Introduction
Breast cancer is one of the most common malignancies
in women worldwide and accounts for >15% of all female cancer
mortalities as a result of tumor proliferation and metastasis
(1). Excessive proliferation of
tumors is the most serious characteristic of neoplastic cells,
which have a crucial role in the imbalance of tissue homeostasis
(2,3).
Adriamycin is the most effective chemotherapeutic
agent in the treatment of breast cancer (4,5). However,
adriamycin efficacy is often limited by the emergence of resistance
and adverse reactions (6,7). Therefore, improvements in the clinical
application of adriamycin and the reduction of its adverse
reactions are crucial.
Gap junctions (GJs) formed by connexins are the only
communication junctions identified in animal tissues, and are
responsible for the direct trafficking of ions, molecules and
several second messengers, including inositol 1,4,5-trisphosphate,
Ca2+, glutathione and cyclic adenosine monophosphate (8). Connexins that form GJ channels are
involved in the exchange of molecular signals in the cytoplasm of
neighboring cells (9,10). Decreased expression of connexins
and/or absence of GJ intercellular communication (GJIC) have been
associated with tumor phenotype (11,12).
Intercellular junctions are important in the maintenance of
cellular homeostasis, cell differentiation and cellular death
(13). In normal mammary tissues,
connexin 43 (Cx43) was shown to be mostly present in the
myoepithelial cells and to be required for myoepithelial
differentiation (14). Lack of Cx43
was a common feature of human mammary cancer tissues compared with
non-neoplastic breast tissues surrounding primary tumors (15). Additionally, connexins have been
reported to have functions independent of GJIC (16,17). Qin
et al (18) reported that the
tumor growth of human breast cancer cells (MDA-MB-231) transfected
with the Cx43 gene was suppressed independently of GJIC.
In the present study, the expression of Cx43 was
determined in breast cancer cells with different malignancy degree.
GJ potentiators/inhibitors and Cx43 mall interfering RNA (siRNA)
were used to regulate the function of GJs in order to certify
whether the modulation of adriamycin cytotoxicity was dependent or
independent on GJs. In summary, the current study will illustrate
the association between GJIC and the antineoplastic effect of
adriamycin in breast cancer cells.
Materials and methods
Materials
Adriamycin, retinoic acid (RA), oleamide and
18-α-glycyrrhetinic acid (18-α-GA) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Anti-Cx43
(cat. no. C8093) and anti-β-actin (cat. no. A5441) primary
antibodies, and alkaline phosphatase-conjugated goat anti-mouse
secondary antibodies, were acquired from Sigma-Aldrich (Merck
Millipore). IgG-fluorescein isothiocyanate (FITC) for
immunofluorescence (cat. no. LK-GAR4882) was purchased from
Sigma-Aldrich (Merck Millipore). Calcein-acetoxymethyl ester
(Calcein-AM) and Lipofectamine™ 2000 were acquired from Invitrogen
(Thermo Fisher Scientific, Inc.). All other reagents were obtained
from Sigma-Aldrich (Merck Millipore) unless stated otherwise.
Cell lines and cell culture
Human breast cancer cell lines (Hs578T, MDA-MB-231
and SK-BR-3) (American Type Culture Collection, Manassas, VA, USA)
were grown in Dulbecco's modified Eagle's medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin/streptomycin. MCF-7 cells were maintained in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing
10% (v/v) FBS. All cell lines were grown at 37°C in a humidified
atmosphere containing 95% air and 5% carbon dioxide.
Chemicals
All chemicals were prepared as stock solutions and
stored at −20°C in aliquots. Working solutions were freshly diluted
at the time of the experiment. Stock solutions of adriamycin were
prepared at 1 mmol/l in PBS. All exposures to adriamycin were
performed in the dark. 18-α-GA was dissolved in dimethyl sulfoxide
(DMSO) at 10 mM and diluted to a final concentration of 10 µM in
culture medium, while oleamide was dissolved in DMSO at 25 mM and
diluted to a final concentration of 25 µM in culture medium, and
then they were added to the cells prior to adriamycin
treatment.
Modulation of GJIC
For potentiation, cells were incubated with the GJ
potentiator RA (10 µM in DMSO) 24 h prior to adriamycin exposure
and during the adriamycin treatment. For inhibition, cells were
incubated with two GJ inhibitors, 18-α-GA (10 µM in DMSO) and
oleamide (25 µM in DMSO) prior to adriamycin exposure and during
the adriamycin treatment. Control cells were incubated with DMSO
alone.
Western blotting
Western blot assays were conducted as reported in
previous studies (19). Cells were
washed three times with cold PBS. Then, cell lysates were prepared
with cell wash buffer (Beyotime Institute of Biotechnology, Haimen,
China) followed by 1-h incubation in lysis buffer [Tris·HCl (pH
7.4) 20 mM, NaCl 150 mM, EDTA 1 mM, Triton 1%, sodium pyrophosphate
2.5 mM, Na3VO4 1 mM, β-glycerophosphate 1 mM
and protease inhibitors 1:1,000] on ice. Protein concentration was
determined using the BCA Protein Assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). A total of 20 µg of protein from each
sample was separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
(w/v) skimmed dry milk in wash buffer [0.01 mol/l PBS (pH 7.4) and
0.05% (v/v) Tween 20]. Next, the membranes were incubated with
monoclonal antibodies against Cx43 (1:4,000) or β-actin (1:2,000)
at 4°C overnight, followed by incubation with the alkaline
phosphatase-conjugated goat anti-mouse secondary antibody (1:4,000)
for 2 h at room temperature. The immunopositive bands were
visualized using the Immobilon Western™ Chemiluminescent HRP
Substrate (Merck Millipore) and quantified using Quantity One 4.62
software (Bio-Rad Laboratories, Inc.).
Immunofluorescence
Cells were grown to confluence on 6-well plates.
Cells were fixed with 3.7% neutral buffered formalin in PBS
containing Tween 20 (PBST) and permeabilized with ice-cold methanol
for 30 min at −20°C. Cover slips were blocked with 5% bovine serum
albumin (BSA) (Sigma-Aldrich; Merck Millipore) in PBST for 2 h, and
then incubated with the primary antibody overnight at 4°C.
Anti-Cx43 antibody diluted 1:100 in 2% BSA in PBST was used.
Samples were washed three times with PBST the next day, followed by
the addition of secondary anti-mouse IgG FITC-conjugated antibody
at 1:200 dilution in 2% BSA in PBST for 2 h at room temperature (in
the dark). Cover slips were subsequently washed three times with
PBST for 5 min and then stained with DAPI (100 ng/ml)
(Sigma-Aldrich; Merck Millipore) in order to count the cells.
‘Parachute’ dye-coupling assay
The assay for GJ function was performed as described
by Hong et al (20) and Tong
et al (21). Donor cells and
receiver cells were grown to confluence in 12-well plates. Donor
cells from one well were double-labeled with 5 µM calcein-AM, which
is converted intracellularly into the GJ-permeable dye calcein.
