Introduction
Regulation of mRNA decay is an important mechanism
underlying the control of gene expression (1). The control of mRNA stability depends on
sequences in the transcript, and on the RNA-binding proteins that
dynamically bind to these sequences (2). Human antigen R (HuR) is a member of the
embryonic lethal abnormal visual family of RNA-binding proteins.
HuR has numerous functions, but one of the best characterized is
the regulation of mRNA turnover and stability (3). Various molecules that are associated
with cell proliferation, migration, immune response and
angiogenesis have previously been identified as targets of HuR
(3–5).
Increased expression levels of HuR are significantly associated
with malignant aggressiveness and poor survival in various types of
cancer, including urothelial cancer (UC) (6–10).
Another important function of HuR is to increase the
protein expression of deoxycytidine kinase (dCK), a key enzyme
involved in metabolizing the prodrug GEM into its active
metabolites through phosphorylation (11). Consequently, increased expression
levels of dCK may be associated with certain anticancer effects of
GEM. A role for dCK in activating GEM cytotoxicity has also been
indicated by the finding that suppression of dCK activity is
associated with resistance to GEM in various types of cancer
(12,13). Increased expression levels of HuR may
increase the cytotoxicity of GEM and reduce chemoresistance to this
drug in a variety of malignant cell types. A previous in
vitro study demonstrated that the modulation of dCK expression
via HuR overexpression markedly sensitized pancreatic cancer cells
to GEM (11). Similar results were
identified in human gallbladder cancer cells (14), and an in vivo study has also
demonstrated that the status of HuR expression in various cancer
cells is closely associated with response to GEM and GEM-based
chemotherapy in patients with pancreatic cancer (11,15). Thus,
HuR may have an important role in the GEM-based chemotherapy of
patients with cancer (16).
In patients with advanced UC, a cisplatin
(CDDP)-based regimen is the most commonly used first-line
chemotherapy (17). Combined
chemotherapy with methotrexate, vinblastine, doxorubicin and CDDP
(MVAC) has also been established as one of most useful regimens for
the treatment of patients with advanced UC since the 1980s
(18). From 2000, combined
gemcitabine (GEM) and CDDP therapy (GC) has become another standard
chemotherapy regimen for the treatment of patients with UC, as it
has been demonstrated to exert similar antitumor effects with
reduced toxicity, as compared to MVAC therapy (19). However, GC therapy is limited with
respect to the degree and duration of its anticancer effects,
particularly in patients with metastatic UC (20,21).
Furthermore, the efficacy and safety of this regimen as a second
line chemotherapy approach, following CDDP-based therapy, has yet
to be established, despite numerous clinical trials of various
drugs and regimens (22–25). A number of previous studies have,
therefore, investigated the potential of non-CDDP agents, including
GEM, as second- or third-line chemotherapy agents (24,25);
single-drug therapy with GEM, and combination therapy with GEM and
paclitaxel (PTX), have been reported (24,25).
Therefore, GEM is an essential chemotherapeutic agent for the
treatment of patients with advanced and recurrent UC, and
chemosensitivity to GEM is an important determinant of tumor
suppression, treatment outcomes and survival in these patients.
Based on the results of these previous studies, it
was hypothesized that HuR expression may be a useful predictive
marker of antitumor effects in patients with advanced UC; however,
there is currently limited evidence to support this hypothesis. The
primary purpose of the present study was to clarify the prognostic
role of HuR expression in first- and second-line chemotherapy, with
respect to tumor size and progression free survival. The
association between anticancer effects and HuR intracellular
localization (nuclear or cytoplasmic) and the use of GEM in the
therapeutic regimen, was also evaluated. Finally, the predictive
potential of HuR expression levels were analyzed, with respect to
the treatment outcomes of patients with advanced UC who were
treated with a GEM-based chemotherapy regimen, using multivariate
analyses that included pathological features.
Materials and methods
Patients
A total of 50 patients with advanced UC (male, 32;
female, 18), who were treated with chemotherapy in Nagasaki
University Hospital (Nagasaki, Japan), were analyzed
retrospectively. These patients were selected as they had received
a CDDP-based first-line chemotherapy regimen, followed by a
GEM-based second-line chemotherapy regimen. For first-line
chemotherapy, MVEC and GC regimens were administered to 34 (68.0%)
and 16 patients (32.0%), respectively. Following a diagnosis of
tumor progression, 45 patients (90.0%) were treated with combined
GEM and PTX therapy, and 5 patients (10.0%) were treated using GEM
monotherapy. The relevant clinicopathological features are
presented in Table I. The median
patient age was 70 years (range, 39–88 years), and 27 (54.0%), 22
(44.0%) and 1 (2.0%) patient(s) had UC of the urinary bladder,
upper urinary tract and both locations, respectively. With regard
to pathological features, 43 (86.0%) and 41 (82.0%) patients were
diagnosed as having muscle invasive and metastatic disease,
respectively. A total of 7 patients (14.0%) with upper urinary
tract cancer were determined to have non-muscle invasive disease.
However, these patients were unable to receive radical surgery as
they were elderly or had metastatic disease.
 | Table I.Clinicopathological features according
to the first-line chemotherapy regimen. |
Table I.
