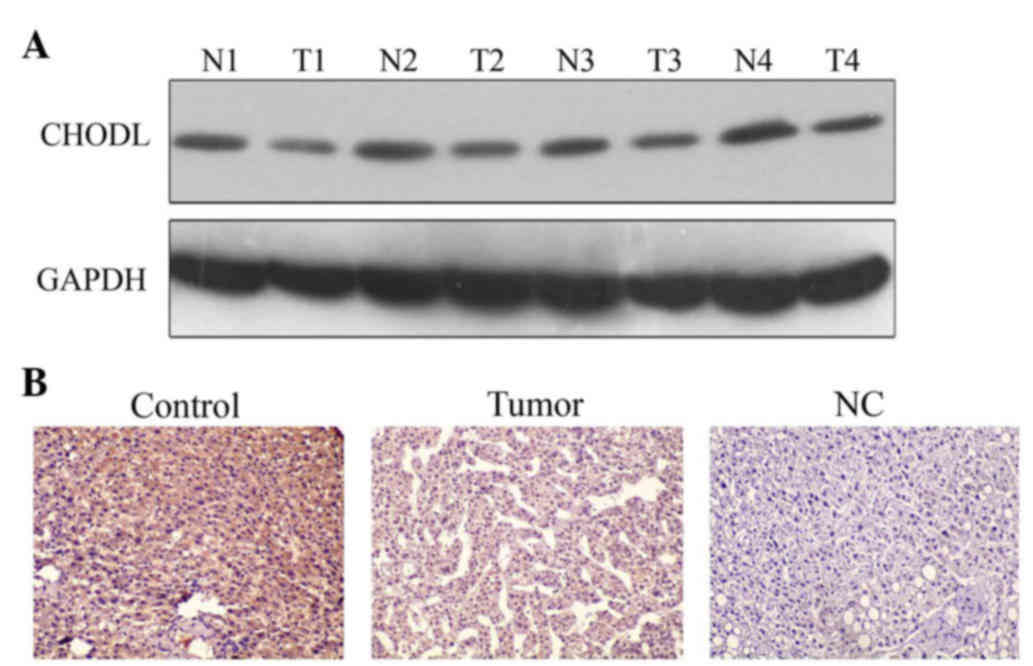
A western blot assay was used to detect protein
expression of CHODL. The results showed that CHODL expression in
all four HCC clinical samples was lower than in the normal liver
tissues (Fig. 2A). IHC staining was
performed to investigate the expression of CHODL in the four HCC
clinical samples. In accord with the western blot results, CHODL
staining in human normal liver tissues was positive (uniformly
yellow), but was negative in HCC samples (Fig. 2B).
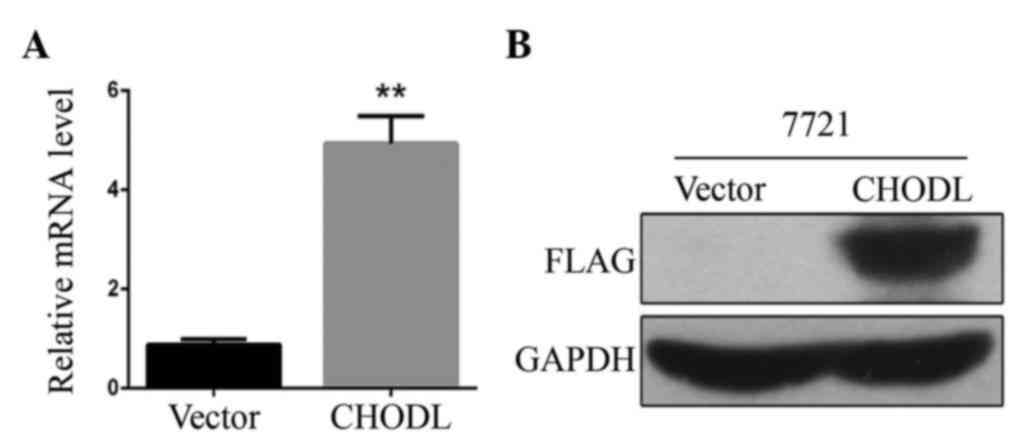
Lentiviral vector mediates
overexpression of CHODL in SMMC7721 cells
SMMC7721 cells were transduced with the recombinant
lentiviral CHODL overexpression vector or with empty vector.