Calcein-AM was freshly made as a solution of 10 µg/ml. Donor cells
were then trypsinized and seeded onto the receiver cells at a 1:150
donor:receiver ratio. Donor cells were allowed to attach to the
monolayer of receiver cells and form GJs for 4–5 h at 37°C, and
then examined with a fluorescence microscope (CK40; Olympus
Corporation, Tokyo, Japan) and photographed. For each experimental
condition, the average number of receiver cells containing dye per
donor cell was visually determined and normalized to that of
control cultures.
MTT assay
Cells were plated in a 96-well plate at a density of
1×104 cells/ml and, after 24 h, the cells were pretreated with RA
for 24 h, oleamide for 1 h or 18-α-GA for 1 h prior to treatment
with 6 µM adriamycin for 24 h. Cell viability was assessed by MTT
(Sigma-Aldrich; Merck Millipore) reduction assay at the appropriate
time points, and the absorbance was read at 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
RNA interference
Chemically synthesized siRNAs, which were acquired
from Shanghai GenePharma Co., Ltd. (Shanghai, China) were
transfected using TransMessenger Transfection Reagent (Qiagen GmbH,
Hilden, Germany) according to the manufacturer's specifications.
Briefly, cells were subcultured into 6-well plates containing glass
cover slip inserts and incubated under their normal growth
conditions. On the day of transfection, cells were washed with
Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.) twice. Then,
Opti-MEM and siRNA complex mixed with TranMessenger Transfection
Reagent was applied to the cells and incubated for 6 h without
antibiotics. For transiently transfected cells, a synthetic siRNA
targeting Cx43 (target sequence, 5′-GGAAGCACCAUCUCUAACUTT-3′) or
negative control siRNA (target sequence,
5′-UUCUCCGAACGUGUCACGUTT-3′) were transfected into cells by
LipofectamineTM 2000. Cells were changed to a regular cell culture
medium 48 h later. Cells were either fixed for immunocytochemical
studies, or the cell lysates were collected and prepared for
western blot analysis.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical significance was determined with one-way
analysis of variance using SPSS version 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
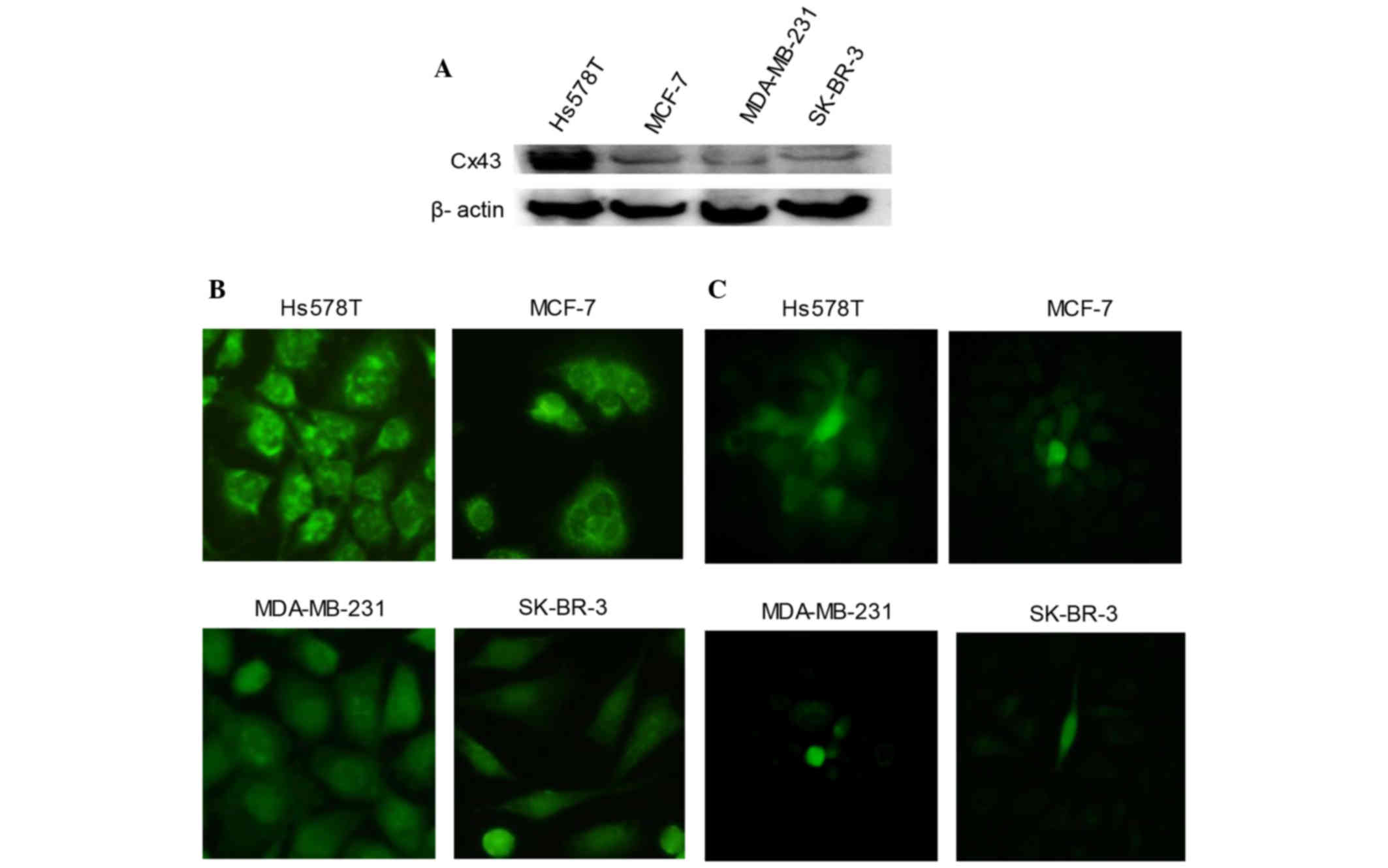
Expression of Cx43 in breast cancer
cells
‘Parachute’ dye-coupling assay was used to detect
the function of GJ in human breast cancer cells. Fig. 1 indicates that Hs578T and MCF-7 formed
complete GJs. However, there was no effective GJ formed in
MDA-MB-231 or SK-BR-3 cells.
In four types of human mammary cancer cells, western
blotting and immunofluorescence were used to detect total Cx43
protein (Fig. 1A) and Cx43 protein on
the membrane of cells (Fig. 1B),
respectively. Fig. 1A and B
demonstrate that Hs578T cells expressed the highest level of Cx43,
followed by MCF-7 and SK-BR-3 cells, while MDA-MB-231 cells
expressed the lowest level of Cx43.