Clinicopathological features according
to the first-line chemotherapy regimen.
|
|
| First-line
chemotherapy |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Total (n=50) | GC (n=16) | MVEC (n=34) | P-value |
|---|
| Gender, n (%) |
|
|
| 0.157 |
|
Male | 32 (64.0) | 8 (50.0) | 24 (70.6) |
|
Female | 18 (36.0) | 8 (50.0) | 10 (29.4) |
| Age, years |
|
|
| 0.205 |
| Median
(range) | 70 (39–88) | 66 (39–88) | 73 (45–80) |
| Site of primary
tumor, n (%) |
|
|
| 0.603 |
|
Bladder | 27 (54.0) | 10 (62.5) | 17 (50.0) |
| Upper
tract | 22 (44.0) | 6
(37.5) | 16 (47.1) |
| Bladder
and upper tract | 1 (2.0) | 0 (0.0) | 1 (2.9) |
| Grade, n (%) |
|
|
| 0.138 |
| Low or
grade 1+2 | 9
(18.0) | 1 (6.3) | 8 (23.5) |
| High or
grade 3 | 41 (82.0) | 15 (93.7) | 26 (76.5) |
| T stage, n (%) |
|
|
| 0.361 |
| T1 | 7 (14.0) | 4 (25.0) | 3 (8.8) |
| T2 | 18 (36.0) | 6 (37.5) | 12 (35.3) |
| T3 | 18 (36.0) | 5 (31.3) | 12 (35.3) |
| T4 | 7 (14.0) | 1 (6.3) | 6 (17.6) |
| Metastasis, n
(%) |
|
|
| 0.094 |
|
Absence | 9 (18.0) | 5 (31.3) | 4 (11.8) |
|
Presence | 41 (82.0) | 11 (68.7) | 30 (88.2) |
Chemotherapy
The MVEC regimen consisted of methotrexate (30
mg/m2 on days 1, 15 and 22), vinblastine (3
mg/m2 on days 2, 15 and 22), epirubicin (30
mg/m2 on day 2) and CDDP (70 mg/m2 on day 2),
administered by intravenous infusion over a 28-day cycle. GC
therapy consisted of GEM (1,000 mg/m2 on days 1, 8 and
15) and CDDP (70 mg/m2 on day 2) and was also
administered by intravenous infusion over a 28-day cycle. For
second-line chemotherapy, GP therapy consisted of GEM (700
mg/m2) and paclitaxel (70 mg/m2),
administered by intravenous infusion on day 1 and 8 of each 28-day
cycle. GEM monotherapy (1,000 mg/m2 on day 1 and 8) was
also administered by intravenous infusion over a 28-day cycle. In
the current study, all patients received ≥2 cycles of first-line
chemotherapy, and the median number of treatment cycles was two for
MVAC and GC therapy.
Immunohistochemistry
HuR expression was evaluated as previously described
(8). Briefly, immunohistochemical
analyses were performed using formalin-fixed, paraffin-embedded
tissue sections. The tissue sections (5 µm) were deparaffinized in
xylene and rehydrated in solutions of graded ethanol. Antigen
retrieval was performed by heating at 100°C for 15 min in 0.01 M
sodium citrate buffer (pH 6.0). All tissue sections were then
immersed in 3% hydrogen peroxide for 30 min to block endogenous
peroxidase activity. The tissue sections were incubated overnight
with the primary antibody [HuR (H-280): sc-20694, 1:100; Santa Cruz
Biotechnology, Dallas, TX, USA] at 4°C, followed by washing in
0.05% Tween-20 in phosphate-buffered saline. Subsequently, the
tissue sections were incubated with peroxidase using the labeled
polymer method with Dako EnVision+™ Peroxidase (Dako North America,
Inc., Carpinteria, CA, USA) for 60 min at room temperature
according to the manufacturer's instructions. The peroxidase
reaction was visualized using a liquid 3,3′-diaminobenzidine
tetrahydrochloride substrate kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The tissue sections were then
counterstained using hematoxylin. As previously described (8), formalin-fixed and paraffin-embedded
liver tissue samples (comprising resected and stored specimens
obtained from Nagasaki University Hospital between January 2012 and
December 2014) were used as the positive controls. A consecutive
section from each tissue sample was processed without the primary
antibody to be used as the negative controls.
HuR expression was evaluated based on an
immunoreactive staining score, as previously reported (8,11). HuR
expression was evaluated separately in cancer cell cytoplasm and
nuclei. Briefly, HuR immunostaining in cytoplasm of cancer cells
was scored as follows: 0, no staining; 1, weak or focal staining in
<10% of cells; 2, moderate or intense staining in 10–50% of
cells; 3, moderate or intense staining in >50% of cells. Nuclear
HuR expression was scored as follows: 0, no staining; 1, <10% of
cells stained; 2, 10–50% of cells stained; 3, >50% of cells
stained. A score of 0 or 1 was considered to indicate low HuR
expression, whereas a score of 2 or 3 was determined to indicate
high HuR expression. This evaluation was performed by two
independent investigators blinded to the clinical features and
survival data. The tissue sections were observed using an E-400
light microscope (Nikon Corporation, Tokyo, Japan) to obtain
digital images. In addition, the computer-aided image analysis
system WinROOF version 5.0 (Mitani Corporation, Fukui, Japan) was
used to evaluate HuR expression.
Treatment response
Between 8 and 16 weeks following chemotherapy, all
patients underwent a computed tomography scan or magnetic resonance
imaging to determine the in-field tumor response. The local
response was assessed using the Response Evaluation Criteria in
Solid Tumors (RECIST) guidelines version 1.1 (26). Based on these guidelines, the complete
response (CR) was defined as the disappearance of all target
lesions and the reduction in size of any pathological lymph nodes
to <10 mm in the short axis. Partial response (PR) was defined
as a decrease in the sum of the longest tumor diameters by ≥30%.
