Introduction
Gastric carcinoma (GC) remains a major health issue
worldwide, with 1 million newly diagnosed cases and 700,000
mortalities each year (1). Occurrence
of GC varies with geography, and in Asia or the Pacific Islands,
the incidences and mortality rates of GC can be twice as high as
those in Western countries (2). GC is
considered as a multifactorial disease due to numerous inherited
and environmental factors, including genetic background, infectious
agents and dietary habits (3).
Surgery remains the only curative therapy for GC treatment, and
perioperative and adjuvant chemotherapy can improve the outcome
(4,5).
However, no clear superiority of one strategy over others has been
observed, and serious side effects and dose-limiting toxicities of
chemotherapy treatments are common (6). In addition, >50% of resected GC
patients experience reccurrence and metastases (7). Safe, natural and non-toxic agents that
can interfere with the essential steps of cancer development are
increasingly used for cancer therapy (8,9).
Traditional Chinese medicines (TCMs) have recently drawn great
attention as possible anticancer agents with few side effects.
Trametes robiniophila Murr. (Huaier) is a
fungal species in China that has been applied in TCM for >1,600
years (10). In recent years, the
antitumor effect of Huaier has been demonstrated, and Trametes
robiniophila extracted from the fungus in hot water to
eliminate the free proteins and amino acids has been increasingly
applied in clinical cancer therapy (10,11). The
major active ingredient of Trametes robiniophila is
proteoglycan, including 41.53% polysaccharides, 12.93% amino acids
and 8.72% water (12). However, the
inhibition effect on cancer of proteoglycan and other isolated
ingredients was much lower than that of Trametes
robiniophila (13,14). The antitumor effect of Trametes
robiniophila involves various mechanisms, including induction
of apoptosis, anti-angiogenesis, drug resistance reversal,
anti-metastasis and system immune activation (15). The current study attempted to
investigate the antitumor effect of Trametes robiniophila on
GC using the human GC cell line MKN-45. The apoptosis of the cell
line was examined with acridine orange (AO)/ethidium bromide (EB)
staining and flow cytometry, and the expression level of molecules
involved in the apoptotic and metastatic processes of tumors were
analyzed using reverse transcription-polymerase chain reaction
(RT-PCR) and western blotting.
Materials and methods
Preparation of Trametes
robiniophila
Trametes robiniophila was purchased from
Qidong Gaitianli Pharmaceutical Co., Ltd. (Qidong, China).
Trametes robiniophila (1.0 g) was dissolved in 10 ml
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and sterilized using a 0.22-µm filter to obtain the 100
mg/ml stock solution that was suitable for long-term storage at
37°C.
Preparation of the MKN-45 cell
line
The GC cell line MKN-45 was obtained from the
Shanghai Institute of Cellular Biology of Chinese Academy of
Sciences (Shanghai, China) and was routinely cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (Hangzhou Sijiqing
Bioengineering Material Co., Ltd., Hangzhou, Zhejiang, China), 100
U/ml penicillin and 100 mg/ml streptomycin (Biyuntian, Jiangsu,
China) under the conditions of 5% CO2 at 4°C.
AO/EB staining to detect
apoptosis
The cell concentrations were adjusted to
5×104 cells/ml, and cells were incubated on slides in
24-well plates for 24 h. For experimental use, the Trametes
robiniophila stock solution was diluted to a final
concentration of 5 or 10 mg/ml, with 0 mg/ml serving as control.
AO/EB (4 µg; Amresco, LLC, Solon, OH, USA) was added to each well
after 48 h. The apoptosis of the cell line was detected with a
fluorescence microscope at 510 nm. Each treatment was represented
by three replicates.
Flow cytometric analysis of
apoptosis
Cells were cultured with Trametes
robiniophila for 24, 48 and 72 h as aforementioned. Annexin
V-fluorescein isothiocyanate (5 µl; Kaiji Bio Co., Nanjing, China)
was added to different wells and incubated for 10 min at room
temperature. The cells were resuspended with 1X binding buffer, and
5 µl propidium iodide (PI) was added. Next, the apoptosis of the
cells was analyzed by flow cytometry. Each treatment was
represented by three replicates.
