Introduction
Cholangiocarcinoma (CCA) is a highly lethal type of
cancer that arises from the bile duct epithelium (1). Although CCA is a rare form of cancer,
the global incidence of CCA is increasing, particularly that of the
intrahepatic subtype (2). However,
the highest incidence of CCA has been reported in the northeast of
Thailand where the carcinogenic liver fluke Opisthorchis
viverrini is considered a causative agent (3). The early diagnosis of CCA is challenging
as patients with CCA typically have non-specific symptoms, and CCA
tumor markers are yet to be developed (4). The majority of patients with CCA present
with a late-stage disease that is frequently inoperable, and the
outcomes of conventional chemotherapy or radiotherapy are
unsatisfactory (5). Therefore, a
unique approach to developing novel targeting therapies is required
to reduce CCA-associated mortality.
Cimetidine is an antagonist of histamine type-2
(H2) receptors and is indicated for patients with
gastro-esophageal reflux diseases, peptic ulcers or hypersecretory
conditions (6,7). The effect of cimetidine on the survival
of patients has been reported in various types of cancer, including
gastric cancer (8), colorectal cancer
(6,9,10), renal
cell carcinoma (11), malignant
melanoma (12) and glioblastoma
(13). Numerous molecular mechanisms
underlying the anticancer activities of cimetidine have been
revealed, including the inhibition of cell proliferation by
blocking the cell growth-promoting effect of histamine (14), the inhibition of tumor angiogenesis
(15), the stimulation host immune
responses (16) and the suppression
of cell adhesion (17). However, the
effects of cimetidine in CCA have yet to be demonstrated.
In the present study, the anti-CCA activity of
cimetidine was examined and the antiproliferative and
apoptosis-inducing effects of cimetidine were determined. This was
revealed to be partially due to the suppression of protein kinase B
(Akt) phosphorylation. A CCA transplant xenograft Balb/c
recombination activating gene 2 (Rag-2)/Janus kinase 3 (Jak3)
double deficient (Balb/c R/J) mouse model demonstrated the anti-CCA
effects of cimetidine in vivo. The results of the current
study suggest that cimetidine is a potential anti-CCA agent.
Materials and methods
Cell lines and reagents
The KKU-M055, KKU-M213 and KKU-214 distinct human
CCA cell lines (18) were provided by
Dr. Banchop Spira (Khon Kaen University, Khon Kaen, Thailand). The
cell lines were maintained in Dulbecco's modified Eagle's medium
(DMEM; Wako Pure Chemical Industries, Ltd., Osaka, Japan)
supplemented with 10% fetal bovine serum (HyClone Laboratories,
Inc., Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified incubator at 37°C and 5%
CO2. Cimetidine was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany).
MTT tetrazolium dye assay
The antiproliferative activities of cimetidine in
CCA cell lines were evaluated using an MTT assay (Sigma-Aldrich;
Merck Millipore). Briefly, 5×103 cells were seeded in
100 µl of DMEM onto 96-well plates in triplicate and incubated
overnight at 37°C in an atmosphere of 5% CO2. The medium
was then replaced with fresh DMEM containing various concentrations
of cimetidine (0, 2, 4, 6, 8 or 10 mM) and incubated at 37°C for 24
or 48 h. MTT solution (0.5 mg/ml final concentration) was
subsequently added to each well. Following a 3-h incubation, 100 µl
acidified isopropanol (HCl 34 µl/10 ml isopropanol) was added to
dissolve the formazan crystals. The absorption values at 595 nm
were determined using an automatic microplate reader (Ascent
Software Version 2.6; Multiskan; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The values were normalized to the control
(untreated) samples.
Annexin V binding assay
The number of apoptotic cells was quantified using
an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection
kit (eBioscience, Inc., San Diego, CA, USA). Following treatment
with cimetidine, the dead or unadhered cells were collected and
adherent cells were harvested by trypsinization (trypsin-EDTA
solution; Sigma-Aldrich; Merck Millipore), washed with Annexin V
binding buffer and incubated with Annexin V-FITC at room
temperature for 15 min in the dark. Next, stained cells were
treated with 1 µg/ml propidium iodide (PI; Sigma-Aldrich) prior to
flow cytometry analysis. The cells were analyzed using a BD LSR II™
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data
analysis was performed using FlowJo™ software (version 9.9.3; Tree
Star, Inc., Ashland, OR, USA).