Since MDA-MB-231 and SK-BR-3 cells could not form
effective GJs (Fig. 1C), the
subsequent experiments were conducted on Hs578T and MCF-7 cells,
which expressed higher Cx43 protein levels, to detect the influence
of GJ potentiators/inhibitors on the cytotoxicity of
adriamycin.
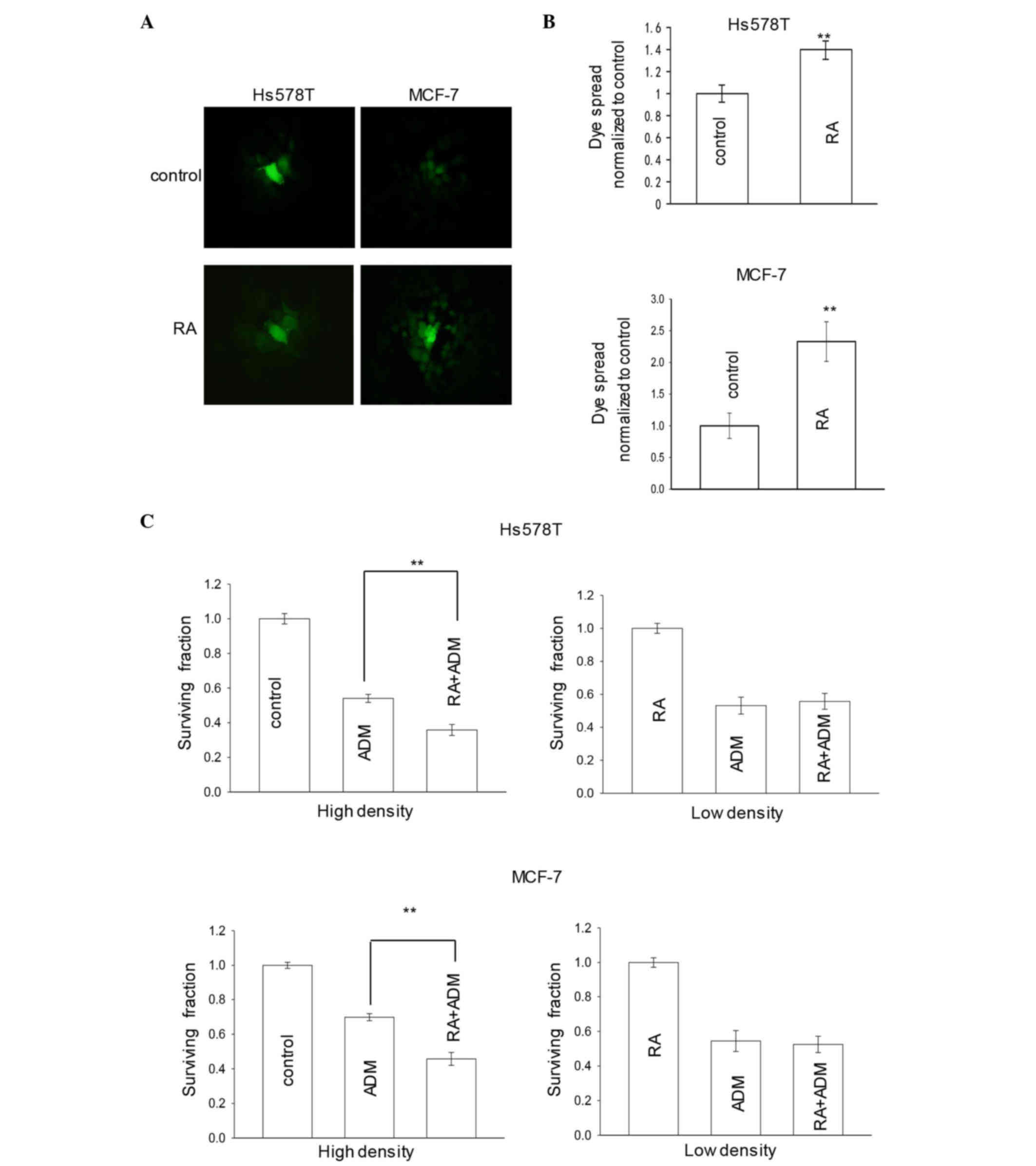
Influence of GJ potentiator on the
cytotoxicity of adriamycin
To determine whether GJIC was required for the
increase of adriamycin-induced cytotoxicity in cells with GJIC, the
breast cancer cells Hs578T and MCF-7 were treated with chemical
modulators of GJs prior and during exposure to 6 µM adriamycin
[inhibitor, oleamide (22,23) or 18-α-GA (24,25);
potentiator, RA (21,26)] at high density (GJ formed) or low
density (no GJ formed).
Fig. 2A and B revealed
that treatment of Hs578T or MCF-7 cells with 10 µM RA for 24 h
increased intercellular dye coupling through GJs. Fig. 2C illustrated that pretreatment of
cells with RA for 24 h increased the cytotoxicity of adriamycin,
leading to a substantially decreased surviving fraction in
high-density cultures. However, at low-cell density, there was very
little effect of RA on adriamycin toxicity. The results suggested
that the enhancement of GJ function by RA increases the effect of
adriamycin cytotoxicity (Fig.
2C).
Overall, the data indicated that the density
dependence of adriamycin responses was a function of GJIC, and that
RA increased the cytotoxicity of adriamycin in breast cancer cell
lines.