Stable disease was defined as insufficient tumor shrinkage to
qualify as PR, or as an insufficient increase in tumor size to
qualify as progressive disease (PD). PD was defined as an increase
in the sum of the longest tumor diameter by ≥20%, and an absolute
increase in tumor size of ≥5 mm. The appearance of new lesions was
also considered to indicate disease progression. The association
between HuR expression levels and progression-free survival (PFS)
was investigated, in addition to its association with overall
survival (OS) following the initiation of second-line chemotherapy.
The study protocol was approved by the Human Ethics Review
Committee of Nagasaki University Hospital (Nagasaki, Japan), and
was conducted according to the Declaration of Helsinki. Written
informed consent was obtained from all patients involved in the
present study prior to their enrollment.
Statistical analysis
Data are expressed as the median and the range. The
Mann-Whitney U test was used for the analysis of continuous
variables. The χ2 test and Fisher's exact test were used
for comparisons of categorical data. Survival analysis was
conducted using Kaplan-Meier analysis and the log-rank test. In
addition, univariate and multivariate Cox proportional hazard
analyses were used to obtain a hazard ratio (HR) with a 95%
confidence interval (CI), and a P-value for survival analyses. All
statistical tests were two-sided and all statistical analyses were
performed using StatView for Windows version 5.0 software (Abacus
Concepts, Berkeley, CA, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
HuR expression patterns and patient
response to first-line chemotherapy
The immunoreactivity of HuR was detected in the
nucleus and cytoplasm of bladder cancer cells. In contrast to the
normal urothelium, moderate-intense cytoplasmic HuR expression
levels were frequently detected in cancer cells. Overall, 78.0%
(39/50) and 72.0% (36/50) of tumors were determined to have
positive nuclear and cytoplasmic HuR expression, respectively
(Table II). With regard to the
characteristics of HuR expression in UC cells, no significant
difference (P=0.529) was observed between bladder cancer cells and
upper urinary tract cancer cells.
 | Table II.Associations between HuR expression
and response to first-line chemotherapy. |
Table II.
Associations between HuR expression
and response to first-line chemotherapy.
|
|
|
| First-line
chemotherapy |
|---|
|
|
|
|
|
|---|
|
| Total (n=50) | GC (n=16) | MVEC (n=34) |
|---|
|
|
|
|
|
|---|
| Expression | Negative | Positive | Negative | Positive | Negative | Positive |
|---|
| Nuclear HuR, n
(%) | 11 (22.0) | 39 (87.0) | 4 (25.0) | 12 (75.0) | 7 (20.6) | 27 (79.4) |
| Response, n
(%) |
|
|
|
|
|
|
|
Complete response | 3 (27.3) | 2 (5.1) | 1 (25.0) | 1 (8.3) | 2 (28.6) | 1 (3.7) |
| Partial