PI staining
CCA cells were seeded at a density of
2×105 cells/well into a six-well plate and incubated
overnight at 37°C in an atmosphere of 5% CO2 followed by
treatment with cimetidine (0, 5 and 10 mM) for 24 and 48 h. The
unadhered cells were collected and adherent cells were harvested by
trypsinization, washed twice with cold phosphate-buffered saline
and fixed in 70% ethanol at 4°C overnight. The fixed cells were
stained with 10 µg/ml PI and incubated at room temperature for 30
min in the dark. The sub-G1 fraction of each cell sample
was analyzed using a BD LSR II™ flow cytometer. Data analysis was
performed using FlowJo™ software.
Protein extraction and western blot
analysis
Cells were lysed in NP-40 lysis buffer (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan), containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1 mM NaF, 1 mM
Na3VO4 (Sigma-Aldrich; Merck Millipore) and a
protease inhibitor cocktail (Nacalai Tesque, Inc., Kyoto, Japan),
and the supernatant was collected. Protein concentrations were
evaluated using a bicinchoninic acid protein assay (Thermo Fisher
Scientific, Inc.). The proteins extracted from the cell lysates (20
µg) were separated using 10% sodium dodecyl sulfate polyacrylamide
gel electrophoresis and transferred onto polyvinylidene fluoride
membranes (GE Healthcare Life Sciences, Chalfont, UK). The membrane
was blocked with 5% skim milk in Tris-buffered saline with 0.1%
Tween 20 (TBST) for 1 h at room temperature. Next, the membrane was
probed with β-actin-C-2 (catalog no., sc-8432; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), cleaved caspase-3 (Asp175;
5A1E; catalog no., 9664; Cell Signaling Technology, Inc., Danvers,
MA, USA), cleaved caspase-8 (Asp931; 18C8; catalog no., 9496; Cell
Signaling Technology, Inc.), caspase-9 (Cell Signaling Technology,
Inc.), protein kinase B (Akt) (human specific, catalog no., 9502;
Cell Signaling Technology, Inc.) and phospho-Akt (Thr308; D25E6;
catalog no., 13038; Cell Signaling Technology, Inc.) primary
antibodies (dilution, 1:1,000) overnight at 4°C. After washing
three times with TBST, the membrane was incubated with horseradish
peroxidase (HRP)-conjugated anti-rabbit (catalog no., 7074; Cell
Signaling Technology, Inc.) and anti-mouse (catalog no., 7076; Cell
Signaling Technology, Inc.) immunoglobulin G secondary antibodies
(dilution, 1:2,000) for 2 h at room temperature. Detection of the
proteins was performed using Chemi-Lumi One Super reagents (Nacalai
Tesque, Inc.). The protein bands were visualized using the
ImageQuant LAS400 system (GE Healthcare Life Sciences).
Xenograft mouse model
Balb/c RJ mice were established as described
previously (19) and were bred,
housed and monitored in the animal research facility at Kumamoto
University (Kumamoto, Japan) according to the institutional
guidelines. In the specific pathogen free conditions, mice were
kept at 22±2°C in 40–80% humidity, with a 12-h light/dark cycle.
Food and UV-treated water were supplied ad libitum. The
Institutional Animal Care and Use Committee of Kumamoto University
approved all experimental procedures and protocols used in the
current study. KKU-M213 cells (2×106) were subcutaneously injected
into the flanks of the 6–8 week-old female Balb/c RJ mice. The mice
were administered an intraperitoneal injection of 100 µl dimethyl
sulfoxide (DMSO) or cimetidine (200 mg/kg) one day following cell
transplantation, and then every day for a total of 12 days. Tumor
growth was monitored every 3 days using a vernier caliper. On day
13, mice were sacrificed by cervical dislocation and tumors were
removed and weighed. Body weights were recorded twice a week in
order to observe the condition of the mice.
