Introduction
Retinoblastoma (RB) is the most frequent retinal
tumor worldwide and the most common primary intraocular malignancy
in childhood, accounting for ~4% of all pediatric malignancies
(1). The majority of patients with
retinoblastoma are not diagnosed until the disease has reached an
advanced stage or metastasis has occurred (2). Tumor cells are able to extend through
the optic nerve, sclera and choroid to access the extraocular
space, leading to life-threatening systemic metastasis (3,4).
Metastatic RB has a high mortality rate due to a limited range of
chemo- and radiotherapeutic treatments (5). Although intensive multimodal therapies,
including high-dose chemotherapy with autologous hematopoietic stem
cell rescue, are reported to be effective in the treatment of
metastatic RB, it remains a life threatening disease (6).
Cluster of differentiation 82 (KAI1) was first
identified in prostate cancer cells by Dong et al (7) and has a significant role in the
inhibition of tumor metastasis. As a member of the transmembrane 4
superfamily, KAI1 contains four hydrophobic transmembrane domains
and a large extracellular N-glycosylated structure, which interacts
with integrin, epidermal growth factor receptor and other
tetraspanins (8–10). KAI1 interactions are associated with
cell-cell and cell-extracellular matrix interactions, as well as
cell signaling and motility. Therefore, KAI1 may affect the
invasion and metastasis of tumor cells (11). Decreased KAI1 expression is correlated
with the development of tumor metastasis and poor prognosis in a
wide variety of human malignancies, including prostate (12), breast (13), pancreatic (14) and lung cancer (15). Decreased expression of KAI1 was
reported in metastatic human RB samples (16). However, the biological effect of KAI1
on RB cells remains unclear.
In the present study, KAI1 expression was analyzed
in 26 human retinoblastoma samples, representing non-invasive and
invasive stages. In addition, the in vitro effect of KAI1 on
RB cell migration and invasion was studied in two RB cell
lines.
Materials and methods
Ethics statement
The present study conformed to the Declaration of
Helsinki and was approved by the Ethical Committee of the Xinhua
Hospital Affiliated to Shanghai Jiaotong University School of
Medicine (Shanghai, China). Written consent was obtained from the
patients participating in the study.
Tissue samples
Samples were obtained from RB patients at Xinhua
Hospital (Shanghai, China) between May 2010 and February 2013, who
received no treatment during the enucleation procedure. In total,
14 eyes presenting with no RB invasion of the optic nerve or
choroid were selected, representing the non-invasive group, and 12
eyes presenting with invasion of the optic nerve or choroid were
taken, representing the invasive group.
Detection of KAI1 protein using
western blot analysis
Tumor samples were ground into powder in liquid
nitrogen. Total protein was extracted using an ice-cold SDS
cell-lysis buffer (BiYunTian, P0013G, Shanghai, China), containing
protease inhibitors, and lysate was centrifuged (15,800 × g
for 30 min at 4°C) to remove debris. After quantitative analysis,
all the protein samples were mixed with the loading buffer and then
boiled with the burning water for 5 min. The protein samples were
separated on a 10% SDS-PAGE gel and transferred onto a
polyvinylidene difluoride membrane (GE Healthcare Life Sciences,
Chalfont, UK). The membrane was blocked in TBS and
Tween® 20 with 5% skimmed milk for 90 min, followed by
incubation with monoclonal mouse anti-KAI1 antibody (1:500, sc-4486
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Following three cycles of washing with TBST, the membrane was
incubated with horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (1:1,000, Jackson, 115-035-206, USA) at room
temperature for 1 h. β-actin (1:2,000, Proteintech, 6008-1-Ig, USA)
was used as an endogenous control. The resulting signals were
measured using an enhanced chemiluminescence system. Band intensity
was normalized to the value of β-actin (Image J, NIH, USA). All
experiments were performed in triplicate.
Western blot analysis for protein samples in RB
cells. RB cells incubated in mediums were collected and transferred
into EP tube with the number of 1×106 in each tube.
After centrifuging (4°C, 800 rpm, 10 min), each EP tube were added
with an ice-cold SDS cell-lysis buffer (BiYunTian, P0013G,
Shanghai, China), containing protease inhibitors, and lysate and
were centrifuged (15,800 × g for 30 min at 4°C) to remove
debris. The following steps and experimental conditions were in
consistent with the western blot analysis for tumor samples.
Measuring KAI1 gene expression using
the reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RB tissues were ground into powder in liquid
nitrogen and dissolved using a Trizol® reagent kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
MyiQ and iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), SYBR-Green kit and SYBRR Premix Ex Taq™
kit (Takara Bio, Inc., Otsu, Japan) were used to perform RT-qPCR
and subsequently measure KAI1 mRNA expression. The primer pairs for
KAI1 and GAPDH were as follows: KAI1 forward,
5′-TGTCCTGCAAACCTCCTCCA-3′ and reverse,
5′-CCATGAGCATAGTGACTGCCC-3′; and GAPDH forward,
5′-CCATGGCACCGTCAAGGCTGA-3′ and reverse,
5′-GGGCCATCCACAGTCTTCTGG-3′. Reaction parameters for RT-qPCR were
as follows: 94°C for 5 min followed by 40 cycles of 94°C for 30
sec, 55°C for 30 sec and 72°C for 30 sec. Each reaction was
performed in duplicate. The relative mRNA levels of KAI1 and GAPDH
were calculated using the 2−ΔΔCq method (17).