RT-PCR
Total RNA was isolated from cells treated with
Trametes robiniophila for 24 h using the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) (16). RT-PCR was used to detect the gene
expression level of matrix metalloproteinase (MMP)-2, MMP-9,
B-cell lymphoma (Bcl)-2, Fas, caspase-3 and the reference
gene β-actin. Primers and annealing temperature information
is shown in Table I. The RNA was
reversely transcribed into complementary DNA using an RT-PCR kit
(Thermo Fisher Scientific, Inc.), and the final reaction mixture
(20 µl) contained 4 µl of 5X reaction buffer, 1 µl RNase inhibitor,
1 µl oligo(dT)18 primer, 2 µl 10 mM deoxynucleotides, 1
µl Moloney-murine leukemia virus reverse transcriptase, 2 µl RNA
template and 9 µl double distilled H2O. The thermal
cycling parameters for the amplification were as follows: A
denaturation step at 95°C for 3 min, followed by 30 cycles at 95°C
for 15 sec, the annealing temperature indicated in Table I for 30 sec and 72°C for 45 sec. The
RT-PCR products were semi-quantified with the UVP Gel Imaging
System (UVP, Inc., Upland, CA, USA).
 | Table I.Detailed information of primers. |
Table I.
Detailed information of primers.
| Gene | Sequence
(5′-3′) | Ta
(°C) | Length (bp) |
|---|
| Bcl-2 | Forward
GCTGTCGCAGAGGGGCTAC | 55 | 375 |
|
| Reverse
ATCCTCCCCCAGTTCACCC |
|
|
| Fas | Forward
CTGCCATAAGCCCTGTCCTC | 56 | 316 |
|
| Reverse
GGTGTTGCTGGTGAGTGTGC |
|
|
| Caspase-3 | Forward
CAAATGGACCTGTTGACCTGA | 56 | 351 |
|
| Reverse
ATTCTGTTGCCACCTTTCGG |
|
|
| MMP-2 | Forward
GATGCCGCCTTTAACTGG | 55 | 278 |
|
| Reverse
TCAGCAGCCTAGCCAGTCG |
|
|
| MMP-9 | Forward
CAGTACCGAGAGAAAGCCTATTTCTG | 54 | 101 |
|
| Reverse
TAGGTCACGTAGCCCACTTGGT |
|
|
| β-actin | Forward
GGGAAATCGTGCGTGACATT | 55 | 183 |
|
| Reverse
GGAAGGAAGGCTGGAAGAGTG |
|
|
Western blot analysis
The cells and Trametes robiniophila were
cultured as aforementioned. Total protein was extracted from the
cells using a 400 µl single detergent lysis buffer (including
phenylmethylsulfonyl fluoride) and radioimmunoprecipitation buffer
(Sangon Biotech, Shanghai, China). The lysis mixture was then added