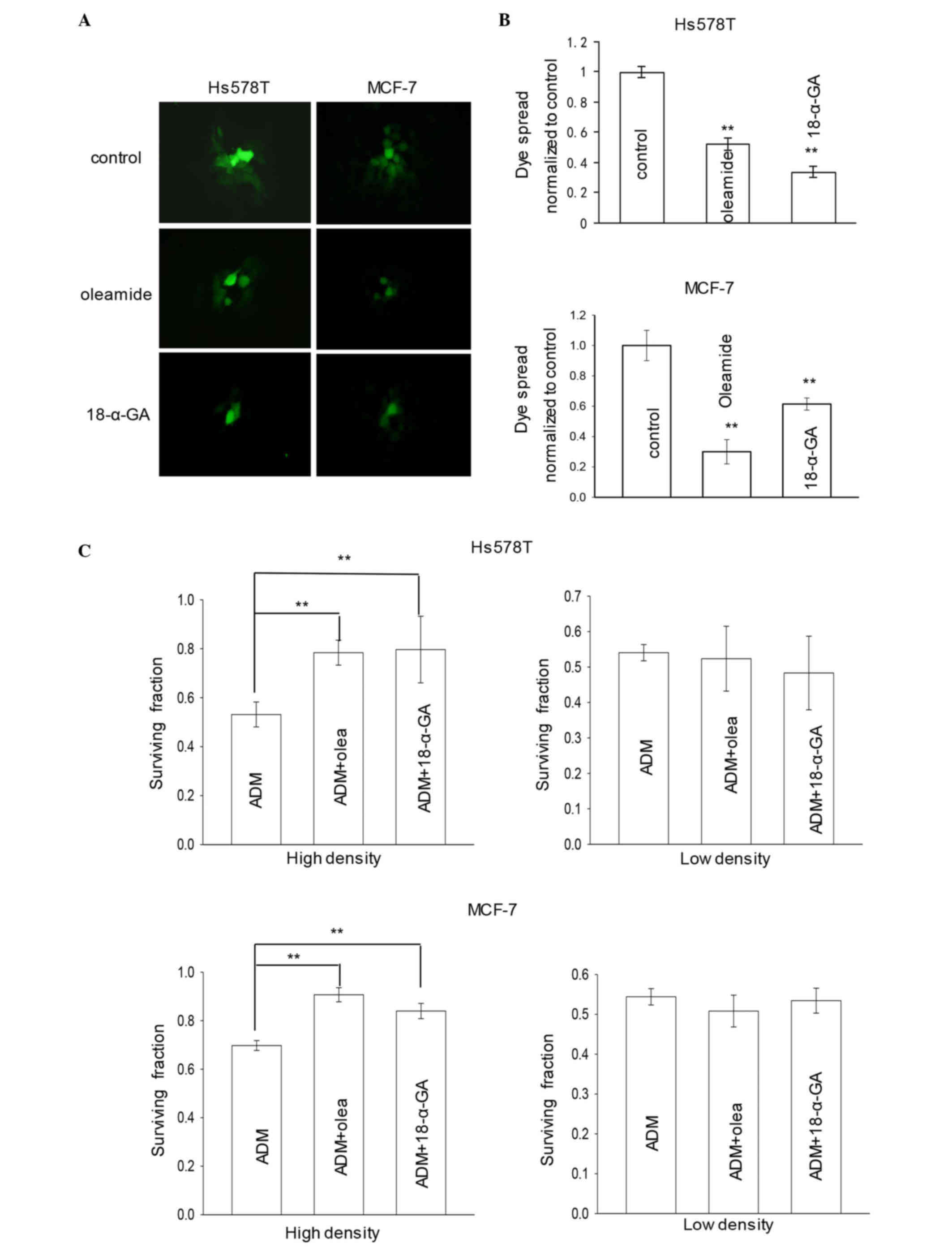
Influence of GJ inhibitors on the
cytotoxicity of adriamycin
The ‘parachute’ dye-coupling assay was used to
determine the effect of oleamide or 18-α-GA on the GJIC of mammary
cancer cells expressing Cx43. Fig. 3A and
B indicated that treatment with 25 µM oleamide or 10 µM 18-α-GA
for 1 h significantly inhibited GJIC in the cells, according to the
results of the dye-transfer assay. As presented in Fig. 3C, pretreatment of cells with oleamide
or 18-α-GA for 1 h reduced the cytotoxicity of adriamycin,
resulting in substantially increased survival in high-density
cultures. However, at low density, there was very little effect of
GJ inhibitor on adriamycin toxicity (Fig.
3C). The results suggest that inhibition of GJ function by
oleamide or 18-α-GA decreases adriamycin cytotoxicity in breast
cancer cell lines.
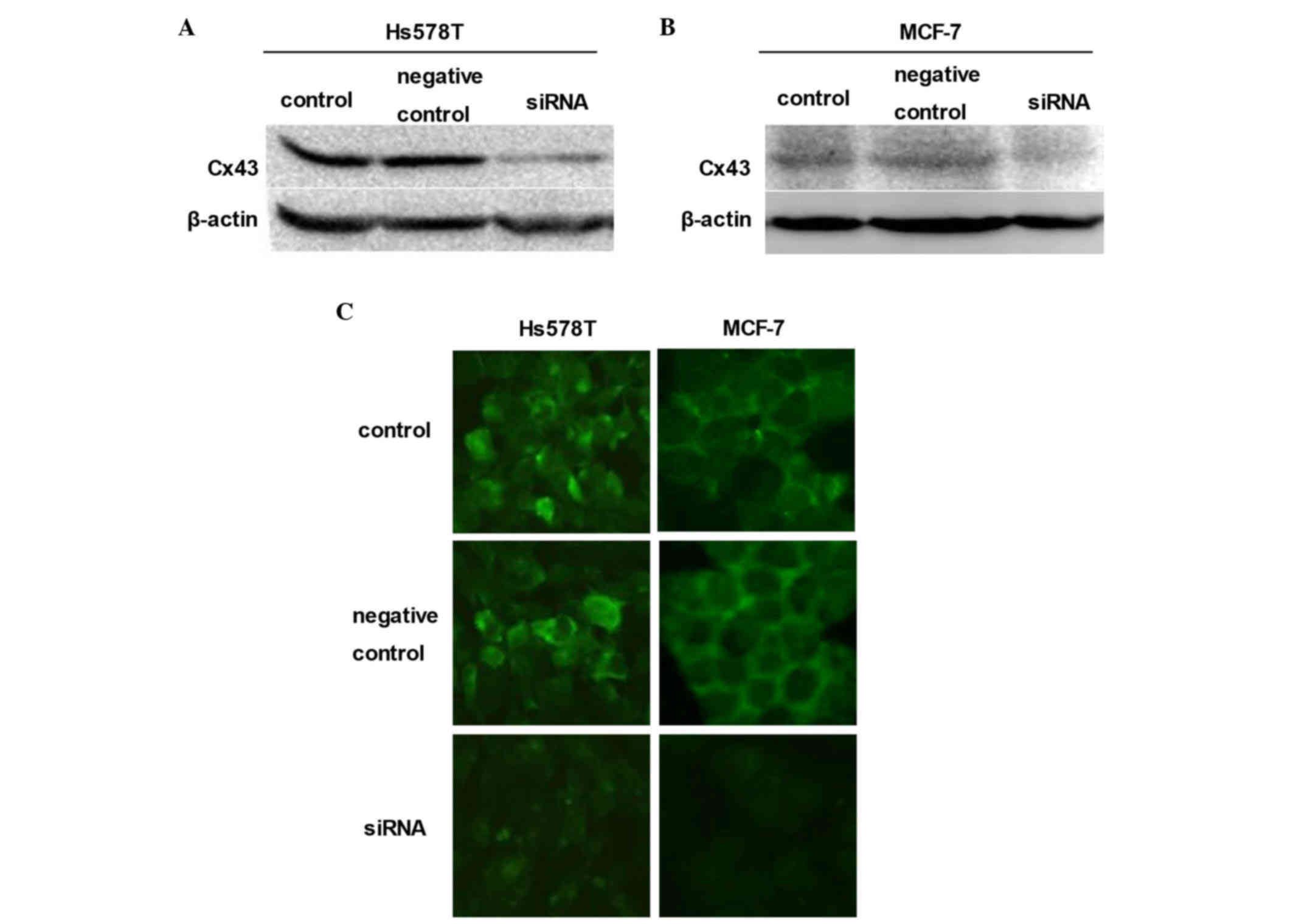
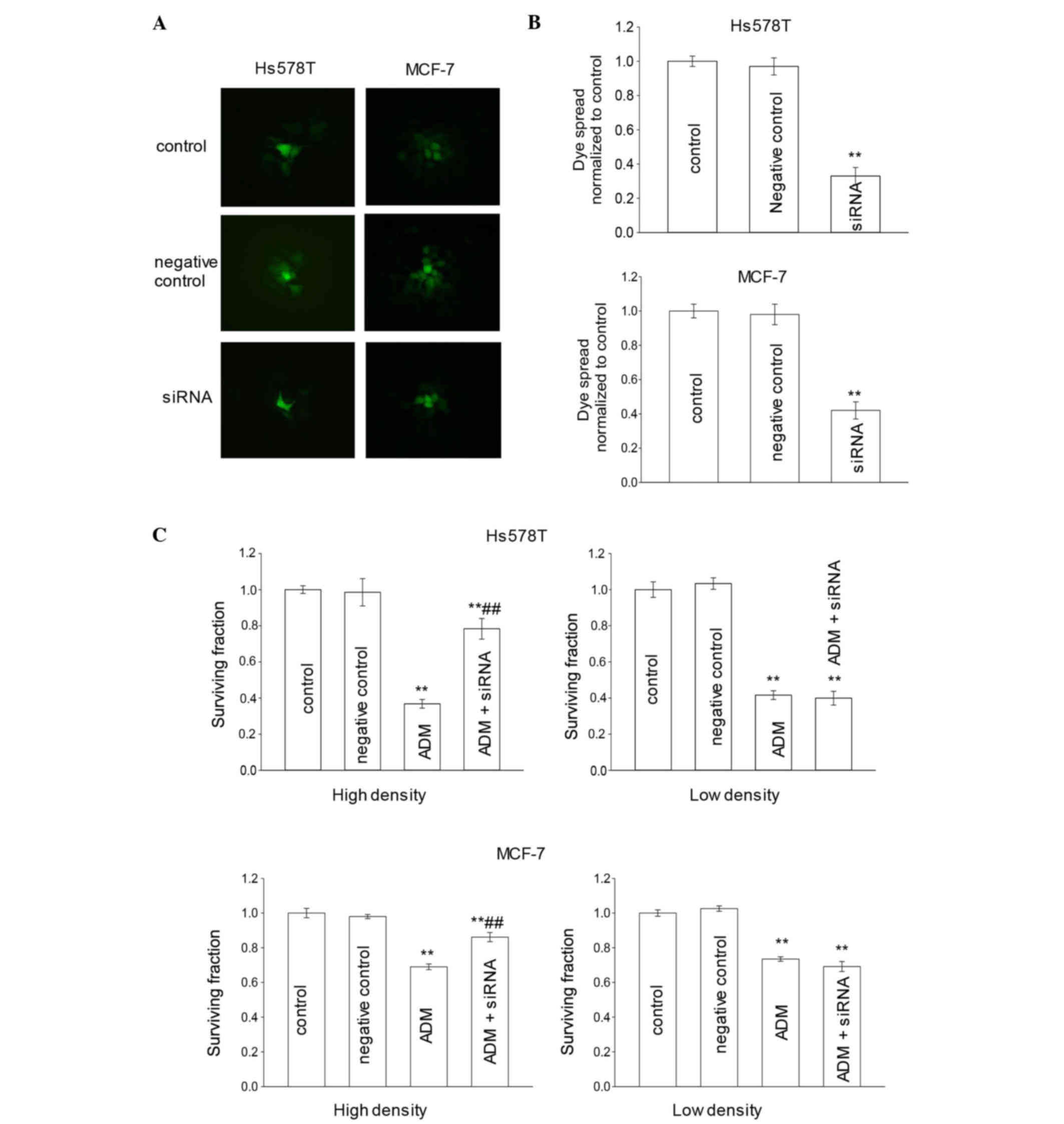
Influence of siRNA on Cx43 expression
and function of GJ
To further assess the role of GJIC in cell
density-dependent adriamycin sensitivity, siRNA was used to
downregulate the expression of Cx43 in these cells (27,28).
Downregulation was achieved by transient transfection of Hs578T and
MCF-7 cells with synthetic Cx43-targeted siRNA. Cells were
pretreated with siRNA negative control or Cx43 siRNA for 48 h.
Western blot analyses confirmed that, in the two breast cancer cell
lines (Hs578T and MCF-7), the expression of Cx43 was markedly
reduced relative to their untreated counterparts (control groups)
and siRNA negative controls (Fig. 4A and
B). In order to form an integrated GJ, connexins must be
transferred to the membrane of cells (29,30).
Consistent with the results of western blotting, the expression of
Cx43 on the membrane determined by immunofluorescence assay was
also reduced (Fig. 4C).
