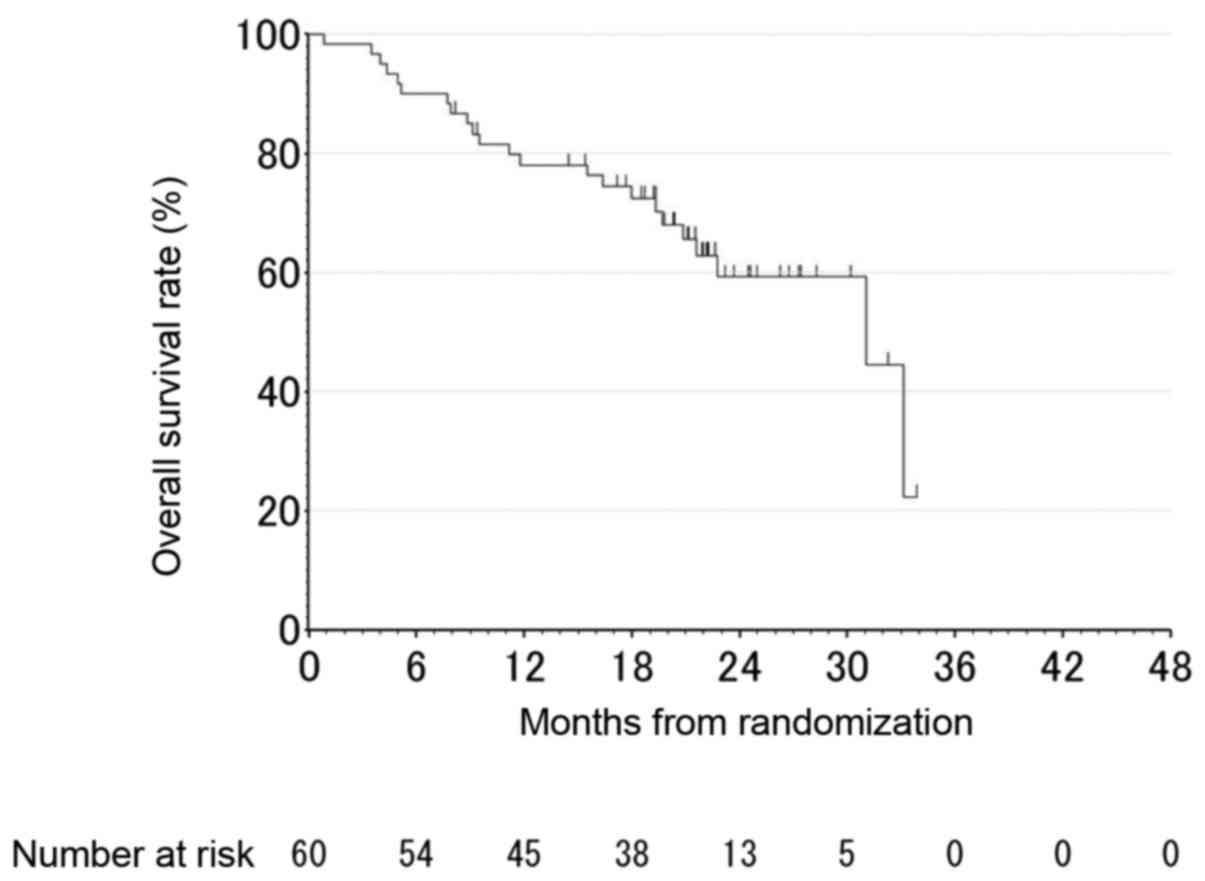
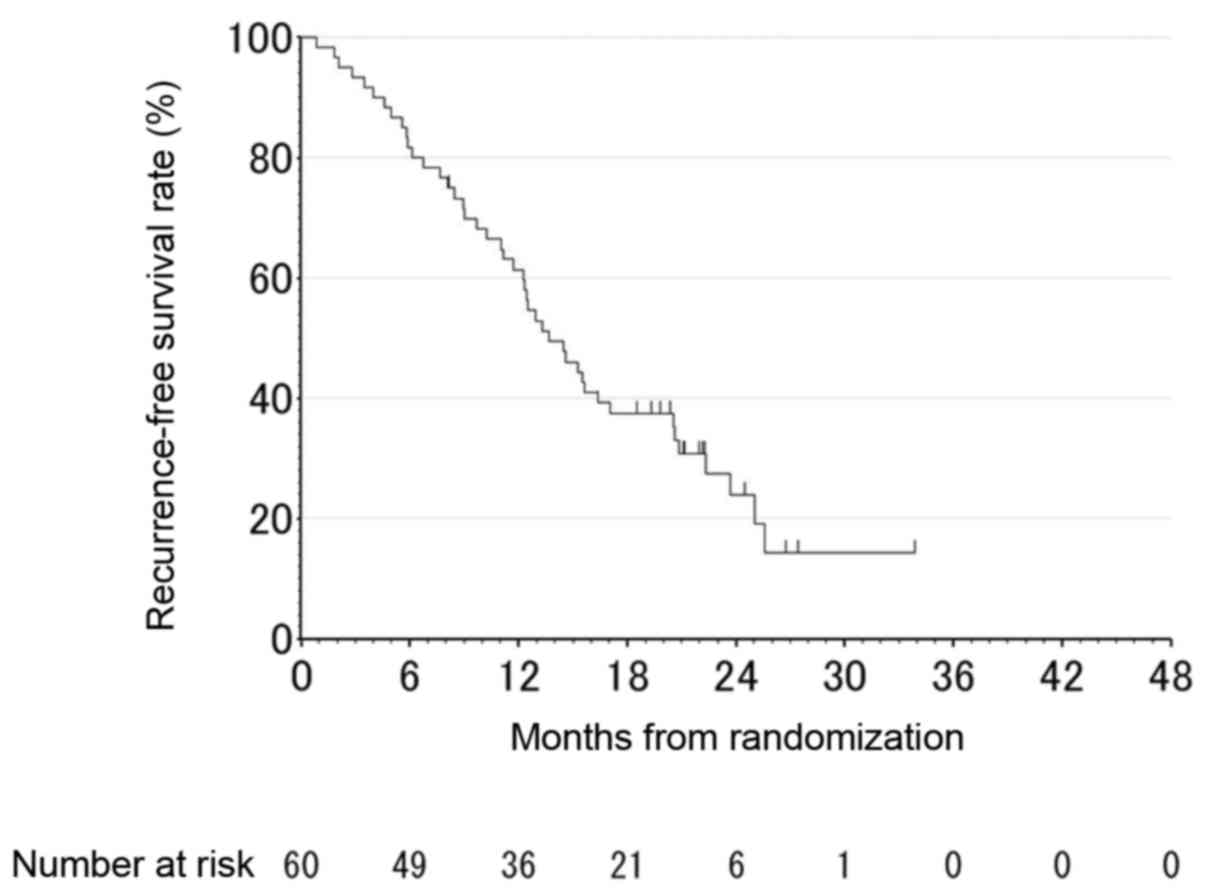
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Köhne CH, van Cutsem E, Wils J, Bokemeyer
C, El-Serafi M, Lutz MP, Lorenz M, Reichardt P, Rückle-Lanz H,
Frickhofen N, et al: Phase III study of weekly high-dose infusional
fluorouracil plus folinic acid with or without irinotecan in
patients with metastatic colorectal cancer: European Organisation
for Research and Treatment of Cancer Gastrointestinal Group Study
40986. J Clin Oncol. 23:4856–4865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, CortesFunes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000.PubMed/NCBI
|
|
6
|
Sunada H, Magun BE, Mendelsohn J and
MacLeod CL: Monoclonal antibody against epidermal growth factor
receptor is internalized without stimulating receptor
phosphorylation. Proc Natl Acad Sci USA. 83:3825–3829. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein NS and Armin M: Epidermal growth
factor receptor immunohistochemical reactivity in patients with
American Joint Committee on Cancer Stage IV colon adenocarcinoma:
Implications for a standardized scoring system. Cancer.
92:1331–1346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabernero J, Pfeiffer P and Cervantes A:
Administration of cetuximab every 2 weeks in the treatment of
metastatic colorectal cancer: An effective, more convenient
alternative to weekly administration? Oncologist. 13:113–119. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okayama N, Nishioka M, Hazama S, Sakai K,
Suehiro Y, Maekawa M, Sakamoto J, Iwamoto S, Kato T, Mishima H, et
al: The importance of evaluation of DNA amplificability in KRAS
mutation testing with dideoxy sequencing using formalin-fixed and
paraffin-embedded colorectal cancer tissues. Jpn J Clin Oncol.
41:165–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwamoto S, Hazama S, Kato T, Miyake Y,
Fukunaga M, Matsuda C, Bando H, Sakamoto J, Oba K, Mishima H, et