Cell lines and culture conditions
HXO-Rb44-Gl is a green fluorescent protein (GFP) and
luciferase expressing HXO-Rb44 human RB cell line (18). The HXO-Rb44 cell line was originally
established by the Department of Ophthalmology, Hunan Medical
University, Xiangya Hospital, Changsha, China (19). The Y79 human RB cell was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Non-adherent cell lines were cultured in RPMI-1640 supplemented
with 10% fetal bovine serum (FBS) (both Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2 humidified air.
Construction of recombinant lentivirus
expressing KAI1
pCMV-KAI1 was provided by Dr Dong of the National
Institute of Health (Bethesda, MD, USA). The 753 bp KAI1 and the
849 bp enhanced GFP (EGFP) cDNA fragments were amplified from
pCMV-KAI1 and pEGFP-N1 respectively using RT-PCR with the following
primers: KAI1 forward,
ATTCGCCTAGGACTAGTGATCAGCCACCATGGGCTCAGCCTGTATCAA and reverse,
CGGGGTACTTGGGGACCTTGCTGT; EGFP forward,
CAAGGTCCCCAAGTACCCCGGGGGTGGCGGAGGGTCTAGAATGGTGAGCAAGGGCGAG and
reverse, TCGGCGGCCGCTTATTTGTCGTCATCATCCTTATA. The two fragments
were fused together to form a 1585 bp KAI1-EGFP DNA fragment using
PCR, and inserted into the puromycin-resistant lentiviral vector
pMB-Puro (Neuronbiotech Co., Ltd., Shanghai, China), replacing the
firefly luciferase gene. The recombinant plasmid pMB-Puro-KAI1-EGFP
and the two 2nd generation packaging plasmids, psPAX2 and pMD2.G
(Addgene, Inc., Cambridge, MA, USA), were introduced into 293T
cells (donated by American type culture collection), and the
resulting virus (Lenti-MPKG) was harvested and titrated. Briefly,
5×104/well 293T cells were plated onto 6 well plate 1
day before infection. Subsequently, these cells were 10-fold serial
dilutied. 3 days after infection, these cells were digested by EDTA
solution and centrifuged. The titer of virus was determined by
real-time qPCR (20,21). A control virus containing EGFP alone
(Lenti-MPG) was generated in parallel.
Generation of stable Rb-KAI1
cells
HXO-Rb44-Gl cells and Y79 cells were infected with
Lenti-MPKG to generate stable HXO-Rb44-Gl-KAI1+ and
Y79-KAI1+ cells, respectively. The stable Rb-KAI1 cells
were selected using 4 µg/ml puromycin. HXO-Rb44-Gl and Y79 cells
were infected with Lenti-MPG to generate the stable control cell
lines, HXO-Rb44-Gl-KAI1− and Y79-KAI1−.
Stable transduced cells were maintained in RPMI-1640 medium
containing 10% FBS and 2 µg/ml puromycin.
Immunofluorescence staining
analysis
Cultured cells were collected by centrifugation
(15,800 × g for 15 min at 4°C), washed with cold PBS and
fixed in 4% paraformaldehyde. Fixed cells were subsequently blocked
with PBS containing 3% bovine serum albumin (BiYunTian, ST023,
Shanghai, China) for 1 h, and incubated with monoclonal mouse
anti-KAI1 antibody (1:100) overnight at 4°C. Cells were washed with
PBS and centrifuged (3,600 × g for 15 min at 4°C) prior to
incubation with rhodamine-tagged secondary antibody (BiYunTian,
A-0568, Shanghai, China) at 37°C for 1 h. Tagged cells were
subsequently mounted with DAPI-containing mounting solution and
observed using a fluorescence microscope.
Cell proliferation analysis
The Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was used to quantify
cell growth. Cells (HXO-Rb44-GI-KAI1+,
HXO-Rb44-Gl-KAI1−, HXO-Rb44-Gl; Y79-KAI1+,
Y79-KAI1−, Y79) were seeded into 96-well plates at a
density of 1×104 cells per well in 100 µl RPMI-1640 10%
FBS. At the indicated time points (1 day, 2 day, 3 day, 4 day, 5
day, 6 day, 7 day), 10 µl CCK-8 was added to each well and the
plate was incubated for 1 h at 37°C. The optical absorption of each
well at 450 nm was measured using a spectrophotometer.
Cell migration assay
The cell migration assay was performed using a
Transwell® chamber and polyethylene terephthalate
membrane (PET) insert (Corning Inc., Corning, NY, USA) with 8 µm
pores. A total of 100 µl cells (0.5×106/ml)
(HXO-Rb44-GI-KAI1+, HXO-Rb44-Gl-KAI1−,
HXO-Rb44-Gl; Y79-KAI1+, Y79-KAI1−, Y79) and
200 µl serum free RPMI-1640 media were added to the top chamber. A
total of 500 µl RPMI-1640 media with 10% FBS was added to the
bottom chamber as a chemoattractant. Each cell line was plated in
three duplicate wells. Following 24 h incubation (37°C, 5%
CO2), the migratory cells in the bottom chambers were
counted using a light microscope in five randomly selected visual
fields.