to a homogenizer, and centrifuged at 13,500 × g and 4°C for 5 min.
The supernatant was subpackaged into Eppendorf tube and stored at
−20°C. The extracts were boiled with loading buffer for 5 min and
then subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 10% gels. Targeted proteins were transferred
onto polyvinylidene difluoride membranes. The membranes were washed
with Tris-buffered saline containing Tween 20 three times, for 20
min each time. Then, the membranes were incubated overnight with
antibodies against Bcl-2 (catalog no. 15071), caspase-3 (catalog
no. 9668) and Fas (catalog no. 8023) (all Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C. Following an additional
three washes, anti-mouse immunoglobulin G, horseradish
peroxidase-linked secondary antibodies (catalog no. 7076; 1:3,000
dilution; Cell Signaling Technology, Inc.) were added and incubated
at room temperature for 1 h. Upon three final washes, the blots
were developed using Beyo ECL Plus reagent (Beyotime Institute of
Biotechnology, Haimen, China) and the results were detected in the
UVP Gel Imaging System. β-actin was used as reference. The
expression levels were calculated with Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All the data were expressed as the mean ± standard
deviation. Differences between the groups were calculated using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. All statistical analyses
were conducted using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Cell apoptosis is induced by Trametes
robiniophila
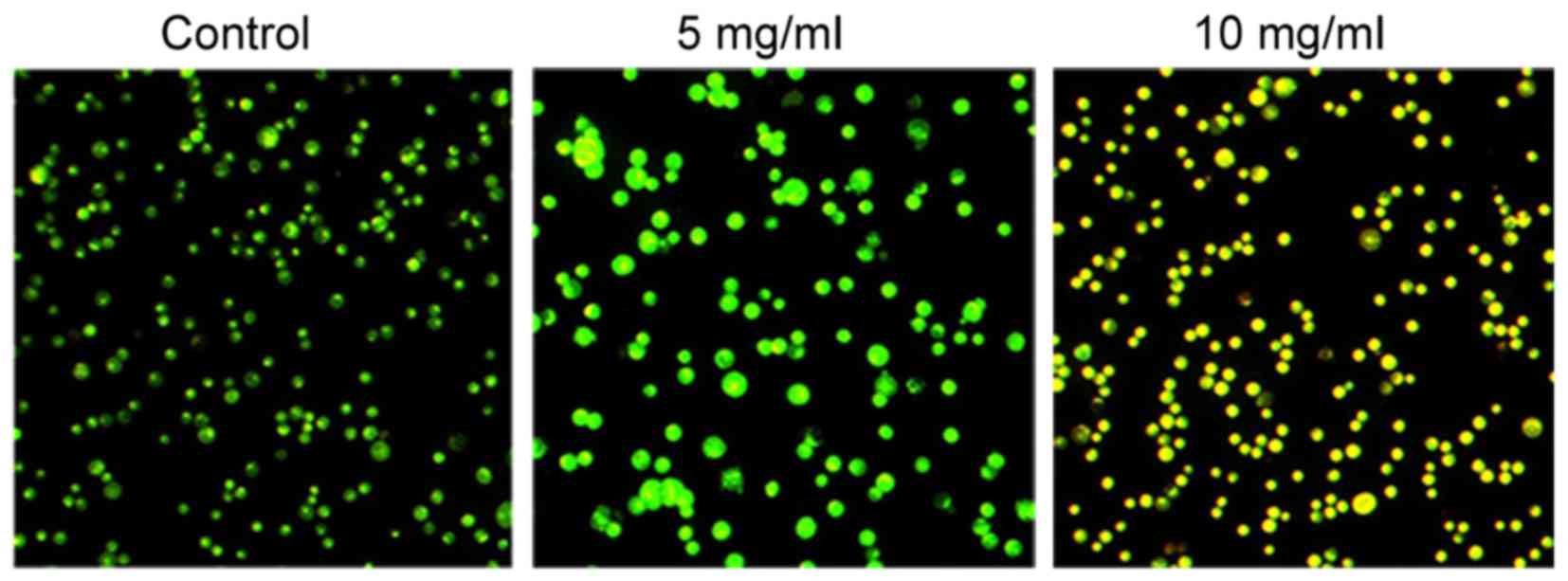
Morphological changes in the human GC cell line
MKN-45 following treatment with different concentrations of
Trametes robiniophila (0, 5 and 10 mg/ml) for 24 h are shown
in Fig. 1. Compared with the control
group, the majority of the Trametes robiniophila-treated
cancer cells became enlarged and irregular-shaped, and exhibited
vacuolated changes in the cytoplasm. These morphological changes
demonstrated cell damage subsequent to Trametes robiniophila
treatment. MKN-45 cells treated with 5 mg/ml Trametes
robiniophila were yellow-dyed, indicating the occurrence of
apoptosis (Fig. 1).
