Introduction
Dendritic cells (DCs) are the most potent
antigen-presenting cells; thus, investigation of these cells is
crucial to elucidate the functions of the immune system. The
maturation state of DCs has a pivotal role in their activity, since
it influences their ability to uptake, process and present
antigens, as well as their migration to lymph nodes that drain
tumour sites and their stimulation of a potent T-cell-mediated
anti-tumour response. Immature DCs are more efficient at capturing
and processing antigens than mature DCs. Furthermore, immature DCs
have been shown to migrate more effectively to lymph nodes where
they undergo maturation and upregulate the expression of
costimulatory and major histocompatibility complex (MHC) molecules,
resulting in a T-cell response (1–4).
Tumour escape from the immune response may be due to
the decreased or absent expression of MHC molecules and/or
costimulatory molecules. In addition, the secretion of
immunosuppressive factors by tumours, such as transforming growth
factor-β (TGF-β), interleukin (IL)-10, vascular endothelial growth
factor and IL-4, as well as prostaglandin E2, nitric oxide, soluble
IL-2 receptors, complement inhibitors, proteases, gangliosides,
hexosamines, α-fetoprotein, fibronectin and phosphatidylserine, may
inhibit the anti-tumour immune response (5–8).
Previous studies have suggested that there is an
important association between physical activity (PA) and the immune
system (9–12), although the underlying mechanisms are
unknown. LaVoy et al (9)
demonstrated that a single session of dynamic exercise could
increase the differentiation of monocyte-derived DCs in healthy
individuals. Furthermore, the practice of certain activities was
hypothesized to contribute to the prevention of, or serve as an
adjunct to the treatment of, diseases such as cancer, type 2
diabetes, arthritis and other chronic diseases (10–12).
Chronic diseases represent the leading causes of mortality
worldwide (13), and the clinical use
of non-pharmacological interventions for immune activation may
improve the quality of life of patients with chronic diseases.
With respect to cancer, there have been
long-standing queries regarding the influence of PA on the risk of
developing cancer; however, previous studies have shown that PA
decreases the risk of breast cancer development in women and
increases their 5-year survival rate (14,15). In
addition, it was demonstrated that DCs could induce an improved
tumour-specific response in vitro following a short period
of exercise (16). Furthermore,
previous studies from our group demonstrated that moderate PA was
able to potentiate the polarization of cluster of differentiation
(CD)4+ T-cell and macrophage secretion profiles to
anti-tumour patterns: Th1 and M1, respectively (17,18).
Considering the immunosuppressive features of
tumours during their development and progression (19), the authors of the present study
hypothesise that prolonged PA may improve the anti-tumour activity
of the immune system. Therefore, the present study aimed to
investigate DC maturation in a mouse model of
7,12-dimethyl-benzanthracene (DMBA)-induced immunosuppression that
was subjected to PA.
Materials and methods
Animals
A total of 56 adult female Balb/c mice (8-weeks-old)
from the Institute for Research in Oncology were used. The mice
were housed in groups in plastic cages under a light/dark cycle at
21±3°C, with food and water available ad libitum. Following
the experimental period, the mice were sacrificed with an overdose
of 50 mg/kg ketamine and 15 mg/kg xylazine. This study was approved
by the Federal University of Triângulo Mineiro (Uberaba, Brazil)
Ethics Committee on the Use of Animals (registration no. 160).
Experimental groups and DMBA
induction
The mice were divided into the following four groups
(n=14 per group): i) Non-DMBA/non-PA group (GI); ii) non-DMBA/PA
group (GII); iii) DMBA/non-PA group (GIII); and iv) DMBA/PA group
(GIV). In the DMBA groups, the mice received daily oral gavage with
1 mg/ml DMBA for 6 weeks. There was a gap of 16 weeks prior to the
initiation of training to permit carcinogenic activity.
Training
The mice underwent swimming training for 5 days per
week for 8 weeks. Following a 15-day period of adaptation in water,
mice in the trained groups were placed in water for 45 min of
swimming on each training day. The water was changed daily and the
water temperature was maintained in the range of 30–35°C.
Bone marrow collection and
culturing
Following sacrifice, bone marrow was removed from
the femur and tibia of the mice, immediately homogenized, washed
three times in incomplete Iscove's Modified Dulbecco's Medium
(IMDM), and centrifuged at 290 × g for 10 min at 4°C.
Following centrifugation, the cells were counted and re-suspended
in complete IMDM.