Cell invasion assay
The cell invasion assay was performed using a
modified Transwell chamber. PET inserts with 8 µm pores were
pre-coated with 30 µl 20% Matrigel® (BD Biosciences,
Franklin Lakes, NJ, USA) diluted in RPMI-1640 media. A total of 100
µl cells (1×106/ml) (HXO-Rb44-GI-KAI1+,
HXO-Rb44-Gl-KAI1−, HXO-Rb44-Gl; Y79-KAI1+,
Y79-KAI1−, Y79) and 200 µl serum free RPMI-1640 media
were added to the top chamber. A total of 500 µl RPMI 1640 media
with 10% FBS was added to the bottom chamber. Each cell line was
plated in three duplicate wells. Following 48 h incubation (37°C,
5%CO2), the invasive cells in the bottom chambers were
counted using a light microscope in five randomly selected visual
fields.
Statistical analysis
Values are presented as the mean ± standard
deviation. Data was analyzed using the Student's t-test (Two groups
comparisons), or one-way analysis of variance (ANOVA) test
(multigroups comparisons) with post hoc contrasts by
Student-Newman-Keuls test, and using Statistical Analysis Software
(SAS 9.3 Institute, Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in KAI1 expression in human RB
tissues
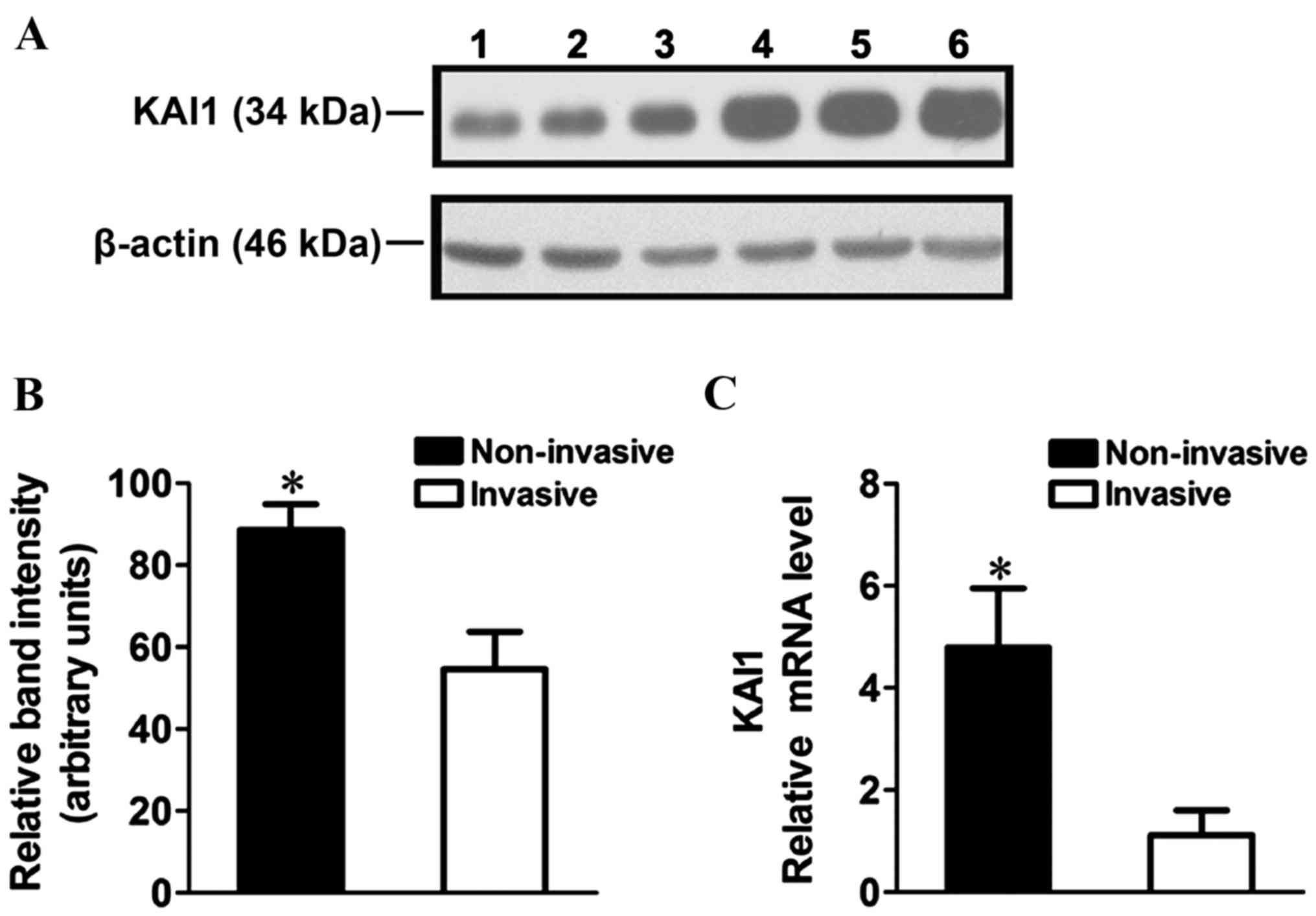
KAI1 mRNA and protein expression in human RB tissue
was analyzed using RT-qPCR and western blot analysis. A ~2-fold
decrease in KAI1 protein expression (Fig.