response | 1 (9.1) | 11 (28.2) | 1 (25.0) | 2 (16.7) | 0 (0.0) | 9 (33.3) |
| Stable
disease | 3 (27.3) | 12 (30.8) | 1 (25.0) | 2 (16.7) | 2 (28.6) | 10 (37.0) |
|
Progressive disease | 4 (36.4) | 14 (35.9) | 1 (25.0) | 7 (58.3) | 3 (42.9) | 7 (25.9) |
| P-value | 0.136 | 0.670 | 0.076 |
| Cytoplasmic HuR, n
(%) | 14 (28.0) | 36 (72.0) | 5 (31.3) | 11 (68.8) | 9 (26.5) | 25 (73.5) |
| Response, n
(%) |
|
|
|
|
|
|
|
Complete response | 1 (7.1) | 4 (11.1) | 0 (0.0) | 2 (22.2) | 1 (11.1) | 2 (8.0) |
| Partial
response | 2 (14.3) | 10 (27.8) | 0 (0.0) | 3 (33.3) | 2 (22.2) | 7 (28.0) |
| Stable
disease | 2 (14.3) | 13 (35.1) | 1 (20.0) | 2 (22.2) | 1 (11.1) | 11 (44.0) |
|
Progressive disease | 9 (64.3) | 9 (25.0) | 4 (80.0) | 4 (44.4) | 5 (55.6) | 5 (20.0) |
| P-value | 0.077 | 0.310 | 0.170 |
The anticancer effects of first-line chemotherapy
were evaluated according to the RECIST guidelines and are
summarized in Table II. Of a total
of 50 patients, 5 (10%) and 12 (24%) were determined to exhibit a
CR and PR, respectively. The response rates of MVAC therapy
demonstrated no significant difference (P=0.442) from those of GC
therapy (35.3 and 31.3%, respectively). The anticancer effects
observed in individual patients were not significantly associated
with the localization of HuR expression [nuclear staining
(P=0.136), as compared with cytoplasmic staining (P=0.076)]. When
similar analyses were performed for the first-line chemotherapy
regimen, nuclear and cytoplasmic HuR expression levels were not
determined to be significantly associated with the anticancer
effects of MVAC or GC therapy (Table
II). The median of PFS following the initiation of first-line
MVEC (6 months) and GC therapy (4 months) was also similar
(P=0.172; data not shown). In addition, nuclear and cytoplasmic HuR
expression was not observed to be significantly associated with PFS
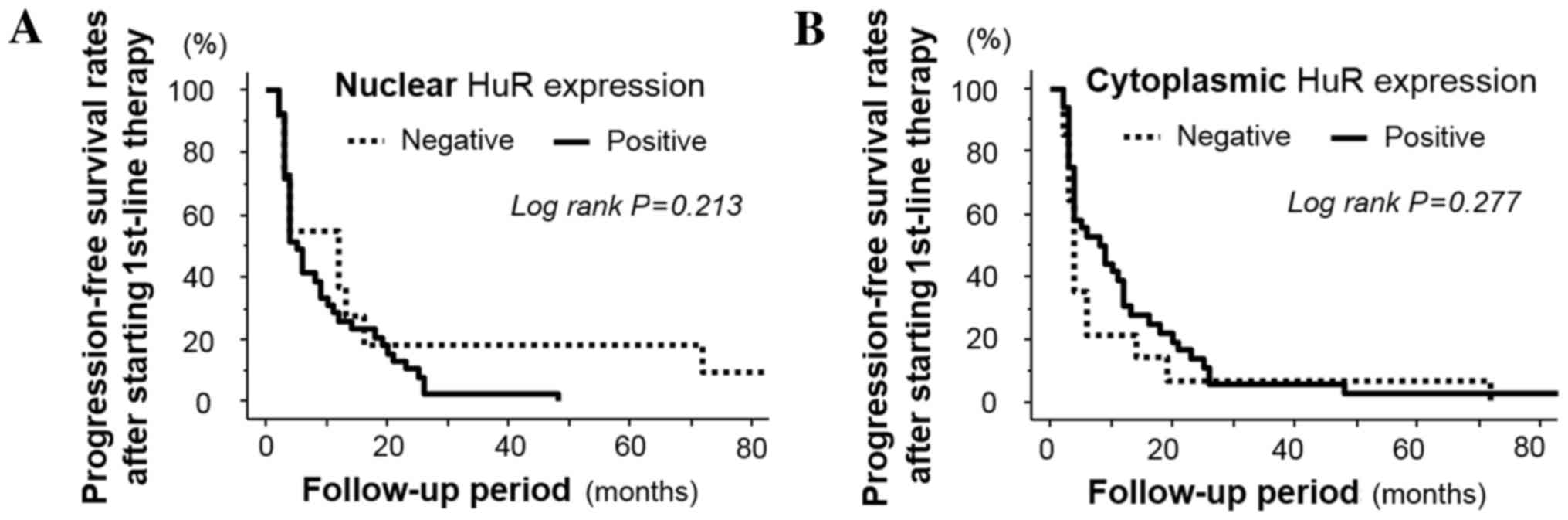
following first-line chemotherapy (P=0.213 and 0.277, respectively;
Fig. 1A and B).
HuR expression and response to second
line chemotherapy
In second-line GEM-based chemotherapy, nuclear HuR
expression was not observed to be significantly associated with
anticancer effects in the first-line MVEC or GC therapy groups
(Table III). Furthermore, as
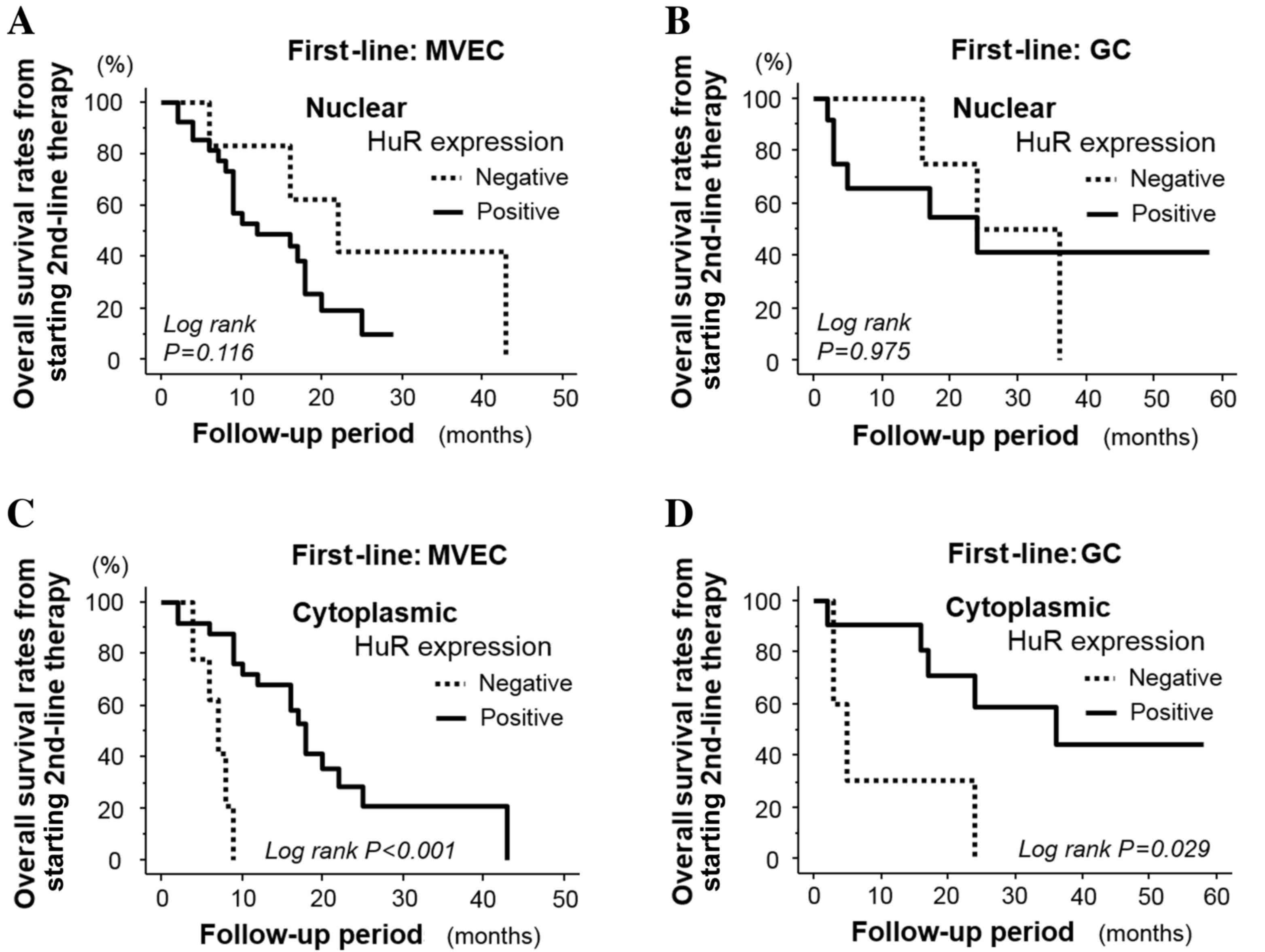
presented in Fig. 2A and B, nuclear
HuR expression levels were not associated with the OS rate from
second-line therapy in patients who had received first-line MVEC
therapy (P=0.116) or first-line GC therapy (P=0.975). However, the
tumor size in patients with positive cytoplasmic HuR tumor
expression was significantly reduced (P=0.002), compared with
patients with negative HuR tumor expression (Table III). Such anticancer effects,
evaluated according to the RECIST guidelines, were also observed in
patients treated with first-line MVAC therapy (P=0.025). A similar