RT-qPCR analysis was performed to quantify the upregulation of
CHODL mRNA levels in SMMC7721 cells. CHODL mRNA expression was
increased by ~5-fold over the control (P<0.01) (Fig. 3A). Western blotting confirmed CHODL
overexpression in SMMC7721 cells. The protein expression level of
CHODL in the overexpression group was significantly upregulated
compared with that in the mock vector group (Fig. 3B). These results showed that CHODL
expression was increased by the lentiviral vectors.
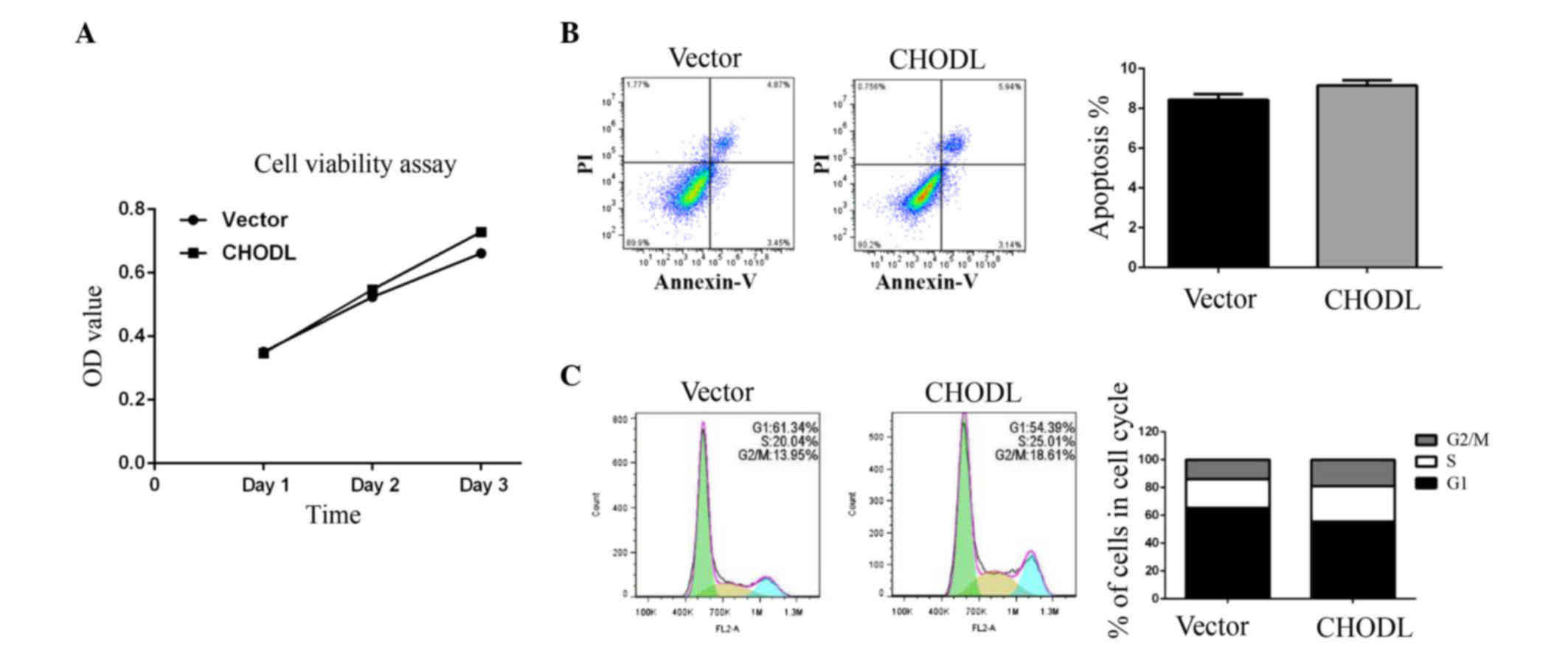
Overexpression of CHODL increases
migration and invasion in SMMC7721 cells
MTT assays and FCM results demonstrated that cell
metabolism (Fig. 4A) and apoptosis
(Fig. 4B) were not significantly
different between CHODL-overexpressing cells and control cells.