1A and B; P=0.0061) and ~4-fold decrease in KAI1 mRNA
expression was observed in the invasive cells compared with the
non-invasive cells (Fig. 1C;
P<0.0001).
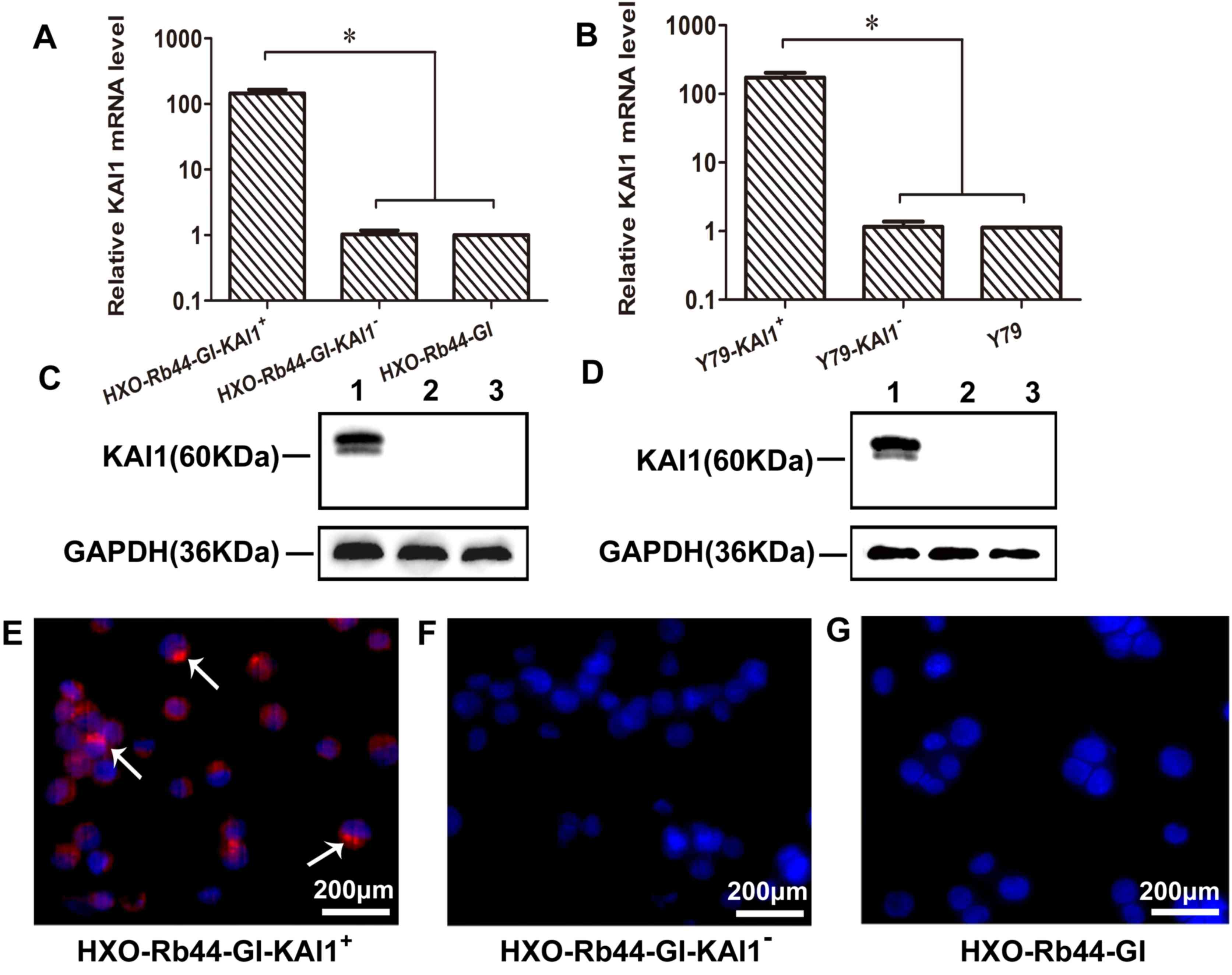
KAI1 overexpression in transduced RB
cell lines
Expression of KAI1 in transduced HXO-Rb44-Gl and Y79
cells was analyzed using RT-qPCR, western blotting and
immunofluorescence staining (Fig. 2).
A >100-fold increase in KAI1 mRNA expression was observed in the
KAI1+ cell lines (Fig. 2A and
B; P<0.0001). Positive KAI1 bands at 60 kDa were observed in
the KAI1+ cell lines and not in the KAI1− or
control cells (Fig. 2C and D). In
addition, KAI1 protein expression was observed in the cell membrane
and nucleus of HXO-Rb44-GI-KAI1+ cells using
immunofluorescence staining but was not observed in the
corresponding KAI− cells (Fig.
2E-G). These results are consistent with immunofluorescence
staining patterns observed in previous studies (22).