Influence of siRNA on adriamycin
cytotoxicity in breast cancer cells
To test whether siRNA would affect the function of
GJs, a ‘parachute’ dye-coupling assay was used to detect dye
coupling in breast cancer cells that were pretreated with siRNAs.
In Hs578T and MCF-7 cells, the GJ-permeable dye calcein passed from
the preloaded cells to various surrounding cells. According to the
results of the ‘parachute’ dye-coupling assay (Fig. 5A and B), when breast cancer cells were
incubated with siRNA for 48 h, the dye coupling through the GJ was
significantly decreased (P<0.01). However, negative control
cells did not exhibit any significant change compared with the
control group (P>0.05).
In order to determine whether siRNA could affect the
adriamycin cytotoxicity in Hs578T cells, the cytotoxicity caused by
6 µM adriamycin was analyzed in Hs578T and MCF-7 cells in
high-density cultures (GJ formed) and low-density cultures (no GJ
formed). Cells were pretreated with siRNA for 48 h. Fig. 5C revealed that, in low-density
cultures, the surviving fraction in the siRNA group was similar to
that in the control group. This result demonstrated that siRNA had
no effect on the adriamycin-induced cytotoxicity in Hs578T and
MCF-7 cells when no GJs were formed. It suggests that, in the
absence of GJIC, downregulation of Cx43 expression had no effect on
the cellular response to adriamycin. Thus, Cx43 expression itself
did not exert modulatory effects on adriamycin cytotoxicity that
were unrelated to GJ formation and function. By contrast, in
high-density cultures, when cells were exposed to 6 µM adriamycin,
the surviving fraction of the siRNA group was increased compared
with that of the control group. This result demonstrated that
knockdown of Cx43 expression by siRNA reduced the cytotoxicity of
adriamycin in mammary tumor cells when GJs were formed (Fig. 5C).