al: Multicenter phase II study of second-line cetuximab plus
folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type
metastatic colorectal cancer: The FLIER study. Anticancer Res.
34:1967–1973. 2014.PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Cancer and Institute Cancer
Therapy Evaluation Program, . Common Terminology Criteria for
Adverse Events v3.0 (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
|
|
17
|
Fernandez-Plana J, Pericay C, Quintero G,
Alonso V, Salud A, Mendez M, Salgado M, Saigi E and Cirera L:
ACROSS Study Group: Biweekly cetuximab in combination with FOLFOX-4
in the first-line treatment of wild-type KRAS metastatic colorectal
cancer: Final results of a phase I. BMC Cancer. 14:8652014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soda H, Maeda H, Hasegawa J, Takahashi T,
Hazama S, Fukunaga M, Kono E, Kotaka M, Sakamoto J, Nagata N, et
al: Multicenter Phase II study of FOLFOX or biweekly XELOX and
Erbitux (cetuximab) as first-line therapy in patients with
wild-type KRAS/BRAF metastatic colorectal cancer: The FLEET study.
BMC Cancer. 15:6952015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brodowicz T, Ciuleanu TE, Radosavljevic D,
ShachamShmueli E, Vrbanec D, Plate S, MrsicKrmpotic Z, Dank M,
Purkalne G, Messinger D and Zielinski CC: FOLFOX4 plus cetuximab
administered weekly or every second week in the first-line
treatment of patients with KRAS wild-type metastatic colorectal
cancer: A randomized phase II CECOG study. Ann Oncol. 24:1769–1777.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tabernero J, Ciardiello F, Rivera F,
RodriguezBraun E, Ramos FJ, Martinelli E, VegaVillegas ME, Roselló
S, Liebscher S, Kisker O, et al: Cetuximab administered once every
second week to patients with metastatic colorectal cancer: A
two-part pharmacokinetic/pharmacodynamic phase I dose-escalation
study. Ann Oncol. 21:1537–1545. 2010. View Article : Google Scholar : PubMed/NCBI
|