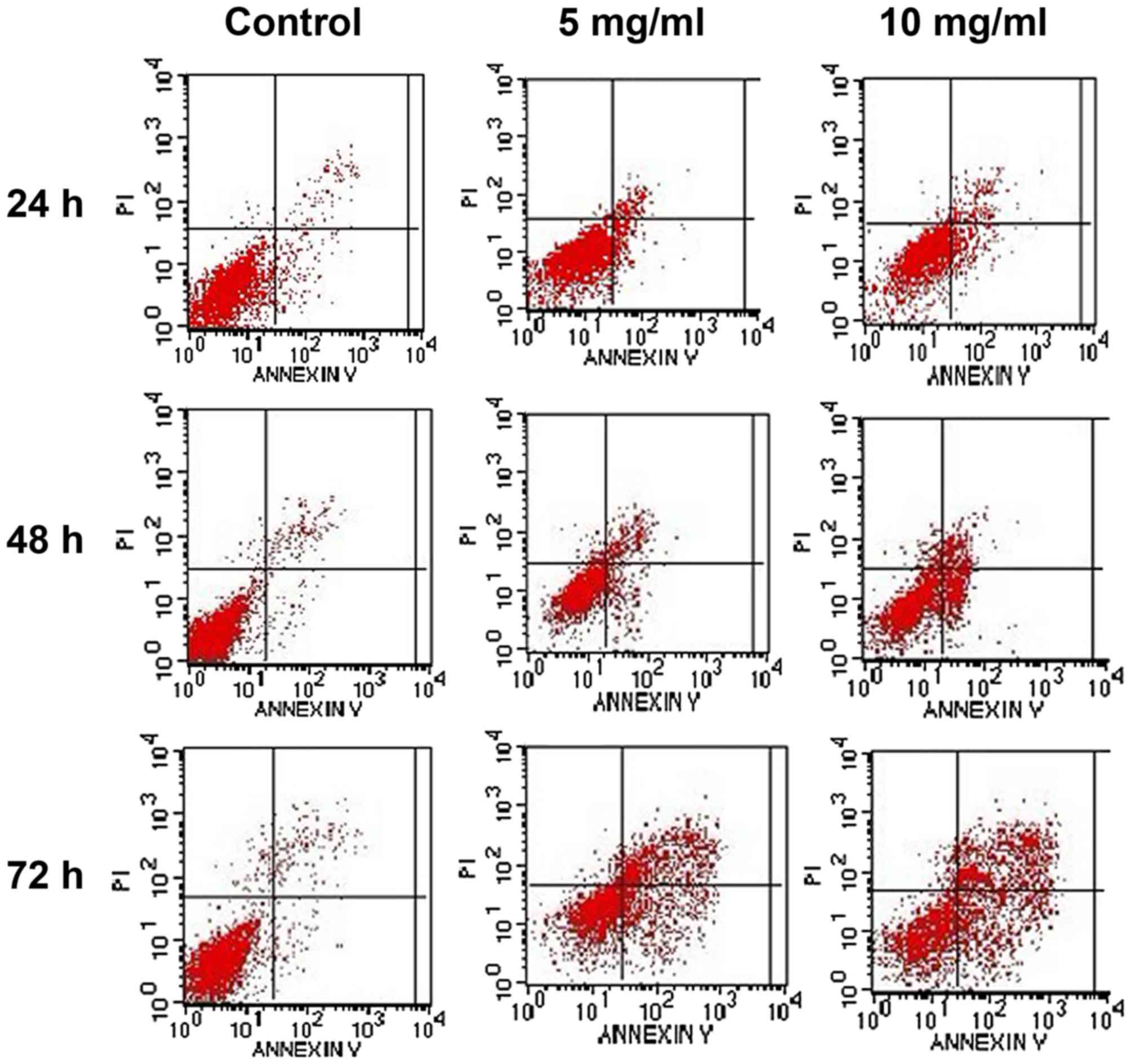
Flow cytometry was used to detect intact cells,
early apoptotic cells, late apoptotic cells and dead cells.
Following treatment with Trametes robiniophila, the cell
death rate [as indicated by the upper right (UR) quadrant, which
represents the percentage of cells in advanced-stage apoptosis] and
the early apoptosis rate [as indicated by the lower right (LR)
quadrant, which represents the percentage of cells in prophase
apoptosis] of the MKN-45 cell line increased in a time- and
dose-dependent manner (Fig. 2).
Apoptotic cells (as indicated by the sum of the UR and LR
quadrants, which represents the percentage of cells in all
apoptosis stages) of the MKN-45 cell line were significantly
different from those of the control group after being exposed to
Trametes robiniophila for 24, 48 and 72 h at drug
concentrations of 5 and 10 mg/ml (all P<0.001) (Table II).
 | Table II.Proportion of apoptotic cells in the
MKN-45 cell line. |
Table II.