However, the CHODL overexpression group displayed more cells in the
G2/M phase than the control group (Fig.
4C).
To determine whether upregulation of CHODL was
correlated to HCC migration, we used Transwell assay to determine
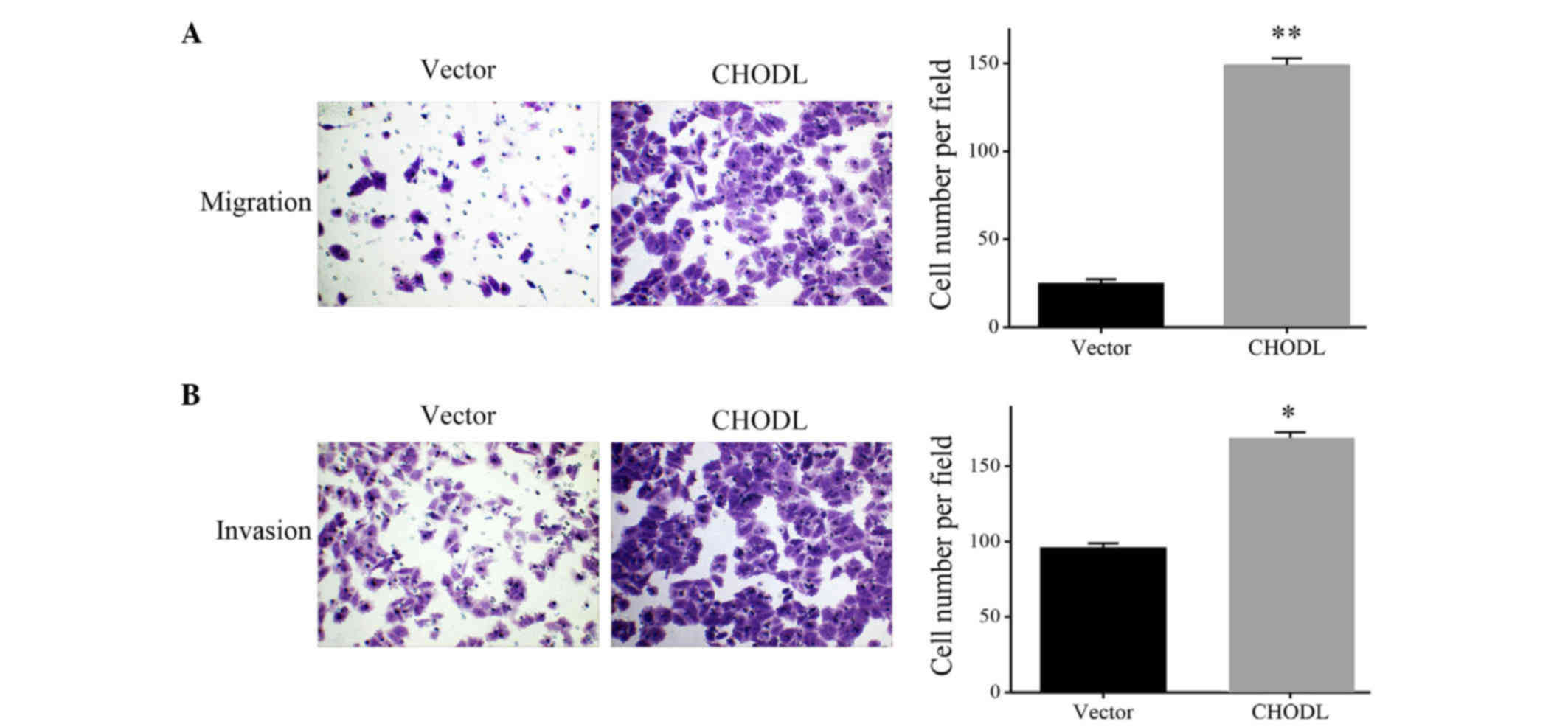
the ability of cell migration and invasion. As shown in Fig. 5A, increased migrating cell numbers
were observed in the CHODL-overexpressing SMMC7221 cells,
indicating a possible association between CHODL and HCC migration
(P<0.01) (Fig. 5A). To further
elucidate the effect of CHODL on HCC cell invasion, cellular
invasion was examined via Matrigel assay. The upregulation of CHODL
in SMMC7721 cells significantly enhanced the cells invasive
activity (Fig. 5B). These results
suggested a contribution of CHODL to the high risk of malignant
metastasis in HCC.