Discussion
The present study demonstrated that there was a
significant GJ-dependent component of adriamycin toxicity in breast
cancer cells. Our results indicated that the expression levels of
Cx43 in breast cancer cells were associated with malignancy. In the
present study, Hs578T cells, which had the lowest malignancy
degree, expressed the highest level of Cx43, followed by MCF-7,
SK-BR-3 and MDA-MB-231 cells, where cells with the highest
malignancy degree expressed the lowest level of Cx43 protein.
Subsequently, Hs578T and MCF-7 cells, which have the ability to
form efficient GJs, were used in order to observe whether
regulation of GJ function could influence the cytotoxicity of
chemical drugs such as adriamycin. RA was used as potentiator of
GJ. Increased cytotoxicity of adriamycin was observed upon RA
treatment in Hs578T and MCF-7 cells. The function of GJ was
downregulated by three different methods: i) Low-density cultures,
which lack functional contacts; ii) drugs, by incubating cells with
the GJIC inhibitors oleamide or 18-α-GA; and iii) molecular
biology, which resulted in Cx43 knockdown by siRNA. These methods
provided the same results: Reduction of adriamycin cytotoxicity via
inhibition of GJ was observed in high-density cultures, in which
functional GJs were effectively formed, but was not observed in
low-density cultures. These results suggested that GJ function
could be enhanced to increase the antitumor activity of therapeutic
agents in mammary cancer cells, which could become a novel
therapeutic target of breast tumors.
Cancer cells (including liver cancer, lung carcinoma
and breast carcinoma) are generally linked to loss of connexin
and/or GJIC (15,31–33).
Numerous studies have revealed the significant role of GJs in
tumors, since recovered expression of connexin that forms GJs or
recovered function of GJs could reduce the cell malignant phenotype
(11,34,35). The
expression of connexin and its derived homotypic GJ suppressed the
invasion of tumor cells (36,37).
In view of this, we attributed both the protective
and toxic effects of GJIC on adriamycin cytotoxicity to the
intercellular propagation of molecular/chemical signals through GJs
in breast cancer cells. Prior reports have indicated that GJs
regulated the cytotoxicity of antineoplastic drugs through various
mechanisms (21,38,39). For
example, heteromeric GJs composed of Cx26 and Cx32 enhanced the
cytotoxicity of cisplatin in HeLa cells, as assessed by
proliferative capacity, cell survival and induction of specific
apoptotic caspases (21). GJIC was
enhanced by simvastatin through protein kinase C-mediated Cx43
phosphorylation, which enhanced etoposide toxicity in Leydig tumor
cells (38). The efficacy of
antineoplastic drugs was increased through a combinatorial
treatment with substituted quinolines, which are specific class of
GJ enhancers (39). This effect was
similarly observed in tumors subjected to X-rays irradiation
treatment. Berberine potentiates the cell apoptosis induced by
X-rays irradiation, probably through enhancement of GJ activity
(40). Apoptosis is currently
recognized as a major mode of antineoplastic-induced or
radiation-induced cell death (41,42). It is
possible that one explanation for these discrepancies is the
GJ-mediated transmission of the ‘death signal’ from apoptotic cells
to neighboring viable cells. This strategy, which is followed in
GJIC-based therapies, depends on the so-called ‘bystander effect’
(BE) (43,44). The BE, a mechanism where a cytotoxic
signal is transferred from targeted cells to neighboring cells, has
been considered as an important therapeutic method (45). Numerous experiments reported that the
BE promotes suicide gene therapy (46,47).
Therefore, several inducers of GJIC such as retinoids have been
used to provide great potential to amplify the efficacy of suicide
gene therapy via the BE (48,49).
However, in normal cells, GJIC could exert a
protective effect, which is a markedly opposite effect to that
exerted in tumor cells (20,50). Nakase et al (50) reported that astrocytic GJs reduce
apoptotic neuronal damage in cerebral ischemia. Hong et al
(20) reported that the modulatory
effect of GJIC on the cisplatin cytotoxic or protective effect
depends on the oncogenic status of the cells, since the toxicity
effect observed in tumor cells is opposite to that observed in
normal cells, and these different effects are mediated by the same
connexin. This phenomenon suggests that the protective effect is
mediated by the transmission of survival signals among normal
cells, the production of which is stimulated by the damaging
effects of cisplatin exposure.
Upregulation of connexins and GJIC has been
suggested as an antineoplastic strategy (19,51).
Conklin et al (51) reported
that genistein and quercetin increase Cx43 expression and suppress
the growth of breast cancer cells. He et al (19) demonstrated that tramadol and
flurbiprofen inhibit the cytotoxicity of cisplatin via their
effects on GJs. This observation suggests that the restoration of
connexins expression and GJIC may have beneficial effects in cancer
therapies.
Connexins are reliable markers for breast cancer
behavior (52). Multiples studies
have revealed that, in breast cancer patients, the expression of
connexin proteins is correlated with clinicopathological
biomarkers, including estrogen receptor (ER), progesterone receptor
(PR) and human epidermal growth factor receptor 2 (HER2), which are
normally used to predict the response of mammary cancer patients to
therapy (33,53). The Hs578T cell line used in the
current study is a type of triple-negative breast cancer (TNBC),
while the MCF-7 cell line is non-TNBC (ER+ and PR+) (54,55). ER
and PR status have traditionally been used to select patients
suitable for tamoxifen treatment (56). Conklin et al (52) reported that there was a significant
positive correlation between Cx43 and PR expression, while both
Cx32 and Cx43 exhibited a strong, positive correlation with ER
status. Previous studies have shown that estrogen-mediated
activation of ER-α suppresses GJIC and Cx43 expression, resulting
in endometrial tumor progression (57). Zhao et al (58) observed that progestin reduces the
transcription of Cx43 in myometrial cells through a mechanism
independent of PR. Whether the expression level of Cx43 in Hs578T
and MCF-7 cells is correlated with ER or PR remains to be
determined. However, the present results demonstrated that the
function of GJ and the expression of Cx43 in breast cancer were not
associated with the levels of any of the above three biomarkers
(ER, PR or HER2), and that the influence of GJ on the antitumor
activity of adriamycin was neither associated with these
biomarkers.
The outcome of our findings implies that regulation
of GJ could be used as a new target in the therapy of breast
cancer. We propose that the expression of Cx43 is associated with
the malignancy degree of breast cancer cells. The cytotoxicity of
adriamycin on breast cancer cells can be regulated by GJ modulators
and Cx43 siRNA, which appears to be dependent on GJIC. Taken
together, these observations indicated that specific modulators of
Cx43 may have therapeutic implications in breast cancer.