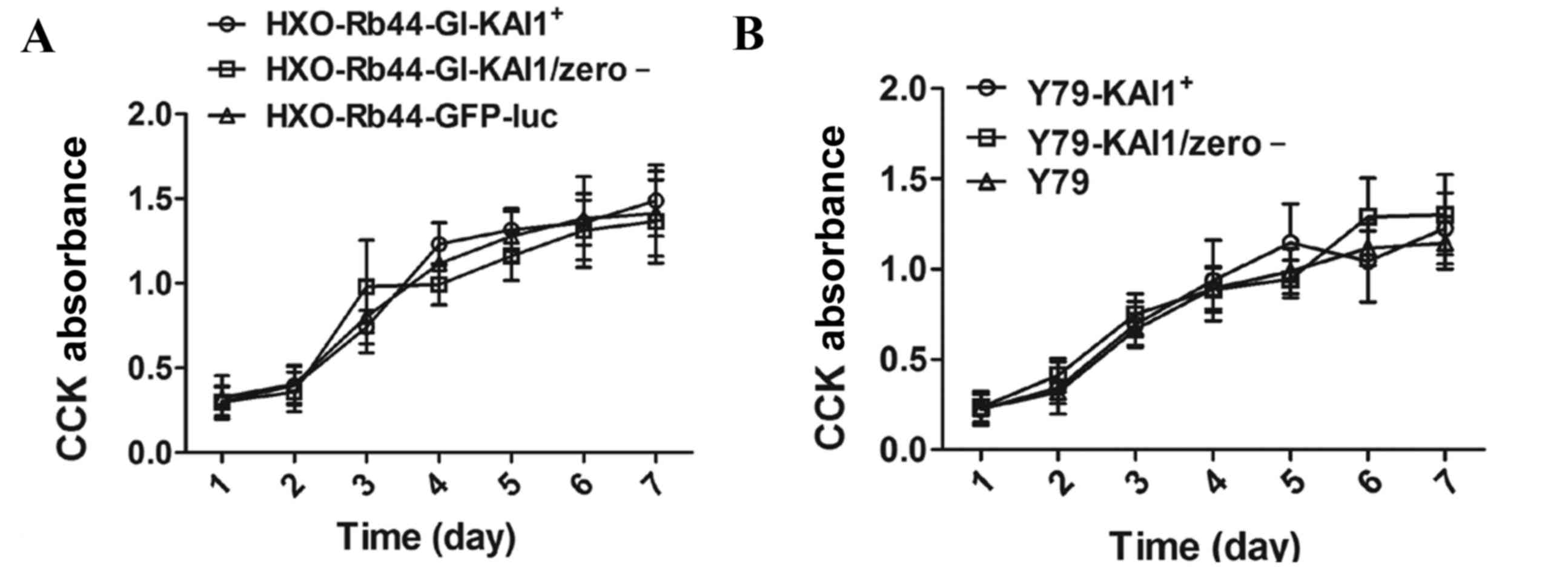
Effect of KAI1 overexpression on cell
growth
CCK-8 analysis was performed on HXO-Rb44-Gl cells
and Y79 cells over 7 consecutive days. No significant difference in
cell growth was observed between the KAI1 overexpressing and
control cells (Fig. 3).
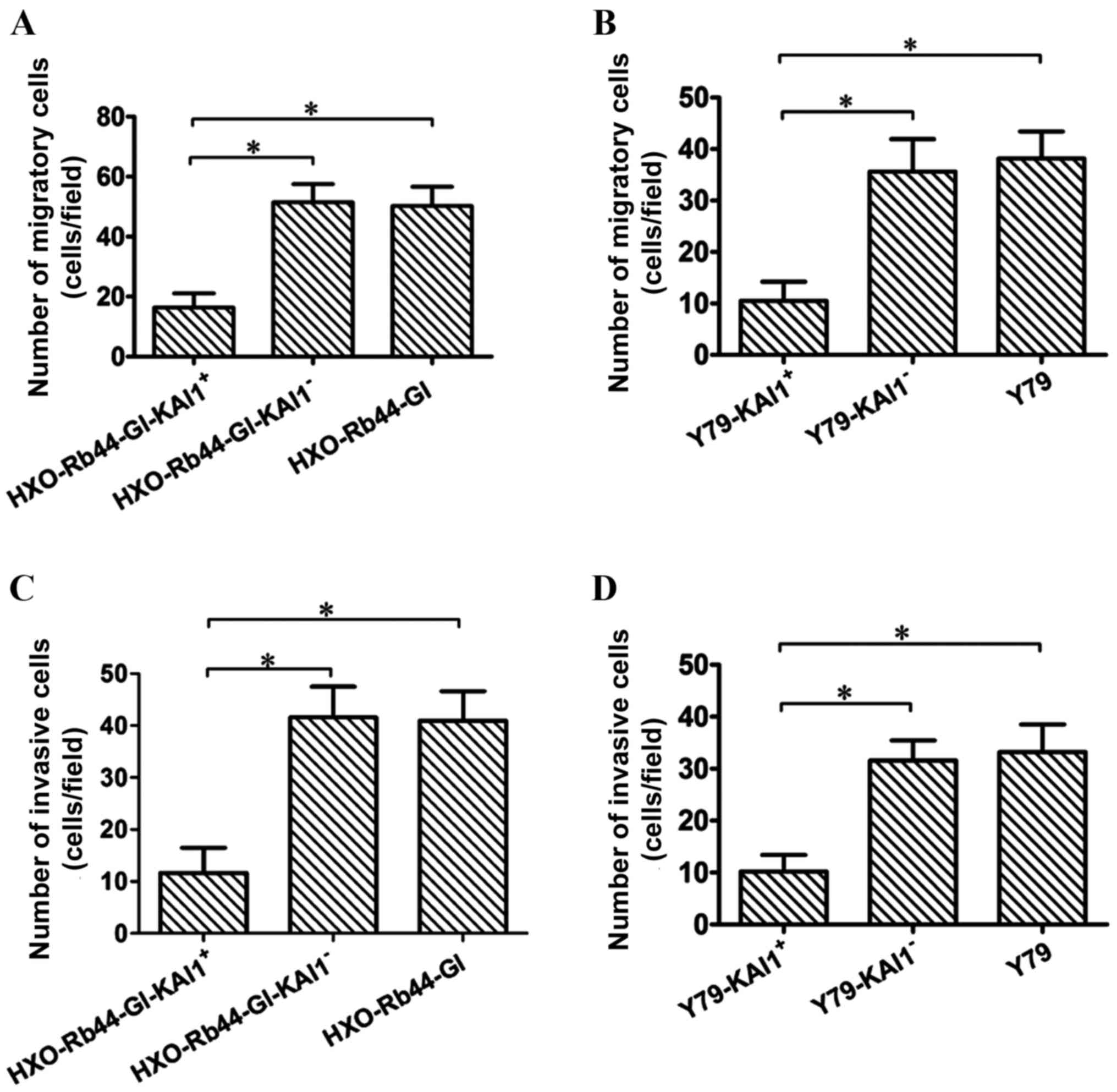
Effect of KAI1 on RB cell migration
and invasion
The migration and invasion of transduced HXO-Rb44-Gl
cells and Y79 cells was compared with control cells. The number of
migratory cells in the HXO-Rb44-Gl-KAI1+ group was
significantly decreased compared with the
HXO-Rb44-Gl-KAI1− and HXO-Rb44-Gl groups (Fig. 4A; P<0.0001). Similarly, the number
of migrated cells in the Y79-KAI1+ group was
significantly decreased compared with the Y79-KAI1− and
Y79 groups (Fig. 4B;
P<0.0001).
A statistically significant reduction in the
invasive ability of the cells was observed in the KAI1 and Y79
transduced cell lines compared with the respective control cell
lines. The number of invasive cells was 11.65±4.85, 41.55±5.92 and
40.95±5.67 in the HXO-Rb44-Gl-KAI1+,
HXO-Rb44-Gl-KAI1− and HXO-Rb44-Gl cell lines,
respectively (Fig. 4C; P<0.0001).
The number of invasive cells was 10.20±3.20, 31.55±3.89 and
33.20±5.32 in the Y79-KAI1+, Y79-KAI1− and
Y79 cell lines, respectively (Fig.
4D; P<0.0001).
Discussion
Poor RB prognosis is primarily due to the occurrence
of distant metastasis and organ infiltration (5). Developing novel methods of metastasis
inhibition is an important aim of cancer research. The KAI1 gene is
a known suppressor of tumor metastasis (23–25) and
has been implicated in the regulation of cell adhesion,
proliferation, motility, fusion, signaling and differentiation
(26). To investigate the effect of
KAI1 on RB migration and invasion, KAI1 expression in RB tissue was