Discussion
HCC is the fifth most common cancer in men and the
seventh in women (15). Liver
cirrhosis and/or chronic viral hepatitis have been proposed as a
predisposing factor for hepatocarcinogenesis (1,6). Chronic
infection with hepatitis B virus (HBV) represents a major risk
factor for HCC in Southeast Asia and Africa, while lingering
infection with hepatitis C virus is the predominant risk factor for
HCC in Western countries and Japan (2,16,17). At the asymptomatic early stages of
HCC, ablative therapies, surgical resection or liver
transplantation are the first-line treatment (18,19).
However, treatment options are limited for most advanced stage
patients due to HCC intra-hepatic expansion and/or poor liver
function (20).
Chronic hepatocyte injury, including continuous
inflammatory and oxidative stress, may cause regenerative stimuli
in hepatic cells (5,17). This kind of cellular proliferation may
lead to mutations in genes controlling proliferation and/or
apoptosis, thus leading to hepatic tumorigenesis (6,17). HCC is
a chemoresistant tumor, and conventional pharmacological
chemotherapy has limited efficacy in clinical benefits and/or
prolonged survival (1,17). Therefore, it is imperative to
investigate HCC biomarkers related to cell proliferation and
migration, which may help to provide curative options for HCC
prevention and intervention.
The human CHODL protein belongs to the C-type lectin
family and contains an N-terminal signal sequence, a single CRD, a
transmembrane region and an intracellular C terminus (8–10). Animal
lectin CRDs have been found in many kinds of proteins, including
selectin, mannose-binding protein, proteoglycan core protein,
lymphocyte immunoglobulin E receptor and hepatic asialoglycoprotein
receptor (10). The C-type CRDs
provide calcium-dependent sugar-binding activity and are involved
in many processes, including cell recognition and communication,
cell-cell adhesion, and ECM-cell interactions, steps that are
involved in and crucial for tumor cell proliferation, migration and
invasion (9,14). The full-length CHODL protein is
expressed in various human tissues and co-localizes with rBet1, a
transmembrane protein that mediates protein transport between the
endoplasmic reticulum (ER) and the Golgi apparatus, indicating an
important role for CHODL in protein processing (10).
Masuda et al showed that CHODL was increased
in NCSLC and was associated with poor prognosis in patients
(14). CHODL protein was mainly
co-localized with calreticulin (an ER marker) and was expressed in
the majority of NSCLC tissues, with the expression of the CHODL
transcript being upregulated 5-fold or greater in 63% of NSCLCs
compared with normal lung tissue. Transfection of exogenous CHODL
into the lung cancer cell line LC319 significantly enhanced cell
growth and invasion, indicating that CHODL was associated with lung
oncogenesis and tumor progression (14).
Our study showed that CHODL was significantly
decreased in HCC clinical samples compared with normal liver tissue
at both the mRNA and protein levels. Similarly, CHODL
downregulation was also observed in HCC cell lines in vitro.
Lentiviral overexpression assays for CHODL revealed that CHODL
could improve HCC cell line SMMC7721 migration and invasion in
vitro, but did not affect HCC proliferation. These data
suggested a possible contribution of CHODL to the high risk of
malignant metastasis in HCC.