Proportion of apoptotic cells in the
MKN-45 cell line.
|
| MKN-45 |
|---|
|
|
|
|---|
| Apoptotic cells
(%) | 24 h | 48 h | 72 h |
|---|
| Control |
6.5 |
7.3 |
7.5 |
| 5 mg/ml | 14.6a | 18.3a | 50.2a |
| 10 mg/ml | 18.0a | 24.5a | 58.0a |
Trametes robiniophila significantly
influences the transcription of MMP-2, MMP-9, Bcl-2, Fas and
caspase-3
The expression of MMP-2, MMP-9, Bcl-2, Fas and
caspase-3 was significantly influenced by Trametes
robiniophila. Analysis of the optical density value revealed
that, for the MKN-45 cell line, the expression of MMP-2, MMP-9 and
Bcl-2 was significantly downregulated (at 5 mg/ml and 10 mg/ml, all
P<0.001 vs. control; at 10 mg/ml, P<0.001, P=0.003 and
P=0.007 vs. 5 mg/ml for MMP-2, MMP-9 and Bcl-2, respectively),
while the expression of Fas and caspase-3 was upregulated (at 5
mg/ml, P=0.326 and P=0.100 vs. control for Fas and caspase-3,
respectively; at 10 mg/ml, P<0.001 and P=0.020 vs. control, and
P=0.017 and P=0.028 vs. 5 mg/ml for Fas and caspase-3,
respectively) (Table III). The
regulation was dose-dependent.
 | Table III.Expression changes in MMP-2, MMP-9,
Bcl-2, Fas and caspase-3 genes induced by Trametes
robiniophila in the MKN-45 cell line. |
Table III.
Expression changes in MMP-2, MMP-9,
Bcl-2, Fas and caspase-3 genes induced by Trametes
robiniophila in the MKN-45 cell line.
|
| MKN-45 |
|---|
|
|
|
|---|
| Gene | Control | 5 mg/ml | 10 mg/ml |
|---|
| MMP-2 | 0.64±0.02 |
0.49±0.01a |
0.36±0.02a,b |
| MMP-9 | 0.71±0.01 |
0.54±0.02a |
0.47±0.02a |
| Bcl-2 | 1.20±0.06 |
0.83±0.05a |
0.64±0.05a,b |
| Fas | 0.67±0.06 |
0.83±0.06a |
0.90±0.04a |
| Caspase-3 | 0.65±0.01 |
0.70±0.03a |
0.99±0.08a,b |
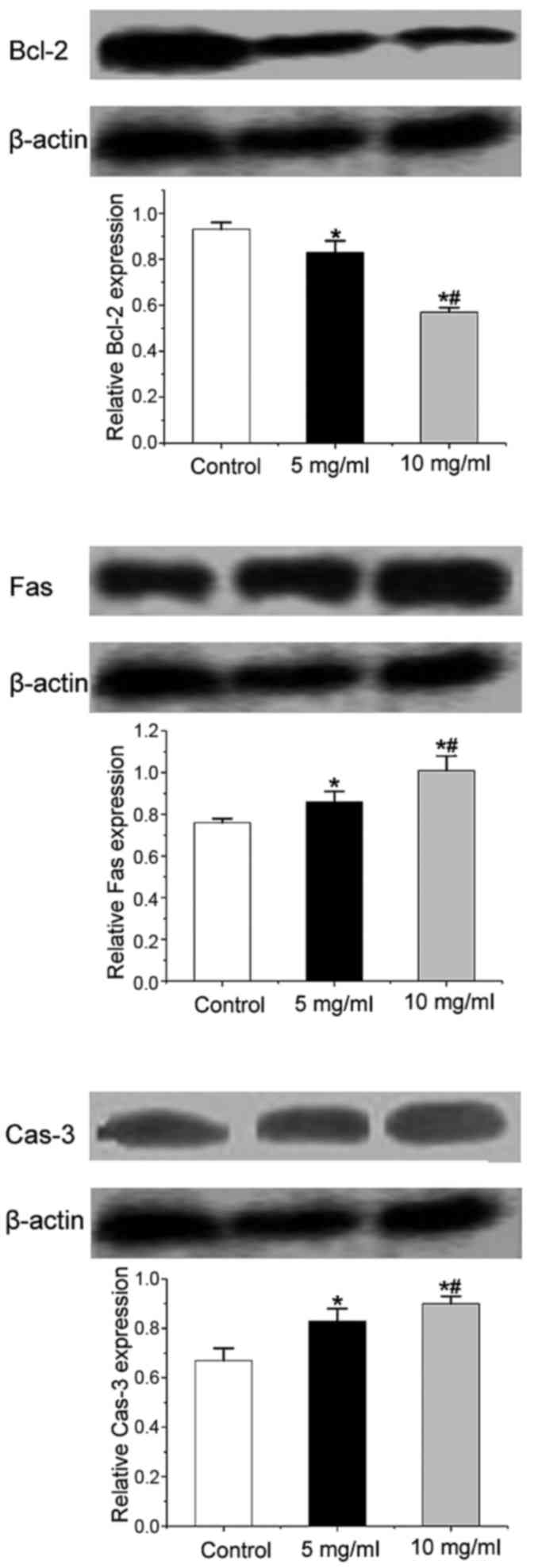
Trametes robiniophila significantly
influences the protein expression of Bcl-2, Fas and caspase-3
The production of Fas and caspase-3 was enhanced
following treatment with Trametes robiniophila in the MKN-45
cell line; however, the synthesis of Bcl-2 decreased. These results
were consistent with the pattern of RT-PCR validation. The
regulation was dose-dependent (Fig.
3).
Discussion
Generally, treatments for GC patients include
surgery, radiotherapy and chemotherapy (17). Certain alternative treatments such as
gene therapy and targeted therapy have also been proposed as
optional treatment methods (18,19).
However, these therapies are usually costly for the majority of
patients and have limited curative effect and serious side effects
(20). In the past years, TCM, which