Statistical analysis
The data are expressed as the mean ± standard
deviation. The significance of differences observed between the
experimental groups was determined using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
result. All of the statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Antiproliferative effects of
cimetidine on CCA cells
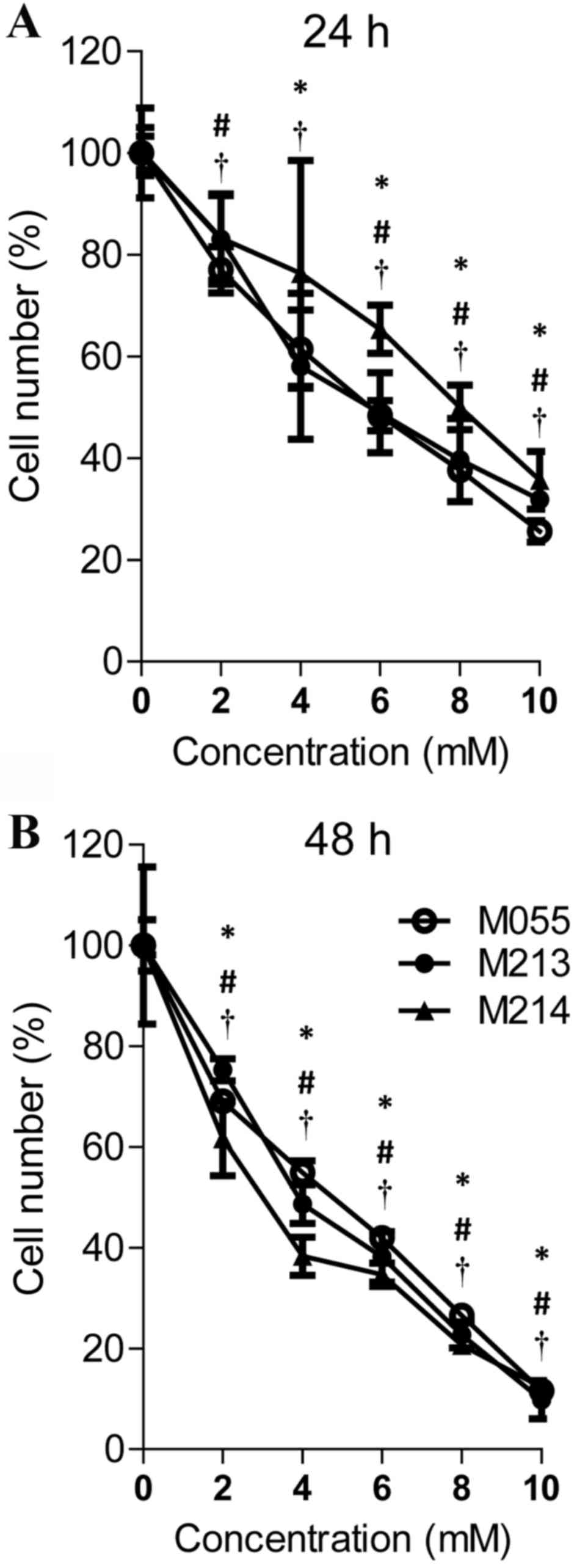
An MTT assay was used to determine whether treatment
with cimetidine induced the inhibition of CCA cell proliferation.
KKU-M055, KKU-M213 and KKU-M214 CCA cells were treated with
cimetidine at various concentrations for 24 or 48 h before the MTT
assay was performed. As shown in Fig.
1, cimetidine effectively inhibited cell proliferation in a
dose- and time-dependent manner. Treatment with 2, 4, 6, 8 and 10
mM cimetidine for 24 h and treatment with 2, 4, 6, 8 and 10 mM
cimetidine for 48 h significantly inhibited cell growth in KKU-M055
cells (P<0.05). The growth suppression effect on KKU-M213 cells
was observed in 4, 6, 8, 10 mM cimetidine treatment at 24 h and in
2, 4, 6, 8, 10 mM cimetidine treatment at 48 h. Cimetidine
effectively inhibited KKU-M214 at 2, 6, 8, 10 mM concentration at
24 h and at 2, 4, 6, 8, 10 mM at 48 h. The half-maximal inhibitory
concentration (IC50) values of cimetidine for KKU-M055,
KKU-M213 and KKU-M214 cells were 5.44, 5.69 and 7.83 mM at 24 h,
and 4.07, 3.95 and 3.18 mM at 48 h, respectively.
Cimetidine induces apoptosis in CCA
cells
In subsequent experiments, it was determined whether
the observed suppressive effects of cimetidine in the MTT assay
were due to the induction of apoptosis. An Annexin V binding assay
was used to detect the apoptotic cells. As presented in Fig. 2A, the proportions of early-stage
(Annexin V-positive/PI-negative) and late-stage (Annexin
V-positive/PI-positive) apoptotic cells increased in a dose- and
time-dependent manner. The sub-G1 population was also
analyzed using flow cytometry. As shown in Fig. 2B, the sub-G1 population
(apoptotic fraction) increased in a dose- and time-dependent
manner. These results suggest that the cell growth inhibition
induced by cimetidine treatment occurs via the stimulation of
apoptosis. To investigate caspase dependency in
cimetidine-dependent apoptosis, a western blot analysis was
performed in order to detect the activation of caspases. Cleaved
caspase-3, cleaved caspase-9 and cleaved caspase-8 expression
levels were determined to be dose- and time-dependently higher in
the cells treated with cimetidine, compared with untreated cells
(Fig. 2C). These results suggest that
cimetidine induces apoptosis in CCA cells via caspase-dependent
intrinsic and extrinsic signaling pathways.