trend was also observed in patients treated with first-line GC
therapy, but this difference was not statistically significant
(P=0.053; Table III). Furthermore,
OS in patients with positive cytoplasmic HuR tumor expression was
significantly longer, compared to those with negative expression,
in patients who received first-line MVAC (P<0.001; Fig. 2C) and first-line GC therapy (P=0.029;
Fig. 2D). Notably, the prognostic
indications of HuR cytoplasmic and nuclear expression for OS
following the initiation of second-line GEM-based chemotherapy were
contrary (Fig. 2A and D).
 | Table III.Associations between HuR expression
and response in second-line chemotherapy. |
Table III.
Associations between HuR expression
and response in second-line chemotherapy.
|
|
|
| First-line
regimen |
|---|
|
|
|
|
|
|---|
|
| Total (n=50) | GC (n=16) | MVEC (n=34) |
|---|
|
|
|
|
|
|---|
| Expression | Negative | Positive | Negative | Positive | Negative | Positive |
|---|
| Nuclear HuR, n
(%) | 11 (22.0) | 39 (87.0) | 4 (25.0) | 12 (75.0) | 7 (20.6) | 27 (79.4) |
| Response, n
(%) |
|
|
|
|
|
|
|
Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial
response | 2 (18.2) | 6 (15.4) | 2 (50.0) | 3 (25.0) | 0 (0.0) | 3 (11.1) |
| Stable
disease | 8 (72.7) | 22 (56.4) | 2 (50.0) | 5 (41.7) | 6 (85.7) | 17 (62.9) |
|
Progressive disease | 1 (9.1) | 11 (28.2) | 0 (0.0) | 4 (33.3) | 1 (14.3) | 7 (25.9) |
| P-value | 0.136 | 0.371 | 0.467 |
| Cytoplasmic HuR, n
(%) | 14 (28.0) | 36 (72.0) | 5 (31.3) | 11 (68.8) | 9 (26.5) | 25 (73.5) |
| Response, n
(%) |
|
|
|
|
|
|
|
Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Partial
response | 0 (0.0) | 8 (22.2) | 0 (0.0) | 5 (45.5) | 0 (0.0) | 3 (12.0) |
| Stable
disease | 6 (42.9) | 24 (66.7) | 2 (40.0) | 5 (45.5) | 4 (44.4) | 19 (76.0) |
|
Progressive disease | 8 (57.1) | 4 (11.1) | 3 (60.0) | 1 (9.1) | 5 (55.6) | 3 (12.0) |
| P-value | 0.002 | 0.053 | 0.025 |
Independent associations between HuR expression and
patient survival from the initiation of second-line chemotherapy,
in univariate and multivariate analysis models that included
clinicopathological features and first-line chemotherapy regimens,
are summarized in Table IV.
Cytoplasmic HuR expression was identified as a significant
predictive factor for longer OS (HR, 0.22; 95% CI, 0.09–0.56;
P=0.001), whereas nuclear HuR expression was not (HR, 1.21; 95% CI,
0.43–3.39; P=0.724, Table IV).
However, this was only determined to be for patients who received
second-line GP therapy (HR, 0.33; 95% CI, 0.12–0.92; P=0.034). When
similar analyses were performed according to tumor type,
significant and independent associations were detected in bladder
cancer (HR, 0.31; 95% CI, 0.10–0.96; P=0.042) and upper urinary
tract cancer (HR, 0.14; 95% CI, 0.09–0.69; P=0.019).
 | Table IV.Predictive value for overall survival
from commencement of second-line chemotherapy. |
Table IV.