has been widely used in China for thousands of years, has shown an
anticancer potential effect (21).
TCM has been demonstrated to reduce toxic side effects, improve the
quality of life of patients, enhance the immune function, and
prevent recurrence and metastasis in cancer patients, with few side
effects (22). In the current study,
AO/EB staining, flow cytometry and RT-PCR were conducted to detect
the effect of Trametes robiniophila on the apoptosis and
metastasis of the human GC cell line MKN-45. The results indicated
that Trametes robiniophila changed the morphology and number
of regular MKN-45 cells in a time- and dose-dependent manner,
suggesting cell damage due to treatment with Trametes
robiniophila (Figs. 1 and
2, Table
II). Our results also demonstrated that Trametes
robiniophila acts as an inducer of the apoptotic process in GC
cells by influencing the expression of Fas, caspase-3
and Bcl-2 (Figs. 2 and
3, Table
III). However, the effect was significantly different between 5
and 10 mg/ml. Considering the potential toxic effect of high doses
of Trametes robiniophila on normal cells, 5 mg/ml should be
taken as a reference for future clinical application.
Generally, apoptosis can be classified into two
types: Apoptotic process mediated by the mitochondrial pathway and
apoptotic process mediated by a membrane receptor signaling pathway
(23). Caspase-3 is one of the key
apoptosis executors, since the majority of factors that trigger
apoptosis ultimately lead to apoptosis through the
caspase-3-mediated signaling pathway (24,25).
Bcl-2, which is mainly distributed in the mitochondrial membrane
and the cytoplasm, is an intracellular anti-apoptotic factor that
can stabilize the mitochondrial membrane, prevent mitochondrial
caspase release and inhibit the oxygen free radical-induced
apoptosis signaling pathway (26).
Fas can initiate the apoptotic process mediated by a membrane
receptor by binding to Fas ligand, and can also recruit caspase-3
to induce apoptosis (27–30). Treatment with Trametes
robiniophila suppressed Bcl-2 expression and increased
caspase-3 expression, which suggested a
mitochondrial-mediated apoptosis. Additionally, the expression of
Fas was also upregulated, indicating activation of the death
receptor pathway of apoptosis. The results in the present study
demonstrated that Trametes robiniophila could induce the
apoptosis of GC cells by different mechanisms, whereas previously,
only apoptosis induced by Trametes robiniophila via the
mitochondrial pathway was reported (11).