Bone marrow cells re-suspended in complete IMDM were
distributed in flat-bottom, 6-well plates at a concentration of
5×106 cells/well. After 2 days of culturing, the cells
were stimulated with granulocyte-macrophage colony-stimulating
factor and IL-4 for 5 days, after which they were stimulated with
tumour necrosis factor-α (TNF-α) and tumour lysate. In addition,
half of the plates were stimulated with 10 µg/ml lipopolysaccharide
(LPS; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). After 48
h of incubation at 37°C in 5% CO2, supernatant samples
were obtained, which were divided into six aliquots per incubation
time point and stored at −80°C. The adherent cells were collected
for flow cytometry.
Flow cytometry
Bone marrow-derived DCs (BMDCs) were immunolabelled
with peridinin chlorophyll-protein complex cyanine 5.5-conjugated
anti-CD11b antibody (rat anti-mouse monoclonal; 550993; undiluted;
BD Biosciences, San Jose, CA, USA) to reveal total DCs,
allophycocyanin (APC)-conjugated anti-CD11c antibody (hamster
anti-mouse monoclonal; 553956; undiluted; BD Biosciences) to reveal
mature DCs, and fluorescein isothiocyanate-conjugated anti-CD80
(hamster anti-mouse monoclonal; 553768; undiluted; BD Biosciences),
APC-conjugated anti-CD86 (rat anti-mouse monoclonal; 558703;
undiluted; BD Biosciences) and phycoerythrin-conjugated anti-I-A
(rat anti-mouse monoclonal; 562010; undiluted; BD Biosciences)
antibodies to detect the expression of costimulatory molecules. The
cells were incubated with each antibody at 4°C for 30 min in the
dark, and then washed with PBS to remove excess antibodies.
Finally, cell aliquots were re-suspended in 500 µl PBS for flow
cytometric analysis using a BD FACSCalibur™ cytometer (BD
Biosciences).
Cytokine levels
The levels of the cytokines interferon (IFN)-γ,
IL-4, IL-12 and TGF-β in the supernatant samples were measured
using ELISAs with pairs of monoclonal antibodies purchased from BD
OptEIA™ (BD Biosciences), according to the manufacturer's protocol.
Results show the index between the supernatants with stimulus (LPS)
and supernatants without stimulus. All cytokines were measured in
pg/ml.
Statistical analysis
Data were assessed for normality of distribution.
For variables in which the distribution was found to be normal,
parametric tests were performed, and for those which the
distribution was not normal, non-parametric tests were conducted.
For comparisons of normally distributed data, Student's t-tests
were performed for two-group comparisons and analysis of variance
was used for comparisons among three or more groups. Normally
distributed data are expressed as the mean ± standard error of
mean. For comparisons of non-normally distributed data,
Mann-Whitney U tests were performed for two-group comparisons and
Kruskal-Wallis and post-hoc Dunn's tests were used for comparisons
among three or more groups. Non-normally distributed data are
expressed as the median (range). P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of DC phenotype
according to the expression of costimulatory molecules
For the phenotypic characterization of the mouse
BMDCs, the cells were labelled with antibodies against CD11b and
CD11c, as well as MHC II and CD80 and CD86 costimulatory molecules,
and analysed by flow cytometry. As shown in Fig. 1, DMBA did not exert a suppressive
effect on the differentiation of DCs. The groups submitted to PA in
the presence (GIV) or absence (GII) of DMBA showed significantly
higher levels of CD11b+/CD11c+ DCs, as
compared with GI and GIII mice (P<0.05). These results suggest a
greater differentiation capacity for BMDCs in vitro
following PA. Similarly, GII and GIV showed significantly higher
expression levels of CD80, CD86 and MHC II, as compared with GI and
GIII (P<0.05), thus suggesting that PA induces the
differentiation and maturation of DCs, even within an
immunosuppressive environment, as induced by DMBA.