investigated in the present study. In addition, the effect of KAI1
overexpression on RB cell migration and invasion was examined.
KAI1 expression levels in RB tissues were evaluated,
and KAI1 mRNA and protein were observed to be expressed at
decreased levels in invasive RB tissue compared with non-invasive
RB tissue. Consistent with the results of the present study, a
previous report demonstrated a reduction in the expression of KAI1
protein in metastasized human RB samples (16). Similar results were reported in other
types of tumor tissue, for example, increased KAI1 protein
expression was observed in early stage colorectal cancer, but the
expression level decreased as the cancer progressed into the later
stages (27). Research on breast
cancer demonstrated a 10-fold decrease in KAI1 mRNA expression in
metastatic lesions compared with the primary tumors (28). Therefore, the reduction in KAI1
expression may be involved in the RB metastatic process.
The results of the present study demonstrated that
overexpression of KAI1 in HXO-Rb44-Gl cells and Y79 cells did not
have any significant effect on cell growth, which is consistent
with observations made in breast and other types of cancer
(29,30). Increased levels of migration and
invasion are associated with cancer metastasis, and previous
research using the Boyden chamber assay indicated that pancreatic
cancer cells infected with KAI1 demonstrate a reduced invasive
ability (30). Similar observations
were reported in studies in hepatocellular carcinoma and breast
cancer (29,31). Consistent with the literature, the
results of the present study demonstrated that migration and
invasion is significantly inhibited in HXO-Rb44-Gl-KAI1+
and Y79-KAI1+ cells compared with KAI1− RB
cells, suggesting that KAI1 suppresses migration and invasion in
RB.