The apoptosis observed in the present study was
associated with downregulation of the expression of MMP-2
and MMP-9. Members of the MMP family are an integral part of
the extracellular matrix's enzymatic arsenal and have been regarded
as major critical molecules that assist tumor cells during
metastasis (31). The expression of
various MMPs has been observed to be upregulated in almost every
type of human cancer, and correlates with advanced stage, invasive
and metastatic properties, and, in general, with poor prognosis
(32). Upon treatment with
Trametes robiniophila, the expression of MMP-2 and
MMP-9 was suppressed. The effect was dose dependent. Ji and
Mai have revealed that the expression level of MMP-2 and
MMP-9 was positively correlated with the metastatic ability
of GC cells (33). In addition, a
previous study has reported the inhibition of metastasis of lung
cancer via the suppression of MMP-2 and MMP-9
(22). Therefore, Trametes
robiniophila has the potential to regulate MMPs and can be
considered a promising target for the therapeutic intervention in
human cancer. Similarly to the majority of TCMs, Trametes
robiniophila also has the advantage of few side effects and low
cost compared with surgery, radiotherapy and chemotherapy (34).
Based on the present study, treatment with
Trametes robiniophila could markedly induce the apoptosis of
the human GC cell line MKN-45. The effect acted through both the
mitochondrial and the member receptor signaling pathways. In
addition, Trametes robiniophila could also suppress the
metastatic ability of GC cells via downregulating the expression of
MMP-2 and MMP-9. In conclusion, we recommend that
Trametes robiniophila is taken into consideration as a
noninvasive therapy of GC in future clinical treatment.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shahrokh I: Gastric cancer as a
multifactorial disease. Ann Mil Health Sci Res. 11:157–164.
2013.
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: MAGIC Trial Participants: Perioperative
Chemotherapy versus surgery alone for resectable gastroesophageal
cancer. New Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: CLASSIC trial
investigators: Adjuvant capecitabine and oxaliplatin for gastric
cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label,
randomised controlled trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monsuez JJ, Charniot JC, Vignat N and
Artigou JY: Cardiac side-effects of cancer chemotherapy. Int J
Cardiol. 144:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Angelica M, Gonen M, Brennan MF,
Turnbull AD, Bains M and Karpeh MS: Patterns of initial recurrence
in completely resected gastric adenocarcinoma. Ann Surg.
240:808–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sagar SM, Yance D and Wong R: Natural
health products that inhibit angiogenesis: A potential source for
investigational new agents to treat cancer-Part 1. Current Oncol.
13:14–26. 2006.
|
|
9
|
Bhat TA and Singh RP: Tumor angiogenesis-a
potential target in cancer chemoprevention. Food Chem. Toxicol.
46:1334–1345. 2008.
|
|
10
|
Wu T, Chen W, Liu S, Lu H, Wang H, Kong D,
Huang X, Kong Q, Ning Y and Lu Z: Huaier suppresses proliferation
and induces apoptosis in human pulmonary cancer cells via
upregulation of miR-26b-5p. FEBS Lett. 588:2107–2114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang F, Zhang Z and Liu Z: Effects of
Huaier aqueous extract on proliferation and apoptosis in the
melanoma cell line A875. Acta Histochem. 115:705–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang T, Wang K, Zhang J, Wang X, Chen Z,
Ni C, Qiu F and Huang J: Huaier aqueous extract inhibits colorectal
cancer stem cell growth partially via downregulation of the
Wnt/β-catenin pathway. Oncol Lett. 5:1171–1176. 2013.PubMed/NCBI
|
|
13
|
Guo Y, Cheng P and Chen Y: Isolation and
analysis of the polysaccharide of Huaier mycelium. Zhong Guo Sheng
Hua Yao Wu Za Zhi. 63:56–59. 1993.(In Chinese).
|
|
14
|
Guo Y, Cheng P and Chen Y: Studies on the
constituents of polysaccharide from the hyphae of Trametes
robiniophila (II)-identification of polysaccharide from the hyphae
of Trametes robiniophila and determination of its molar ratio.