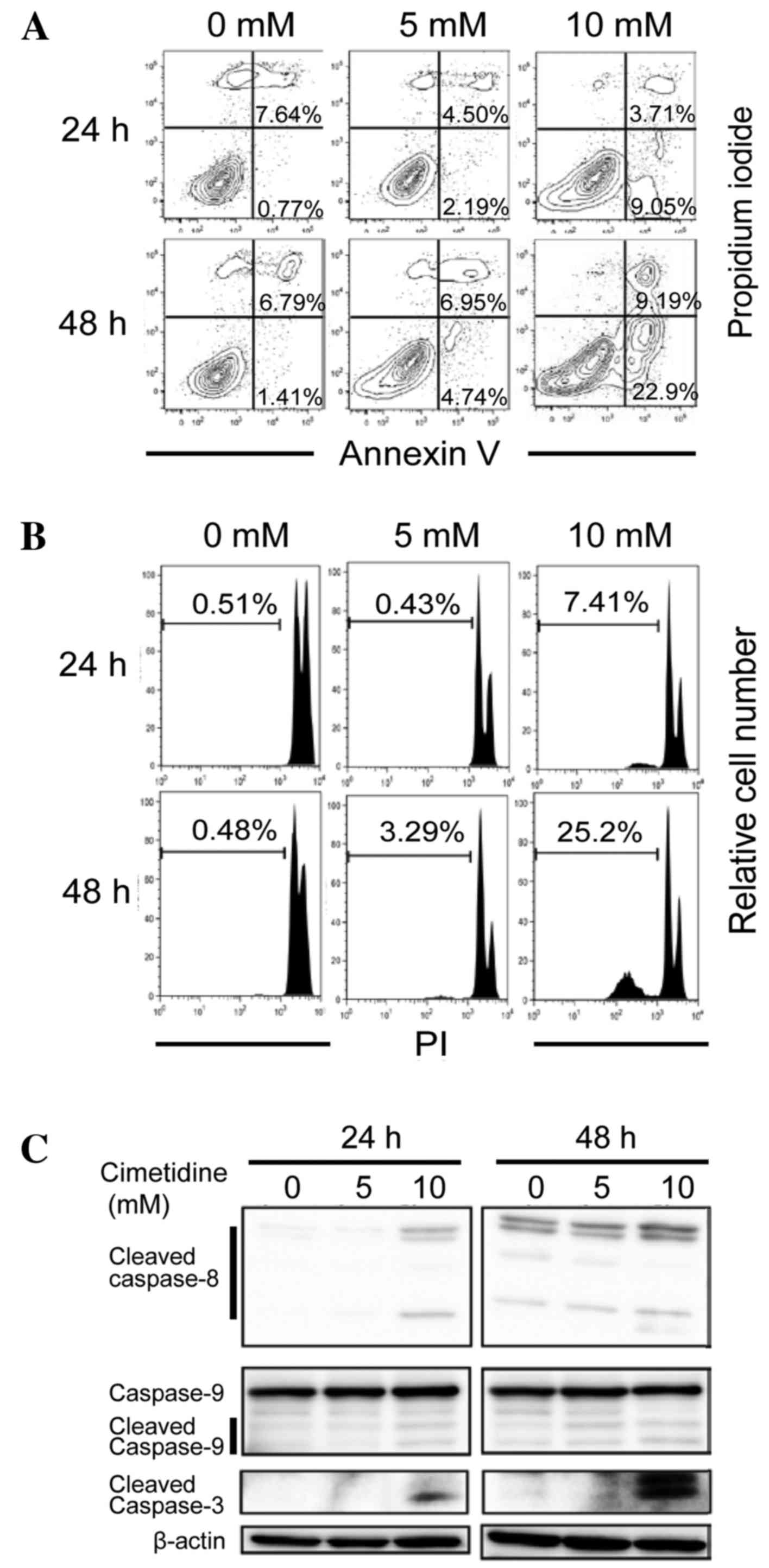 | Figure 2.Effects of cimetidine on the induction
of apoptosis in CCA cells. KKU-M213 cells were treated with
cimetidine at concentrations of 0, 5 and 10 mM for 24 and 48 h. (A)
Apoptotic cell death. At the indicated times, the cells were
harvested, stained with Annexin V-FITC and 1 µg/ml propidium iodide
and analyzed using flow cytometry. Right upper quadrant represents
Annexin V-PI double positive cells (late apoptotic cells) and the
right lower quadrant represents Annexin V positive cells (early
apoptotic cells). Following treatment with 0, 5 and 10 mM
cimetidine, the number of Annexin V positive cells was increased in
dose- and time-dependent manners. (B) Sub-G1 population.