Acknowledgements
The present study was supported by research grants
from the National Natural Science Foundation of China (Beijing,
China; grant no. 81001457), the National Natural Science Foundation
of Anhui (grant no. 1508085QH151), the Natural Science Foundation
of the Provincial Education Department of Anhui (no. KJ2015A147)
and the Scientific Research Project of Bengbu Medical College of
Anhui Province (Bengbu, China; grant nos. BYKY1605ZD and
BYKY1608ZD).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernzweig J, Heiniger B, Prasain K, Lu J,
Hua DH and Nguyen TA: Anti-breast cancer agents, quinolines,
targeting gap junction. Med Chem. 7:448–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Chen Y, Guo W, Yuan L, Zhang D,
Xu Y, Nemeth E, Ganz T and Liu S: Disordered hepcidin-ferroportin
signaling promotes breast cancer growth. Cell Signal. 26:2539–2550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li WJ, Zhong SL, Wu YJ, Xu WD, Xu JJ, Tang
JH and Zhao JH: Systematic expression analysis of genes related to
multidrug-resistance in isogenic docetaxel- and
adriamycin-resistant breast cancer cell lines. Mol Biol Rep.
40:6143–6150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao B, Li M, Zha W, Zhao Q, Gu R, Liu L,
Shi J, Zhou J, Zhou F, Wu X, et al: Metabolomic approach to
evaluating adriamycin pharmacodynamics and resistance in breast
cancer cells. Metabolomics. 9:960–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Wu L, Kou L, Xu M, Sun J, Wang Y,
Fu Q, Zhang P and He Z: Novel nanostructured enoxaparin sodium-PLGA
hybrid carriers overcome tumor multidrug resistance of doxorubicin
hydrochloride. Int J Pharm. 513:218–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao M, Xu Y and Qiu L: Sensitization of
multidrug-resistant malignant cells by liposomes co-encapsulating
doxorubicin and chloroquine through autophagic inhibition. J
Liposome Res. June 7–2016.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Wei CJ, Xu X and Lo CW: Connexins and cell
signaling in development and disease. Annu Rev Cell Dev Biol.
20:811–838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trosko JE and Chang CC: Mechanism of
up-regulated gap junctional intercellular communication during
chemoprevention and chemotherapy of cancer. Mutat Res 480–481.
219–229. 2001. View Article : Google Scholar
|
|
10
|
Trosko JE and Ruch RJ: Gap junctions as
targets for cancer chemoprevention and chemotherapy. Curr Drug
Targets. 3:465–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu M, Zhang C, Li L, Dong S, Zhang N and
Tong X: Cx43 reverses the resistance of A549 lung adenocarcinoma
cells to cisplatin by inhibiting EMT. Oncol Rep. 31:2751–2758.
2014.PubMed/NCBI
|
|
12
|
Li Z, Zhou Z, Welch DR and Donahue HJ:
Expressing connexin 43 in breast cancer cells reduces their
metastasis to lungs. Clin Exp Metastasis. 25:893–901. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saez JC, Berthoud VM, Branes MC, Martinez
AD and Beyer EC: Plasma membrane channels formed by connexins:
Their regulation and functions. Physiol Rev. 83:1359–1400. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLachlan E, Shao Q and Laird DW:
Connexins and gap junctions in mammary gland development and breast
cancer progression. J Membr Biol. 218:107–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choudhary M, Naczki C, Chen W, Barlow KD,
Case LD and Metheny-Barlow LJ: Tumor-induced loss of mural Connexin
43 gap junction activity promotes endothelial proliferation. BMC
Cancer. 15:4272015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aasen T: Connexins: Junctional and
non-junctional modulators of proliferation. Cell Tissue Res.
360:685–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Czyż J, Szpak K and Madeja Z: The role of
connexins in prostate cancer promotion and progression. Nat Rev
Urol. 9:274–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin H, Shao Q, Curtis H, Galipeau J,
Belliveau DJ, Wang T, Alaoui-Jamali MA and Laird DW: Retroviral
delivery of connexin genes to human breast tumor cells inhibits in
vivo tumor growth by a mechanism that is independent of significant
gap junctional intercellular communication. J Biol Chem.
277:29132–29138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong X, Wang Q, Yang Y, Zheng S, Tong X,
Zhang S, Tao L and Harris AL: Gap junctions propagate opposite
effects in normal and tumor testicular cells in response to
cisplatin. Cancer Lett. 317:165–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong X, Dong S, Yu M, Wang Q and Tao L:
Role of heteromeric gap junctions in the cytotoxicity of cisplatin.
Toxicology. 310:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang F, Li S, Gan X, Wang R and Chen Z:
Propofol inhibits gap junctions by attenuating sevoflurane-induced
cytotoxicity against rat liver cells in vitro. Eur J Anaesthesiol.
31:219–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong XH, Dong SY, Jiang GJ and Fan GF:
Influence of Cx26/Cx32 gap junction channel on antineoplastic
effect of etoposide in Hela cells. Nan Fang Yi Ke Da Xue Xue Bao.
32:329–332. 2012.(In Chinese). PubMed/NCBI
|
|
24
|
Babaoglu M, Zumrutbas AE, Acar IC, Hatip
FB, Kucukatay V, Eskicorapci S and Aybek Z: Gap junction expression
and the effects of gap junction inhibitors in overactive bladder
models: Does ovariectomy have a role? Int Urol Nephrol.
45:1001–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Talhouk R, Tarraf C, Kobrossy L, Shaito A,
Bazzi S, Bazzoun D and El-Sabban M: Modulation of Cx43 and gap
junctional intercellular communication by androstenedione in rat
polycystic ovary and granulosa cells in vitro. J Reprod Infertil.
13:21–32. 2012.PubMed/NCBI
|
|
26
|
Wu J, Taylor RN and Sidell N: Retinoic
acid regulates gap junction intercellular communication in human
endometrial stromal cells through modulation of the phosphorylation
status of connexin 43. J Cell Physiol228. 903–910. 2013. View Article : Google Scholar
|
|
27
|
Bier A, Oviedo-Landaverde I, Zhao J,
Mamane Y, Kandouz M and Batist G: Connexin43 pseudogene in breast
cancer cells offers a novel therapeutic target. Mol Cancer Ther.
8:786–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao Q, Wang H, McLachlan E, Veitch GI and
Laird DW: Down-regulation of Cx43 by retroviral delivery of small
interfering RNA promotes an aggressive breast cancer cell
phenotype. Cancer Res. 65:2705–2711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ablasser A, Schmid-Burgk JL, Hemmerling I,
Horvath GL, Schmidt T, Latz E and Hornung V: Cell intrinsic
immunity spreads to bystander cells via the intercellular transfer
of cGAMP. Nature. 503:530–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vliagoftis H, Ebeling C, Ilarraza R,
Mahmudi-Azer S, Abel M, Adamko D, Befus AD and Moqbel R: Connexin
43 expression on peripheral blood eosinophils: Role of gap
junctions in transendothelial migration. Biomed Res Int.