However, the exact mechanism of KAI1-mediated cancer
metastasis inhibition remains unclear. It has been reported that
KAI1 may suppress tumor metastasis by linking to cell surface
molecules, including tetraspanins, integrins, epidermal growth
factor receptor and protein kinase C (32,33).
Additionally, KAI1 has been demonstrated to stabilize E-cadherin
and β-catenin complexes in malignant cells, inhibiting tumor
metastasis (34).
Epithelial-mesenchymal transition (EMT) increases levels of cell
migration, causing epithelial cells to lose epithelial
characteristics and gain a mesenchymal phenotype (35). Previously, KAI1 has been demonstrated
to cause certain EMT-associated genetic changes, including
upregulation of cadherin 1 and catenin-α 1, and downregulation of
hepatocyte growth factor and fibronectin 1 (22). In addition, upregulation of matrix
metalloproteinases, and downregulation of TIMP metalloproteinase
inhibitor 1 and SRC proto-oncogene, non-receptor tyrosine kinase
are implicated in the inhibition of KAI1 in metastatic tumors
(22). Therefore, KAI1 may suppress
metastasis by regulating EMT, and regulating the migratory and
invasive abilities of RB cells, however further research into the
molecular mechanisms of KAI1-mediated metastasis inhibition in RB
is required.
In conclusion, KAI1 may serve an essential role in
the regulation of malignant cell migration and invasion in RB. The
results of the present study may aid in the development of novel
treatments to prevent and regulate RB metastasis.
Acknowledgements
The authors thank all staff of the central
laboratory of Xinhua Hospital (Shanghai, China), in particular Dr
Rang Xu, for providing various experimental services. The authors
thank Dr Dafeng Xu (Molecular Biology Department, East China
University Of Science and Technology) for assistance with the
production of the manuscript and Dr Hui Wang (Department of
Orthopedics, Xinhua Hospital Affiliated to Shanghai Jiaotong
University School of Medicine) for help with the data analysis.
References
|
1
|
Kiss S, Leiderman YI and Mukai S:
Diagnosis, classification, and treatment of retinoblastoma. Int
Ophthalmol Clin. 48:135–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Q, Wang Y, Wang H, Liu Y, Liu T and
Kunda PE: Tandem therapy for retinoblastoma: Immunotherapy and
chemotherapy enhance cytotoxicity on retinoblastoma by increasing
apoptosis. J Cancer Res Clin Oncol. 139:1357–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shields CL and Shields JA: Basic
understanding of current classification and management of
retinoblastoma. Curr Opin Ophthalmol. 17:228–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Moura LR, Marshall JC, Di Cesare S,
Fernandes BF, Antecka E and Burnier MN: The effect of imatinib
mesylate on the proliferation, invasive ability, and
radiosensitivity of retinoblastoma cell lines. Eye (Lond).
27:92–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chawla B, Hasan F, Azad R, Seth R,
Upadhyay AD, Pathy S and Pandey RM: Clinical presentation and
survival of retinoblastoma in Indian children. Br J Ophthalmol.
100:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunkel IJ, Khakoo Y, Kernan NA, Gershon T,
Gilheeney S, Lyden DC, Wolden SL, Orjuela M, Gardner SL and
Abramson DH: Intensive multimodality therapy for patients with
stage 4a metastatic retinoblastoma. Pediatr Blood Cancer. 55:55–59.
2010.PubMed/NCBI
|
|
7
|
Dong JT, Lamb PW, RinkerSchaeffer CW,
Vukanovic J, Ichikawa T, Isaacs JT and Barrett JC: KAI1, a
metastasis suppressor gene for prostate cancer on human chromosome
11p11.2. Science. 268:884–886. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JJ, Jin YB, Lee YJ, Lee JS, Lee YS,
Ko YG and Lee M: KAI1 suppresses HIF-1α and VEGF expression by
blocking CDCP1-enhanced Src activation in prostate cancer. BMC
Cancer. 12:812012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HA, Park I, Byun HJ, Jeoung D, Kim YM
and Lee H: Metastasis suppressor KAI1/CD82 attenuates the matrix
adhesion of human prostate cancer cells by suppressing fibronectin
expression and β1 integrin activation. Cell Physiol Biochem.
27:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gellersen B, Wolf A, Kruse M, Schwenke M
and Bamberger AM: Human endometrial stromal cell-trophoblast
interactions: Mutual stimulation of chemotactic migration and
promigratory roles of cell surface molecules CD82 and CEACAM1. Biol
Reprod. 88:802013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng J, Huang C, Wren JD, Wang DW, Yan J,
Zhang J, Sun Y, Han X and Zhang XA: Tetraspanin CD82: A suppressor
of solid tumors and a modulator of membrane heterogeneity. Cancer
Metastasis Rev. 34:619–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouras T and Frauman AG: Expression of the
prostate cancer metastasis suppressor gene KAI1 in primary prostate
cancers: A biphasic relationship with tumour grade. J Pathol.
188:382–388. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mooez S, Malik FA, Kayani MA, Rashid R,
Zahid A and Khan A: Expressional alterations and transcript
isoforms of metastasis suppressor genes (KAI1 and KiSS1) in breast
cancer patients. Asian Pac J Cancer Prev. 12:2785–2791.