Cells were fixed in 70% ethanol, stained with 10 µg/ml propidium
iodide and the sub-G1 population was determined by flow
cytometry. Percentages of the sub-G1 population were indicated in
the graphs, which revealed that the sub-G1 populations were
increased in a time-dependent manner following treatment with
cimetidine. (C) Caspase-dependent apoptosis. Total proteins were
extracted and western blotting was performed. Cimetidine treatment
induced the expression of cleaved caspase-8,-9 and −3 in a
time-dependent manner. The data are representative of three
independent experiments. CCA, cholangiocarcinoma; FITC, fluorescein
isothiocyanate. |
Cimetidine suppresses the
phosphorylation of Akt signaling
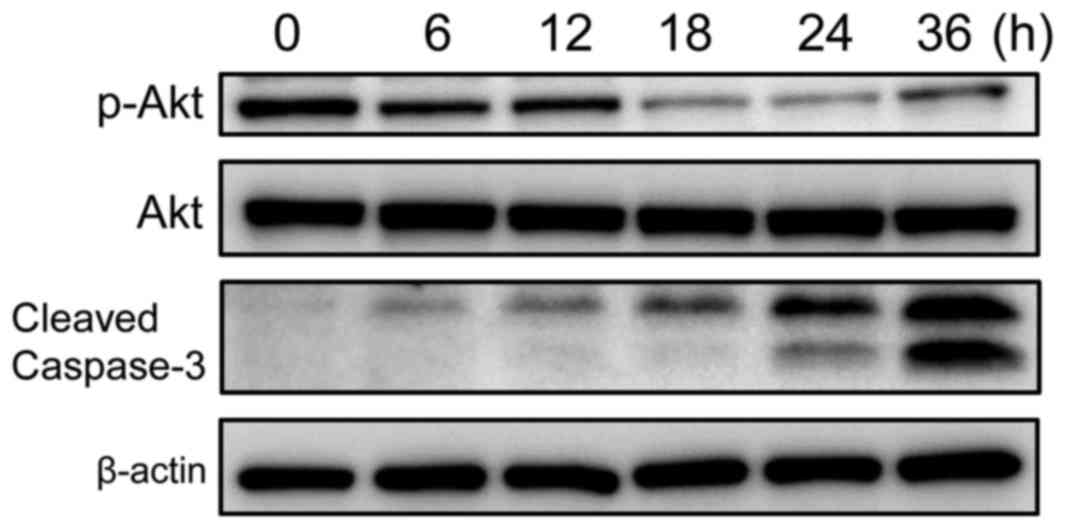
To explore the molecular mechanisms underlying
cimetidine-induced apoptosis in the KKU-M213 CCA cell line, and to
determine whether the Akt signaling pathway was involved in this
effect, KKU-M213 cells were treated with 10 mM cimetidine at
various time points (0–36 h). The results of the western blot
analysis are presented in Fig. 3.
Cimetidine significantly suppressed the phosphorylation of Akt in a
time-dependent manner, but did not affect total Akt expression
levels. This result suggested that cimetidine induces apoptosis in
KKU-M213 cells by inactivating the Akt signaling pathway.