Predictive value for overall survival
from commencement of second-line chemotherapy.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinical
feature | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Male gender | 0.54 | 0.26–1.12 | 0.096 | 0.29 | 0.11–0.78 | 0.014 |
| Age, years | 0.99 | 0.97–1.03 | 0.958 | 1.00 | 0.96–1.03 | 0.787 |
| High grade/grade
3 | 1.01 | 0.42–2.42 | 0.983 | 0.88 | 0.31–2.50 | 0.814 |
| Tumor stage 4 | 1.35 | 0.39–4.68 | 0.641 | 0.57 | 0.14–2.37 | 0.443 |
| Presence of
metastasis | 1.42 | 0.54–3.70 | 0.478 | 1.48 | 0.45–4.65 | 0.523 |
| First-line
MVEC | 1.77 | 0.82–3.90 | 0.153 | 2.48 | 0.95–6.78 | 0.063 |
| Positive C-HuR | 0.27 | 0.12–0.59 | 0.001 | 0.22 | 0.09–0.56 | 0.001 |
| Positive N-HuR | 1.56 | 0.66–3.69 | 0.308 | 1.21 | 0.43–3.39 | 0.724 |
Discussion
HuR is primarily detected in the nucleus under
normal physiological conditions, but relocates to the cytoplasm in
response to various stimuli, including certain signaling pathways
activated during carcinogenesis (27). Similar findings have also been
observed in patients with various malignancies, including bladder
cancer (8). Therefore, the
pathological role and biological characteristics of HuR in numerous
types of malignancy have been investigated in vivo and in
vitro (5–10,28).
To the best of our knowledge, the first study that
identified upregulated HuR expression as a significant marker for
an improved response to GEM-based chemotherapy in patients with
pancreatic cancer was published in 2009 (11). The study also observed that high HuR
expression levels predicted a favorable prognosis in these patients
(11). This result was notable as
increased HuR expression was previously considered to predict
progression and shorter survival in numerous types of malignancy
(9). Subsequently, the molecular
mechanisms underlying the anticancer effects of GEM were identified
in pancreatic cancer cells (29).
Although high HuR expression levels were significantly associated
with a high T stage, it was also indicated to be a potent marker of
clinical outcomes for patients with resected pancreatic cancer who
were undergoing GEM therapy (15).
These results demonstrated that HuR expression had conflicting
prognostic indications with respect to malignant potential and the
response to GEM-based chemotherapy in pancreatic cancer. In
addition to pancreatic cancer, HuR has been reported to have
important roles in the chemosensitivity of human gallbladder cancer
cells to GEM (14). Therefore, it was
hypothesized in the current study that HuR expression in UC cells
may be a useful predictive marker for the efficacy of GEM-based
therapy in patients with UC.
To the best of our knowledge, the present study is
the first to assess the association between HuR expression levels
and specific chemosensitivity in human UC tissues. The results
demonstrated that cytoplasmic HuR expression levels are
significantly associated with the anticancer effects of a
second-line GEM-based regimen, but not with a first-line
chemotherapy that included a GC regimen. In addition, cytoplasmic
HuR expression levels were a useful predictive marker for OS from
the initiation of second-line GEM-based chemotherapy, but not for
progression-free survival following first-line chemotherapy.
However, it remains to be elucidated why cytoplasmic HuR expression
was significantly associated with the anticancer effects of a
second-line GEM-based chemotherapy regimen, but not of a first-line
GC therapy, and the design of the present study did not permit this
to be elucidated. However, there are a number of possible reasons
for these findings. Firstly, in GC therapy administered to
chemo-naïve patients with UC, the most effective component may be
CDDP, rather than GEM (30). Among
CDDP-based regimens, the GC regimen is administered at a reduced
frequency and causes fewer severe adverse effects, compared with
the MVAC regimen; however, these two regimens have similar
anticancer effects and prognostic implications in patients with
advanced UC (21). Therefore, the
predictive value of cytoplasmic HuR expression levels for the
anticancer effects of certain first-line chemotherapy regimens is
relatively low, and its effects were primarily determined by
chemosensitivity towards CDDP. Secondly, there is a possibility
that HuR inhibited the anticancer effects of CDDP in first-line
chemotherapy based on a previous report (22). It was identified that, in patients
with UC receiving GEM-based second-line chemotherapy, cytoplasmic
HuR expression levels were positively associated with prolongation
of OS periods. These findings were concordant with the results of
previous studies of other types of cancer (11,14). By
contrast, it has also been suggested that increased HuR expression
levels are associated with decreased sensitivity to CDDP in ovarian
cancer (31). As aforementioned, CDDP
is the principal agent in first-line chemotherapy (21,30). If
this phenomenon also occurs in UC cells, HuR expression may reduce
their sensitivity to GC therapy. Therefore, HuR is able to mediate
and suppress the anticancer effects of GEM. Furthermore, in
addition to GEM, high HuR expression levels may regulate the
response to paclitaxel via regulation of chemoresistance-associated
factors including microRNA (31,32). A
previous in vitro study demonstrated that cytoplasmic HuR
expression was associated with the efficacy of various anticancer
agents (33–35); however, it has also been revealed that
HuR is able to mediate chemoresistance in numerous types of cancer
(36,37). Further studies are required in order
to investigate and elucidate the complex mechanisms underlying the
interaction between HuR expression levels and the response of
patients to chemotherapy. Although numerous previous studies have
reported that the expression of HuR in the cytoplasm has important
roles in tumor aggressiveness, prognosis and the modulation of
chemosensitivity-associated factors (6,8,28,31,32). It
remains to be elucidated whether this is also true of nuclear HuR
expression. Nuclear HuR expression was demonstrated to inhibit the
chemoresistance-associated protein, tubulin beta class 3 (TUBB3),
resulting in a improved prognosis, whereas the expression of
cytoplasmic HuR enhanced TUBB3 expression and was associated with
an improved treatment outcome in patients with ovarian cancer
(31). Furthermore, the association
between HuR expression levels and chemoresistance depends on the
presence of certain binding partners, including acidic leucine-rich
nuclear phosphoprotein 32 family member A (38,39).
Therefore, the localization and availability of co-factors for HuR
in various cancer cells may influence its pathological and
biological characteristics.