2011.PubMed/NCBI
|
|
14
|
Friess H, Guo XZ, BeRBerat P, Graber HU,
Zimmermann A, Korc M and Büchler MW: Reduced KAI1 expression in
pancreatic cancer is associated with lymph node and distant
metastases. Int J Cancer. 79:349–355. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiwu WU, Lan Y, Wenqing S, Lei Z and
Yisheng T: Expression and clinical significance of CD82/KAI1 and
E-cadherin in non-small cell lung cancer. Arch Iran Med.
15:707–712. 2012.PubMed/NCBI
|
|
16
|
Lakshmi S Amirtha, Pushparaj V,
Krishnamurthy V, Biswas J, Krishnakumar S and Shanmugam MP:
Tetraspanin protein KAI1 expression in retinoblastoma. Br J
Ophthalmol. 88:593–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji X, Zhang J, Cheng L, Wei F, Li H, Liu
X, Chen X, Li C, Wang Y and Huang Q: Oncolytic adenovirus
delivering herpes simplex virus thymidine kinase suicide gene
reduces the growth of human retinoblastoma in an in vivo mouse
model. Exp Eye Res. 89:193–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu H, Wang C, Zhu H, Liu S, Xu X and Jiang
Y: Characteristics of an established retinoblastoma cell line
HXO-Rb44. Yan Ke Xue Bao. 11:16–21. 1995.PubMed/NCBI
|
|
20
|
Lv K, Guo Y, Zhang Y, Wang K, Li K, Zhu Y
and Sun S: Transient inhibition of foot-and-mouth disease virus
replication by siRNAs silencing VP1 protein coding region. Res Vet
Sci. 86:443–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Husic N, Lin Y and Snider BJ:
Production of lentiviral vectors for transducing cells from the
central nervous system. J Vis Exp. 24:e40312012.
|
|
22
|
Ji XD, Yan H and Zhao PQ: Expression
changes of tumor metastasis-related genes after overexpression of
KAI1 in retinoblastoma Y79 cells. Zhonghua Yan Ke Za Zhi.
48:1097–1101. 2012.(In Chinese). PubMed/NCBI
|
|
23
|
Zhang B, Liu W, Li L, Lu J, Liu M, Sun Y
and Jin D: KAI1/CD82 and Cyclin D1 as biomarkers of invasion,
metastasis and prognosis of laryngeal squamous cell carcinoma. Int
J Clin Exp Pathol. 6:1060–1067. 2013.PubMed/NCBI
|
|
24
|
Yang X, Wei LL, Tang C, Slack R, Mueller S
and Lippman ME: Overexpression of KAI1 suppresses in vitro
invasiveness and in vivo metastasis in breast cancer cells. Cancer
Res. 61:5284–5288. 2001.PubMed/NCBI
|
|
25
|
Xu JH, Guo XZ, Ren LN, Shao LC and Liu MP:
KAI1 is a potential target for anti-metastasis in pancreatic cancer
cells. World J Gastroenterol. 14:1126–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruseva Z, Geiger PX, Hutzler P, Kotzsch M,
Luber B, Schmitt M, Gross E and Reuning U: Tumor suppressor KAI1
affects integrin alphavbeta3-mediated ovarian cancer cell adhesion,
motility, and proliferation. Exp Cell Res. 315:1759–1771. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lombardi DP, Geradts J, Foley JF, Chiao C,
Lamb PW and Barrett JC: Loss of KAI1 expression in the progression
of colorectal cancer. Cancer Res. 59:5724–5731. 1999.PubMed/NCBI
|
|
28
|
Stark AM, Tongers K, Maass N, Mehdorn HM
and Held-Feindt J: Reduced metastasis-suppressor gene
mRNA-expression in breast cancer brain metastases. J Cancer Res
Clin Oncol. 131:191–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malik FA, Sanders AJ, Kayani MA and Jiang
WG: Effect of expressional alteration of KAI1 on breast cancer cell
growth, adhesion, migration and invasion. Cancer Genomics
Proteomics. 6:205–213. 2009.PubMed/NCBI
|
|
30
|
Liu X, Guo XZ, Zhang WW, Lu ZZ, Zhang QW,
Duan HF and Wang LS: KAI1 inhibits HGF-induced invasion of
pancreatic cancer by sphingosine kinase activity. Hepatobiliary
Pancreat Dis Int. 10:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang JM, Peng ZH, Si SH, Liu WW, Luo YH
and Ye ZY: KAI1 gene suppresses invasion and metastasis of
hepatocellular carcinoma MHCC97-H cells in vitro and in animal
models. Liver Int. 28:132–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu WM and Zhang XA: KAI1/CD82, a tumor
metastasis suppressor. Cancer Lett. 240:183–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miranti CK: Controlling cell surface
dynamics and signaling: How CD82/KAI1 supresses metastasis. Cell
Signal. 21:196–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abe M, Sugiura T, Takahashi M, Ishii K,
Shimoda M and Shirasuna K: A novel function of CD82/KAI-1 on
E-cadherin-mediated homophilic cellular adhesion of cancer cells.
Cancer Lett. 266:163–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|