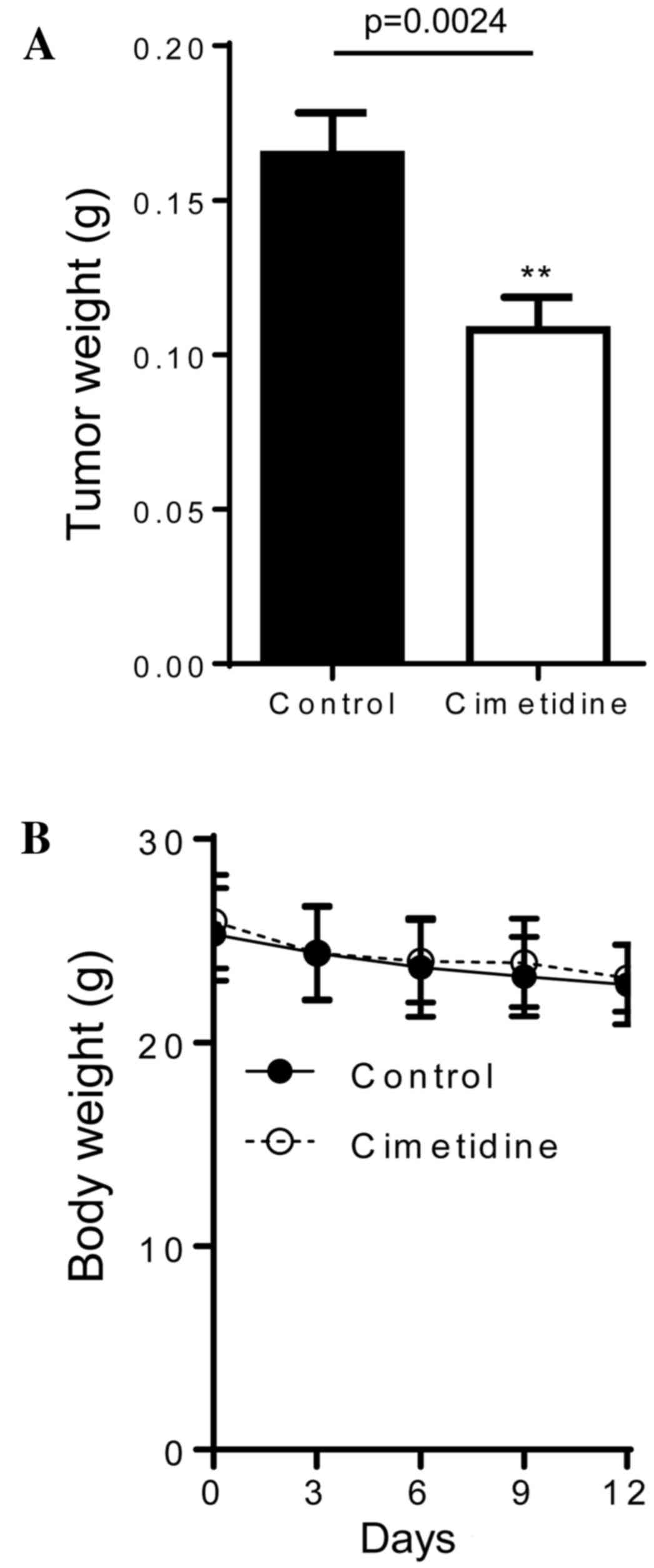
In vivo effects of cimetidine in
severely immunodeficient mice
The results of the in vitro experiments
suggested that cimetidine may be an effective treatment for CCA;
therefore, the in vivo effects of cimetidine in an
immunodeficient mouse model were investigated. Severely
immunodeficient Balb/c R/J mice were subcutaneously injected with
2×106 KKU-M213 cells in the flank. A dose of 200 mg/kg
cimetidine or DMSO alone was administered via an intraperitoneal
injection on day 1 following cell inoculation, and then every day
for 12 days. As shown in Fig. 4A, the
weights of the tumors in cimetidine-treated mice were significantly
lower (0.108±0.05 g; n=22), compared with those in untreated mice
(0.165±0.07 g; n=26; P=0.0024). All mice were observed to be
healthy and no significant differences were identified in body
weights between the treated and control groups (Fig. 4B).
Discussion
In the present study, the H2 receptor
antagonist cimetidine was demonstrated to be a potentially
effective chemotherapeutic agent for CCA. Cimetidine induced
caspase-dependent apoptotic cell death in CCA cells through the
inhibition of Akt phosphorylation. Furthermore, cimetidine
inhibited tumor growth in human CCA-bearing mice with no observable
adverse effects. As cimetidine is frequently used as H2
receptor antagonist without serious adverse effects (20,21), it
will be useful to repurpose cimetidine for the treatment of
CCA.