The pathological aggressiveness and molecular
characteristics of UC are regulated by complex underlying
mechanisms, including external factors (40). A limitation of the present study was
its relatively small study cohort; in addition, the variation and
non-uniformity of the treatment regimens and the patients' clinical
backgrounds must be noted. Therefore, in order to determine the
prognostic role of HuR expression with respect to patient response
to GEM-based chemotherapy and the treatment outcomes in advanced
UC, further detailed in vitro and in vivo studies,
including clinical trials, are essential (15,16).
In conclusion, the present study identified that HuR
expression levels were not significantly associated with antitumor
effects or improved PFS following first-line chemotherapy,
including with GC therapy. By contrast, cytoplasmic HuR expression
was identified to be significantly associated with antitumor
effects, as determined by the RECIST criteria, and it may also
predict the OS of patients with UC who have undergone second-line
GEM-based chemotherapy. This is important with respect to the
selection of treatment approaches for second-line chemotherapy in
these patients. Further and similar studies are required, with a
larger study population, in order to corroborate these results. In
addition, prospective and randomized clinical trials are necessary
to clarify the clinical potential of cytoplasmic HuR expression as
a predictive marker in patients with advanced UC.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Challenging Exploratory Research from the Japan Society for the
Promotion of Science KAKENHI (grant no. JP16K15690).
References
|
1
|
Łabno A, Tomecki R and Dziembowski A:
Cytoplasmic RNA decay pathways-Enzymes and mechanisms. Biochim
Biophys Acta. 1863:3125–3147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Derrigo M, Cestelli A, Savettieri G and Di
Liegro I: RNA-protein interactions in the control of stability and
localization of messenger RNA (Review). Int J Mol Med. 5:111–123.
2000.PubMed/NCBI
|
|
3
|
Hinman MN and Lou H: Diverse molecular
functions of Hu proteins. Cell Mol Life Sci. 65:3168–3181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gately S and Li WW: Multiple roles of
COX-2 in tumor angiogenesis: A target for angiogenenic therapy.
Semin Oncol 31 (2 Suppl 7). 2–11. 2004.
|
|
5
|
Sakuma T, Nakagawa T, Ido K, Takeuchi H,
Sato K and Kubota T: Expression of vascular endothelial growth
factor-A and mRNA stability factor HuR in human meningiomas. J
Neurooncol. 88:143–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim SJ, Lee SH, Joo SH, Song JY and Choi
SI: Cytoplasmic expression of HuR is related to cyclooxygenase-2
expression in colon cancer. Cancer Res Treat. 41:87–92. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Z, Sanders AJ, Ye L, Wang Y and Jiang
WG: Prognostic value of human antigen R (HuR) in human breast
cancer: High level predicts a favourable prognosis. Anticancer Res.
31:303–310. 2011.PubMed/NCBI
|
|
8
|
Miyata Y, Watanabe S, Sagara Y, Mitsunari
K, Matsuo T, Ohba K and Sakai H: High expression of HuR in
cytoplasm, but not nuclei, is associated with malignant
aggressiveness and prognosis in bladder cancer. PLoS One.
8:e590952013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kotta-Loizou I, Gianginis C and Theocharis
S: Clinical significance of HuR expression in human malignancy. Med
Oncol. 31:1612014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ronkainen H, Vaarala MH, Hirvikoski P and
Ristimäki A: HuR expression is a marker of poor prognosis in renal
cell carcinoma. Tumor Biol. 32:481–487. 2011. View Article : Google Scholar
|
|
11
|
Costantino CL, Witkiewicz AK, Kuwano Y,
Cozzitorto JA, Kennedy EP, Dasgupta A, Keen JC, Yeo CJ, Gorospe M
and Brody JR: The role of HuR in gemcitabine efficacy in pancreatic
cancer: HuR up-regulates the expression of the gemcitabine
metabolizing enzyme doxycytidine kinase. Cancer Res. 69:4567–4572.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Haperen VW Ruiz, Veerman G, Eriksson
S, Boven E, Stegmann AP, Hermsen M, Vermorken JB, Pinedo HM and
Peters GJ: Development and characterization of a
2′,2′-difluorodeoxycytidine-resistant variant of the human ovarian
cancer cell line A2780. Cancer Res. 54:4138–4143. 1994.PubMed/NCBI
|
|
13
|
Kroep JR, Loves WJ, van der Wilt CL,
Alvarez E, Talianidis I, Boven E, Braakhuis BJ, van Groeningen CJ,
Pinedo HM and Peters GJ: Pretreatment deoxycytidine kinase levels
predict in vivo gemcitabine sensitivity. Mol Cancer Ther.
1:371–376. 2002.PubMed/NCBI
|
|
14
|
Sekine S, Shimada Y, Nagata T, Moriyama M,
Omura T, Yoshioka I, Hori R, Matsui K, Sawada S, Okumura T, et al:
Establishment and characterization of a new human gallbladder
carcinoma cell line. Anticancer Res. 32:3211–3218. 2012.PubMed/NCBI
|
|
15
|
Richards NG, Rittenhouse DW, Freydin B,
Cozzitorto JA, Grenda D, Rui H, Gonye G, Kennedy EP, Yeo CJ, Brody
JR and Witkiewicz AK: HuR status is a powerful marker for prognosis
and response to gemcitabine-based chemotherapy for resected
pancreatic ductal adenocarcinoma patients. Ann Surg. 252:499–506.