This result was quite different from previous data
in lung cancer (14). In general,
CHODL expression in normal human tissues is very low (8,13,14). In the SMA mouse spinal cord, CHODL-001
isoform expression was reduced before disease onset, and the
reduced expression of CHODL-001 in mice survival motor
neuron-depleted NSC-34 cells improved neurite outgrowth (11). Adult liver cells retain a remarkable
capacity to proliferate in response to liver injury, and can
re-enter the cell cycle and divide once or twice before returning
to a state of quiescence (17).
Hepatic nodules in patients with chronic liver diseases are
subdivided into regenerative nodules, low-grade dysplastic nodules,
high-grade dysplastic nodules, well-differentiated HCC,
moderately-differentiated HCC and poorly-differentiated HCC,
showing an ascending order of histological grades and representing
a sequence of multistep hepatocarcinogenesis (17). Our study showed that CHODL
overexpression led to a higher proportion of cells in the G2/M
phase than the control group, which may explain the lower CHODL
expression in HCC tissues and in HCC cell lines as compared with
the normal liver tissues and cell line (L02), respectively.
Efficient and long-term gene transfer is essential
for clinical gene therapy, of which intensity and duration of
transgenic expression are the two main parameters (21). Chemically synthesized small hairpin
RNAs delivered to target cells are known to be transient, lasting
only 3–4 days, with low transfection efficiency (16,22).
Lentiviral vectors can achieve 70% gene transfer efficacy in both
dividing and nondividing hepatocarcinoma cells over a 16-h
transduction period with a multiplicity of infection of 1 (4,21,23). It is noteworthy that lentiviral
vectors can efficiently transduce HCC cells in the context of HBV
infection, which makes gene therapy protocols in combination with
other therapeutic approaches (surgery and/or chemotherapy) feasible
(16,22). Although there are biosafety concerns,
including potential insertional mutagenesis and vector-related
toxic side effects to the liver, the current third generation human
immunodeficiency virus-1-based lentiviral vectors have minimized
the potential risk (16,24).
In summary, while the exact molecular mechanisms
behind CHODL involvement in HCC tumorigenesis remain to be studied,
our investigation suggests that CHODL plays a role in proliferation
and aggression of HCC. CHODL may be a candidate therapeutic and
prognostic biomarker for liver cancer.
In this study, we found that the expression of CHODL
was significantly lower in HCC clinical samples and in HCC cell
lines than in normal liver tissue. Lentivirus-mediated
overexpression of CHODL in SMMC7721 liver cancer cells
significantly increased cell migration and invasion. Our study
demonstrates for the first time a positive correlation between
CHODL and HCC metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81372562) and the Medical Scientific Research Foundation of
Guangdong Province, China (grant no. A2015462)
References
|
1
|
Ferrin G, Aguilar-Melero P,
Rodríguez-Perálvarez M, Montero-Álvarez JL and de la Mata M:
Biomarkers for hepatocellular carcinoma: Diagnostic and therapeutic
utility. Hepat Med. 7:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong YP, Li ZD, Prasoon P and Zhang Q:
Immunotherapy for hepatocellular carcinoma: From basic research to
clinical use. World J Hepatol. 7:980–992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerolami R, Uch R, Jordier F, Chapel S,
Bagnis C, Bréchot C and Mannoni P: Gene transfer to hepatocellular
carcinoma: Transduction efficacy and transgene expression kinetics
by using retroviral and lentiviral vectors. Cancer Gene Ther.
7:1286–1292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pez F, Lopez A, Kim M, Chapel S, Bagnis C,
Bréchot C and Mannoni P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatia D, Thoppil RJ, Mandal A, Samtani
KA, Darvesh AS and Bishayee A: Pomegranate bioactive constituents
suppress cell proliferation and induce apoptosis in an experimental
model of hepatocellular carcinoma: Role of Wnt/β-catenin signaling
pathway. Evid Based Complement Alternat Med. 2013:3718132013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manu KA, Shanmugam MK, Ong TH, Subramaniam
A, Siveen KS, Perumal E, Samy RP, Bist P, Lim LH, Kumar AP, et al:
Emodin suppresses migration and invasion through the modulation of
CXCR4 expression in an orthotopic model of human hepatocellular
carcinoma. PLoS One. 8:e570152013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weng L, Smits P, Wauters J and Merregaert
J: Molecular cloning and characterization of human chondrolectin, a
novel type I transmembrane protein homologous to C-type lectins.