2014:8032572014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsiao PJ, Jao JC, Tsai JL, Chang WT, Jeng
KS and Kuo KK: Inorganic arsenic trioxide induces gap junction loss
in association with the downregulation of connexin43 and E-cadherin
in rat hepatic "stem-like" cells. Kaohsiung J Med Sci. 30:57–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang D, Chen C, Li Y, Fu X, Xie Y, Li Y
and Huang Y: Cx31.1 acts as a tumour suppressor in non-small cell
lung cancer (NSCLC) cell lines through inhibition of cell
proliferation and metastasis. J Cell Mol Med. 16:1047–1059. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teleki I, Krenacs T, Szasz MA, Kulka J,
Wichmann B, Leo C, Papassotiropoulos B, Riemenschnitter C, Moch H
and Varga Z: The potential prognostic value of connexin 26 and 46
expression in neoadjuvant-treated breast cancer. BMC Cancer.
13:502013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu JF, Ji J, Dong SY, Li BB, Yu ML, Wu DD,
Tao L and Tong XH: Gefitinib enhances oxaliplatin-induced apoptosis
mediated by Src and PKC-modulated gap junction function. Oncol Rep.
Oct 11–2016.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Yu BB, Dong SY, Yu ML, Jiang GJ, Ji J and
Tong XH: Total flavonoids of litsea coreana enhance the
cytotoxicity of oxaliplatin by increasing gap junction
intercellular communication. Biol Pharm Bull. 37:1315–1322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong X, Sin WC, Harris AL and Naus CC: Gap
junctions modulate glioma invasion by direct transfer of microRNA.
Oncotarget. 6:15566–15577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou JZ, Riquelme MA, Gu S, Kar R, Gao X,
Sun L and Jiang JX: Osteocytic connexin hemichannels suppress
breast cancer growth and bone metastasis. Oncogene. 35:5597–5607.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiu HW, Yeh YL, Wang YC, Huang WJ, Chen
YA, Chiou YS, Ho SY, Lin P and Wang YJ: Suberoylanilide hydroxamic
acid, an inhibitor of histone deacetylase, enhances
radiosensitivity and suppresses lung metastasis in breast cancer in
vitro and in vivo. PLoS One. 8:e763402013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shishido SN and Nguyen TA: Gap junction
enhancer increases efficacy of cisplatin to attenuate mammary tumor
growth. PLoS One. 7:e449632012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferraroni M, Bazzicalupi C, Bilia AR and
Gratteri P: X-Ray diffraction analyses of the natural isoquinoline
alkaloids Berberine and Sanguinarine complexed with double helix
DNA d (CGTACG). Chem Commun (Camb). 47:4917–4919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun H, Yang S, Li J, Zhang Y, Gao D and
Zhao S: Caspase-independent cell death mediated by
apoptosis-inducing factor (AIF) nuclear translocation is involved
in ionizing radiation induced HepG2 cell death. Biochem Biophys Res
Commun. 472:137–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jo GH, Bögler O, Chwae YJ, Yoo H, Lee SH,
Park JB, Kim YJ, Kim JH and Gwak HS: Radiation-induced autophagy
contributes to cell death and induces apoptosis partly in malignant
glioma cells. Cancer Res Treat. 47:221–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kong H, Liu X, Yang L, Qi K, Zhang H,
Zhang J, Huang Z and Wang H: All-trans retinoic acid enhances
bystander effect of suicide gene therapy in the treatment of breast
cancer. Oncol Rep. 35:1868–1874. 2016.PubMed/NCBI
|
|
44
|
Yakovlev VA: Role of nitric oxide in the
radiation-induced bystander effect. Redox Biol. 6:396–400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Prise KM and O'Sullivan JM:
Radiation-induced bystander signalling in cancer therapy. Nat Rev
Cancer. 9:351–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li S, Gu C, Gao Y, Amano S, Koizumi S,
Tokuyama T and Namba H: Bystander effect in glioma suicide gene
therapy using bone marrow stromal cells. Stem Cell Res. 9:270–276.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leten C, Trekker J, Struys T, Roobrouck
VD, Dresselaers T, Velde GV, Lambrichts I, Verfaillie CM and
Himmelreich U: Monitoring the Bystander Killing Effect of Human
Multipotent Stem Cells for Treatment of Malignant Brain Tumors.
Stem Cells Int. 2016:40950722016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li S, Gao Y, Pu K, Ma L, Song X and Liu Y:
All-trans retinoic acid enhances bystander effect of suicide-gene
therapy against medulloblastomas. Neurosci Lett. 503:115–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang J, Liu TJ, Jiang YX and Lu Y: ATRA
enhances the bystander effect of suicide gene therapy driven by the
specific promoter LEP 503 in human lens epithelial cells. Mol Vis.
18:2053–2066. 2012.PubMed/NCBI
|
|
50
|
Nakase T, Fushiki S and Naus CC:
Astrocytic gap junctions composed of connexin 43 reduce apoptotic
neuronal damage in cerebral ischemia. Stroke. 34:1987–1993. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Conklin CM, Bechberger JF, MacFabe D,
Guthrie N, Kurowska EM and Naus CC: Genistein and quercetin
increase connexin43 and suppress growth of breast cancer cells.
Carcinogenesis. 28:93–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Conklin C, Huntsman D, Yorida E, Makretsov
N, Turbin D, Bechberger JF, Sin WC and Naus CC: Tissue microarray
analysis of connexin expression and its prognostic significance in
human breast cancer. Cancer Lett. 255:284–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sulkowska U, Wincewicz A, Kanczuga-Koda L,
Koda M and Sulkowski S: Eventual proapoptotic or anti-apoptotic
impact of aberrantly expressed Cx43 and Cx26 can depend on ER-alpha
overexpression in human endometrioid adenocarcinoma. Gynecol
Endocrinol. 31:604–608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tarasewicz E, Rivas L, Hamdan R, Dokic D,
Parimi V, Bernabe BP, Thomas A, Shea LD and Jeruss JS: Inhibition
of CDK-mediated phosphorylation of Smad3 results in decreased
oncogenesis in triple negative breast cancer cells. Cell Cycle.
13:3191–3201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Collins DC, Cocchiglia S, Tibbitts P,
Solon G, Bane FT, McBryan J, Treumann A, Eustace A, Hennessy B,
Hill AD and Young LS: Growth factor receptor/steroid receptor cross
talk in trastuzumab-treated breast cancer. Oncogene. 34:525–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ciocca DR and Elledge R: Molecular markers
for predicting response to tamoxifen in breast cancer patients.
Endocrine. 13:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Saito T, Tanaka R, Wataba K, Kudo R and
Yamasaki H: Overexpression of estrogen receptor-alpha gene
suppresses gap junctional intercellular communication in
endometrial carcinoma cells. Oncogene. 23:1109–1116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao K, Kuperman L, Geimonen E and
Andersen J: Progestin represses human connexin43 gene expression
similarly in primary cultures of myometrial and uterine leiomyoma
cells. Biol Reprod. 54:607–615. 1996. View Article : Google Scholar : PubMed/NCBI
|