2010.PubMed/NCBI
|
|
16
|
Maréchal R and van Laethem JL: HuR
modulates gemcitabine efficacy: New perspectives in pancreatic
cancer treatment. Expert Rev Anticancer Ther. 9:1439–1441. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. Jun 23–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sternberg CN, Yagoda A, Scher HI, Watson
RC, Ahmed T, Weiselberg LR, Geller N, Hollander PS, Herr HW and
Sogani PC: Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985.PubMed/NCBI
|
|
19
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemicitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI
|
|
20
|
Saxman SB, Propert KJ, Einhorn LH,
Crawford ED, Tannock I, Raghavan D, Loehrer PJ Sr and Trump D:
Long-term follow-up of a phase III intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 15:2564–2569.
1997.PubMed/NCBI
|
|
21
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyata Y, Asai A, Mitsunari K, Matsuo T,
Ohba K and Sakai H: Safety and efficacy of combination therapy with
low-dose gemcitabine, paclitaxel, and sorafenib in patients with
cisplatin-resistant urothelial cancer. Med Oncol. 32:2352015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massari F, Santoni M, Ciccarese C,
Brunelli M, Conti A, Santini D, Montironi R, Cascinu S and Tortora
G: Emerging concepts on drug resistance in bladder cancer:
Implications for future strategies. Crit Rev Oncol Hematol.
96:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stadler WM, Kuzel T, Roth B, Raghavan D
and Dorr FA: Phase II study of single-agent gemcitabine in
previously untreated patients with metastatic urothelial cancer. J
Clin Oncol. 15:3394–3398. 1997.PubMed/NCBI
|
|
25
|
Miyata Y, Nomata K, Ohba K, Matsuo T,
Sagara Y, Kanetake H and Sakai H: Use of low-dose combined therapy
with gemcitabine and paclitaxel for advanced urothelial cancer
patients with resistance to cisplatin-containing therapy: A
retrospective analysis. Cancer Chemother Pharmacol. 70:451–459.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MY, Hur J and Jeong S: Emerging roles
of RNA and RNA-binding protein network in cancer cells. BMB Rep.
42:125–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitsunari K, Miyata Y, Asai A, Matsuo T,
Shida Y, Hakariya T and Sakai H: Human antigen R is positively
associated with malignant aggressiveness via upregulation of cell
proliferation, migration, and vascular endothelial growth factors
and cyclooxygenase-2 in prostate cancer. Transl Res. 175:116–128.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pineda DM, Rittenhouse DW, Valley CC,
Cozzitorto JA, Burkhart RA, Leiby B, Winter JM, Weber MC, Londin
ER, Rigoutsos I, et al: HuR's post-transcriptional regulation of
death receptor 5 in pancreatic cancer cells. Cancer Biol Ther.
13:946–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bellmunt J and Albiol S: Chemotherapy for
metastatic or unresectable bladder cancer. Semin Oncol. 34:135–144.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prislei S, Martinelli E, Mariani M,
Raspaglio G, Sieber S, Ferrandina G, Shahabi S, Scambia G and
Ferlini C: MiR-200c and HuR in ovarian cancer. BMC Cancer.
13:722013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Li D, Wang B and Wu Y: Predictive
and prognostic significance of cytoplasmic expression of ELAV-like
protein HuR in invasive breast cancer treated with neoadjuvant
chemotherapy. Breast Cancer Res Treat. 141:213–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Yu J, Du D, Fu S, Chen Y, Yu F and
Gao P: Involvement of post-transcriptional regulation of FOXO1 by
HuR in 5-FU-induced apoptosis in breast cancer cells. Oncol Lett.
6:156–160. 2013.PubMed/NCBI
|
|
34
|
Latorre E, Tebaldi T, Viero G, Spartà AM,
Quattrone A and Provenzani A: Downregulation of HuR as a new
mechanism of doxorubicin resistance in breast cancer cells. Mol
Cancer. 11:132012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lal S, Burkhart RA, Beeharry N,
Bhattacharjee V, Londin ER, Cozzitorto JA, Romeo C, Jimbo M, Norris
ZA, Yeo CJ, et al: HuR posttranscriptionally regulates WEE1:
Implication for the DNA damage response in pancreatic cancer cells.
Cancer Res. 74:1128–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raspaglio G, De Maria I, Filippetti F,
Martinelli E, Zannoni GF, Prislei S, Ferrandina G, Shahabi S,
Scambia G and Ferlini C: HuR regulates beta-tubulin isotype
expression in ovarian cancer. Cancer Res. 70:5891–5900. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Li D, Wang B and Wu Y: Predictive
and prognostic significance of cytoplasmic expression of ELAV-like
protein HuR in invasive breast cancer treated with neoadjuvant
chemotherapy. Breast Cancer Res Treat. 141:213–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Williams TK, Costantino CL, Bildzukewicz
NA, Richards NG, Rittenhouse DW, Einstein L, Cozzitorto JA, Keen
JC, Dasgupta A, Gorospe M, et al: pp32 (ANP32A) expression inhibits
pancreatic cancer cell growth and induces gemcitabine resistance by
disrupting HuR binding to mRNAs. PLoS One. 5:e154552010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imamachi K, Higashino F, Kitamura T,
Kakuguchi W, Yanagawa-Matsuda A, Ishikawa M, Kitagawa Y, Totsuka Y
and Shindoh M: pp32r1 controls the decay of the RNA-binding protein
HuR. Oncol Rep. 31:1103–1108. 2014.PubMed/NCBI
|
|
40
|
Miyata Y, Mitsunari K, Akihiro A, Watanabe
SI, Mochizuki Y and Sakai H: Smoking-induced changes in
cancer-related factors in patients with upper tract urothelial
cancer. Mol Clin Oncol. 3:287–294. 2015.PubMed/NCBI
|