Journal of China Pharmaceutical University. 23:155–157. 1992.
|
|
15
|
Zhang F, Zhang ZY and Liu Z: Effects of
Huaier aqueous extract on proliferation and apoptosis in the
melanoma cell line A875. Acta Histochemica. 115:705–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Li X, Kong X, Moran MS, Su P,
Haffty BG and Yang Q: Testin is a tumor suppressor and prognostic
marker in breast cancer. Cancer Sci. 103:2092–2101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Wang W, Chen Y, Huang K, Shuai X,
Chen Q, Li X and Lian G: The investigation of polymer-siRNA
nanoparticle for gene therapy of gastric cancer in vitro. Int J
Nanomedicine. 5:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang TT, Qian XP and Liu BR: Survivin:
Potential role in diagnosis, prognosis and targeted therapy of
gastric cancer. World J Gastroenterol. 13:2784–2790.
2007.PubMed/NCBI
|
|
20
|
Li HW, Yang JK and Zhao AG: Adjuvant
therapy for gastric cancer. Shijie Huaren Xiaohua Zazhi.
22:4921–4927. 2014.(In Chinese).
|
|
21
|
Song X, Li Y, Zhang H and Yang Q: The
anticancer effect of Huaier (Review). Oncol Rep. 34:12–21.
2015.PubMed/NCBI
|
|
22
|
Hu B, Du Q, Shen K and Xu L: Principles
and scientific basis of traditional Chinese medicine in cancer
treatment. J Bioanal Biomed. S6:22012.
|
|
23
|
Salahudeen AK, Huang H, Joshi M, Moore NA
and Jenkins JK: Involvement of the mitochondrial pathway in cold
storage and rewarming-associated apoptosis of human renal proximal
tubular cells. Am J Transplant. 3:273–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao RV, Hermel E, CastroObregon S, del Rio
G, Ellerby LM, Ellerby HM and Bredesen DE: Coupling endoplasmic
reticulum stress to the cell death program mechanism of caspase
activation. J Biol Chem. 276:33869–33874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin L, Amatya VJ, Takeshima Y, Shrestha L,
Kushitani K and Inai K: Evaluation of apoptosis and
immunohistochemical expression of the apoptosis-related proteins in
mesothelioma. Hiroshima J Med Sci. 59:27–33. 2010.PubMed/NCBI
|
|
26
|
Bagci E, Vodovotz Y, Billiar T, Ermentrout
G and Bahar I: Bistability in apoptosis: Roles of bax, bcl-2 and
mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu D, Wang S and Feng Y: Expression of
matrix metalloproteinase-7 and Fas and their significances in
gastric carcinoma. Shi Jie Hua Ren Xiao Hua Za Zhi. 14:32372006.(In
Chinese).
|
|
28
|
Morimoto Y, Hizuta A, Ding EX, Ishii T,
Hongo T, Fujiwara T, Iwagaki H and Tanaka N: Functional expression
of Fas and Fas ligand on human intestinal intraepithelial
lymphocytes. Clin Exp Immunol. 116:84–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Wang YR and Yang J: Significance
of integrin β_3 expression in human hemangioma (J). Chinese Journal
of Aesthetic Medicine. 5:0312009.
|
|
30
|
Cai CF, Feng L, Wang L, Kong QZ and Zhao
YF: Tetrazolium violet induces apoptosis via caspases-8, −9
activation and Fas/FasL up-regulation in Rat C6 glioma cells. Arch
Pharm Res. 32:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rucci N, Sanità P and Angelucci A: Roles
of metalloproteases in metastatic niche. Curr Mol Med. 11:609–622.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hesek D, Toth M, Meroueh SO, Brown S, Zhao
H, Sakr W, Fridman R and Mobashery S: Design and characterization
of a metalloproteinase inhibitor-tethered resin for the detection
of active MMPs in biological samples. Chem Biol. 13:379–386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji DL and Mai DH: Effect of huaier granule
on immunity and quality of life in patients with gastric cancer
undergoing postoperative concurrent radiochemotherapy. Zhong Guo
Zhong Liu. 19:e62010.(In Chinese).
|
|
34
|
Song XY, YingDong LI, Shi YP, Jin L and
Chen J: Quality control of traditional Chinese medicines: A review.
Chin J Nat Med. 11:596–607. 2013. View Article : Google Scholar : PubMed/NCBI
|