CCA is the second most common type of liver cancer
and exhibits aggressive characteristics, including a general
resistance to conventional chemotherapy (5). The molecular signature of CCA has
previously been investigated, which revealed that increased
expression of inflammation-related proteins was often observed in
CCA (22). Numerous signaling
pathways are involved in the carcinogenesis of CCA, including
transforming growth factor-β/mothers against decapentaplegic
homolog, interleukin-6/signal transducer and activator of
transcription, phosphoinositide 3-kinase/Akt, Wnt, rapidly
accelerated fibrosarcoma/mitogen-activated protein kinase
kinase/mitogen-activated protein kinase and Notch (23). Activated Akt and phospho-Akt are
recognized transcription activation and anti-apoptotic signaling
pathway components (24). Akt
signaling is a critical pathway in CCA and its potential use as a
target for CCA treatment has previously been suggested (25). In the current study, it was
demonstrated that the phosphorylation of Akt was suppressed by
cimetidine, and corresponded to the induction of apoptosis in CCA.
Therefore, it was hypothesized that cimetidine induces apoptosis in
CCA cell lines via the inhibition of Akt phosphorylation (25).
Cimetidine is considered to improve the survival of
patients with malignant tumors such as gastric and colorectal
cancers (6,8,9). It has
been demonstrated to inhibit tumor growth by several underlying
mechanisms, including the inhibition of cancer cell proliferation
(20), the blockade of tumor
angiogenesis (15) and the
enhancement of immune activity (26).
A previous study demonstrated that cimetidine induced
myeloid-derived suppressor cell apoptosis in mice (27). These results suggested that cimetidine
directly suppresses tumor growth via various antitumor effects and
indirectly via specific modifications of the tumor
microenvironment, including angiogenesis, and of the host immune
responses. Therefore, combination treatment with cimetidine may
potentiate the effects of current chemotherapy regimens
(5-fluorouracil or gemcitabine with oxaliplatin) that are employed
to treat patients with CCA.
In conclusion, the present study demonstrated the
potent anti-CCA activity of cimetidine in vitro and in
vivo. To the best of our knowledge, this study is the first to
demonstrate that cimetidine induces caspase-dependent apoptosis via
the suppression of the Akt signaling pathway. These results suggest
that cimetidine is a potentially effective candidate for the
treatment of patients with CCA.
Acknowledgements
The authors would like to thank Ms. I. Suzu and Ms.
S. Fujikawa (Division of Hematopoiesis, Center for AIDS Research,
Kumamoto University, Kumamoto, Japan) for their technical
assistance and Ms. Y. Kanagawa (Division of Hematopoiesis, Center
for AIDS Research, Kumamoto University, Kumamoto, Japan) for
secretarial assistance. This study was supported in part by
Grants-in-Aid for Scientific Research (grant no. 25114711) from the
Ministry of Education, Science, Sports and Culture of Japan, and by
the Royal Golden Jubilee-PhD Program co-funding with Khon Kaen
University, Thailand (to Ms. Paweena Dana and Dr. Sopit Wongkham;
no. PHD/0192/2552).
Glossary
Abbreviations
Abbreviations:
|
CCA
|
cholangiocarcinoma
|
|
FACS
|
fluorescence- activated cell
sorting
|
|
PI
|
propidium iodide
|
References
|
1
|
Vatanasapt V, Sriamporn S and Vatanasapt
P: Cancer control in Thailand. Jpn J Clin Oncol. 32:(Suppl).
S82–S91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou
Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, et
al: Intrahepatic cholangiocarcinoma: Rising frequency, improved
survival, and determinants of outcome after resection. Ann Surg.
248:84–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wongkham S and Silsirivanit A: State of
serum markers for detection of cholangiocarcinoma. Asian Pac J
Cancer Prev. 13:(Suppl). S17–S27. 2012.
|
|
5
|
Anderson CD, Pinson CW, Berlin J and Chari
RS: Diagnosis and treatment of cholangiocarcinoma. Oncologist.
9:43–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams WJ and Morris DL: Short-course
cimetidine and survival with colorectal cancer. Lancet.