Genomics. 80:62–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weng L, Hubner R, Claessens A, Smits P,
Wauters J, Tylzanowski P, Van Marck E and Merregaert J: Isolation
and characterization of chondrolectin (Chodl), a novel C-type
lectin predominantly expressed in muscle cells. Gene. 308:21–29.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weng L, Van Bockstaele DR, Wauters J, Van
Marck E, Plum J, Berneman ZN and Merregaert J: A novel alternative
spliced chondrolectin isoform lacking the transmembrane domain is
expressed during T cell maturation. J Biol Chem. 278:19164–19170.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sleigh JN, Barreiro-Iglesias A, Oliver PL,
Biba A, Becker T, Davies KE, Becker CG and Talbot K: Chondrolectin
affects cell survival and neuronal outgrowth in in vitro and in
vivo models of spinal muscular atrophy. Hum Mol Genet. 23:855–869.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong Z, Ohnmacht J, Reimer MM, Bach I,
Becker T and Becker CG: Chondrolectin mediates growth cone
interactions of motor axons with an intermediate target. J
Neurosci. 32:4426–4439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Claessens A, Van de Vijver K, Van
Bockstaele DR, Wauters J, Berneman ZN, Van Marck E and Merregaert
J: Expression and localization of CHODLDeltaE/CHODLfDeltaE, the
soluble isoform of chondrolectin. Cell Biol Int. 31:1323–1330.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuda K, Takano A, Oshita H, Akiyama H,
Tsuchiya E, Kohno N, Nakamura Y and Daigo Y: Chondrolectin is a
novel diagnostic biomarker and a therapeutic target for lung
cancer. Clin Cancer Res. 17:7712–7722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dang H, Steinway SN, Ding W and Rountree
CB: Induction of tumor initiation is dependent on CD44s in c-Met+
hepatocellular carcinoma. Bmc Cancer. 15:1612015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng L, Li G, Xi L, Yin A, Gao Y, You W,
Wang X and Sun B: Hepatitis B virus inhibition in mice by
lentiviral vector mediated short hairpin RNA. BMC Gastroenterol.
9:732009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altimari A, Fiorentino M, Gabusi E,
Gruppioni E, Corti B, D'Errico A and Grigioni WF: Investigation of
ErbB1 and ErbB2 expression for therapeutic targeting in primary
liver tumours. Dig Liver Dis. 35:332–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forner A, Hessheimer AJ, Real M Isabel and
Bruix J: Treatment of hepatocellular carcinoma. Crit Rev Oncol
Hematol. 60:89–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK,
Yeung C and Wong J: Hepatectomy for hepatocellular carcinoma:
Toward zero hospital deaths. Ann Surg. 229:322–330. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang LJ, Urlacher V, Iwakuma T, Cui Y and
Zucali J: Efficacy and safety analyses of a recombinant human
immunodeficiency virus type 1 derived vector system. Gene Ther.
6:715–728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Butterfield LH, Fu X, Song Z, Zhang
X, Lu C, Ding G and Wu M: Lentivirally engineered dendritic cells
activate AFP-specific T cells which inhibit hepatocellular
carcinoma growth in vitro and in vivo. Int J Oncol. 39:245–253.
2011.PubMed/NCBI
|
|
23
|
Wang Q, Liu QY, Liu ZS, Qian Q, Sun Q and
Pan DY: Lentivirus mediated shRNA interference targeting MAT2B
induces growth-inhibition and apoptosis in hepatocelluar carcinoma.
World J Gastroenterol. 14:4633–4642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kafri T, Blomer U, Peterson DA, Gage FH
and Verma IM: Sustained expression of genes delivered directly into
liver and muscle by lentiviral vectors. Nat Genet. 17:314–317.
1997. View Article : Google Scholar : PubMed/NCBI
|