344:1768–1769. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freston JW: Cimetidine: II. Adverse
reactions and patterns of use. Ann Intern Med. 97:728–734. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tønnesen H, Knigge U, Bülow S, Damm P,
Fischerman K, Hesselfeldt P, Hjortrup A, Pedersen IK, Pedersen VM
and Siemssen OJ: Effect of cimetidine on survival after gastric
cancer. Lancet. 2:990–992. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams W and Morris D: Cimetidine and
colorectal cancer. Dis Colon Rectum. 39:111–112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly MD, King J, Cherian M, Dwerryhouse
SJ, Finlay IG, Adams WJ, King DW, Lubowski DZ and Morris DL:
Randomized trial of preoperative cimetidine in patients with
colorectal carcinoma with quantitative assessment of
tumor-associated lymphocytes. Cancer. 85:1658–1663. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dexeus FH, Logothetis CJ, Sella A, Fitz K,
Amato R, Reuben JM and Dozier N: Phase II study of coumarin and
cimetidine in patients with metastatic renal cell carcinoma. J Clin
Oncol. 8:325–329. 1990.PubMed/NCBI
|
|
12
|
Morton RF, Creagan ET, Cullinan SA,
Mailliard JA, Ebbert L, Veeder MH and Chang M: Phase II studies of
single-agent cimetidine and the combination
N-phosphonacetyl-L-aspartate (NSC-224131) plus L-alanosine
(NSC-153353) in advanced malignant melanoma. J Clin Oncol.
5:1078–1082. 1987.PubMed/NCBI
|
|
13
|
Lefranc F, Yeaton P, Brotchi J and Kiss R:
Cimetidine, an unexpected anti-tumor agent, and its potential for
the treatment of glioblastoma (review). Int J Oncol. 28:1021–1030.
2006.PubMed/NCBI
|
|
14
|
Adams WJ, Lawson JA and Morris DL:
Cimetidine inhibits in vivo growth of human colon cancer and
reverses histamine stimulated in vitro and in vivo growth. Gut.
35:1632–1636. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Natori T, Sata M, Nagai R and Makuuchi M:
Cimetidine inhibits angiogenesis and suppresses tumor growth.
Biomed Pharmacother. 59:56–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahasrabudhe DM, McCune CS, O'Donnell RW
and Henshaw EC: Inhibition of suppressor T lymphocytes (Ts) by
cimetidine. J Immunol. 138:2760–2763. 1987.PubMed/NCBI
|
|
17
|
Kobayashi K, Matsumoto S, Morishima T,
Kawabe T and Okamoto T: Cimetidine inhibits cancer cell adhesion to
endothelial cells and prevents metastasis by blocking E-selectin
expression. Cancer Res. 60:3978–3984. 2000.PubMed/NCBI
|
|
18
|
Seubwai W, Vaeteewoottacharn K, Hiyoshi M,
Suzu S, Puapairoj A, Wongkham C, Okada S and Wongkham S:
Cepharanthine exerts antitumor activity on cholangiocarcinoma by
inhibiting NF-kappaB. Cancer Sci. 101:1590–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ono A, Hattori S, Kariya R, Iwanaga S,
Taura M, Harada H, Suzu S and Okada S: Comparative study of human
hematopoietic cell engraftment into BALB/c and C57BL/6 strain of
rag-2/jak3 double-deficient mice. J Biomed Biotechnol.
2011:5397482011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kubecova M, Kolostova K, Pinterova D,
Kacprzak G and Bobek V: Cimetidine: An anticancer drug? Eur J Pharm
Sci. 42:439–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris DL and Adams WJ: Cimetidine and
colorectal cancer-old drug, new use? Nat Med. 1:1243–1244. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaeteewoottacharn K, Seubwai W,
Bhudhisawasdi V, Okada S and Wongkham S: Potential targeted therapy
for liver fluke associated cholangiocarcinoma. J Hepatobiliary
Pancreat Sci. 21:362–370. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maemura K, Natsugoe S and Takao S:
Molecular mechanism of cholangiocarcinoma carcinogenesis. J
Hepatobiliary Pancreat Sci. 21:754–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitz KJ, Lang H, Wohlschlaeger J,
Sotiropoulos GC, Reis H, Schmid KW and Baba HA: AKT and ERK1/2
signaling in intrahepatic cholangiocarcinoma. World J
Gastroenterol. 13:6470–6477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams WJ, Morris DL, Ross WB, Lubowski DZ,
King DW and Peters L: Cimetidine preserves non-specific immune
function after colonic resection for cancer. Aust N Z J Surg.
64:847–852. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Xu M, Li X, Jia J, Fan K and Lai
G: Cimetidine suppresses lung tumor growth in mice through
proapoptosis of myeloid-derived suppressor cells. Mol Immunol.
54:74–83. 2013. View Article : Google Scholar : PubMed/NCBI
|