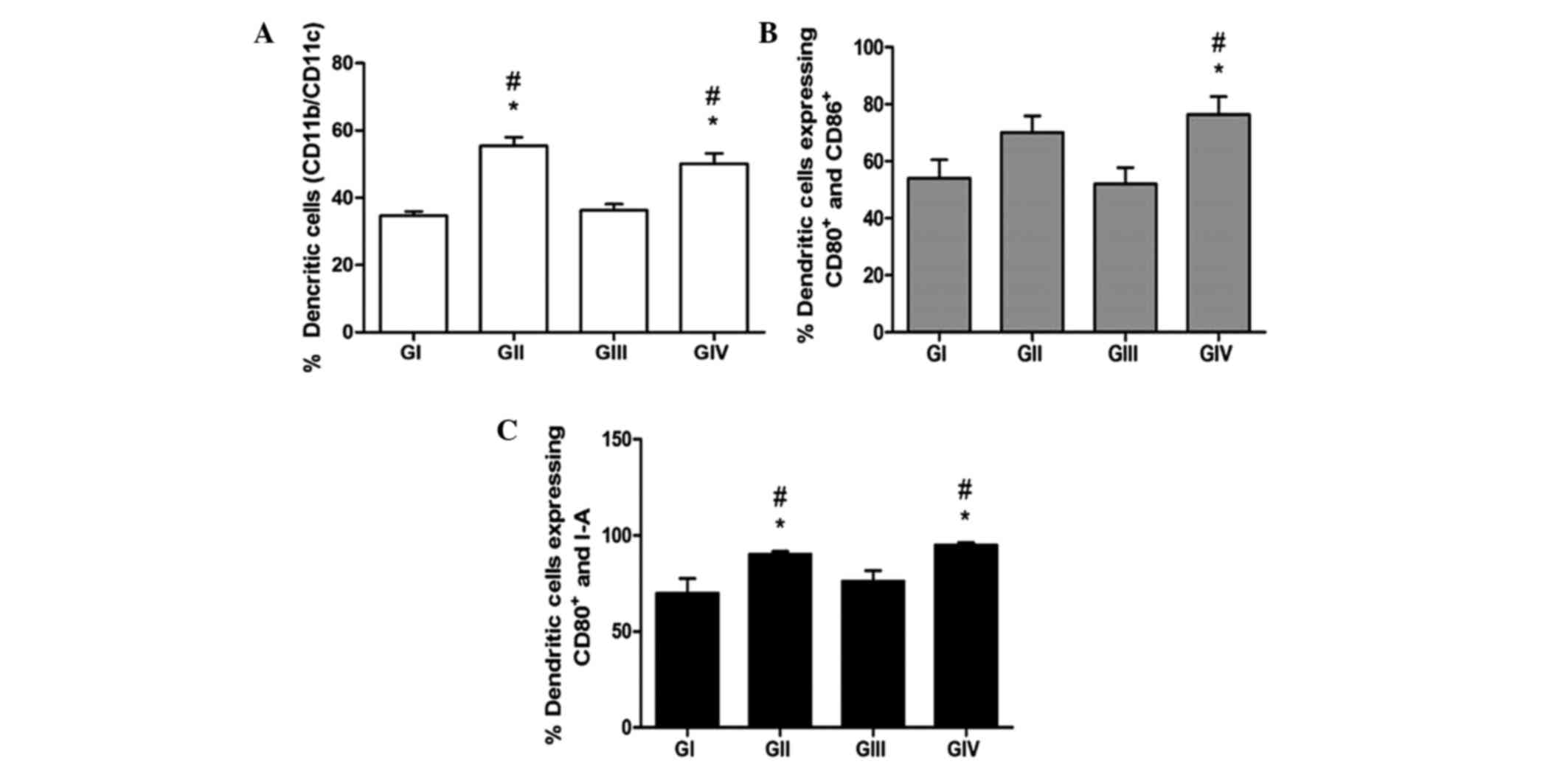 | Figure 1.Immunolabelling of bone marrow-derived
dendritic cells (BMDCs) from the various groups. (A) BMDCs were
labelled with anti-CD11b and anti-CD11c antibodies to reveal total
and mature DCs, respectively. (B) Co-expression of CD80 and CD86,
and (C) co-expression of CD80 and major histocompatibility complex
class II, were assessed by immunolabelling and flow cytometry. Data
are presented as the mean ± standard error of the mean.
#P<0,05 vs. GI. *P<0,05 vs. GIII. GI,
non-7,12-dimethyl-benzanthracene (DMBA)/non-physical activity (PA)
group; GII, non-DMBA/PA group; GIII, DMBA/non-PA group; GIV,
DMBA/PA group; CD, cluster of differentiation. |
Synthesis of cytokines by DCs
The cytokine profile of BMDCs was analysed using
ELISAs. As is shown in Fig. 2, BMDCs
from the GII and GIV mice showed higher synthesis levels of IL-12,
as compared with those from the GI and GII mice. Furthermore, the
secretion levels of IL-12 were markedly higher for the GIV mice
compared with the GIII mice (P=0.063).
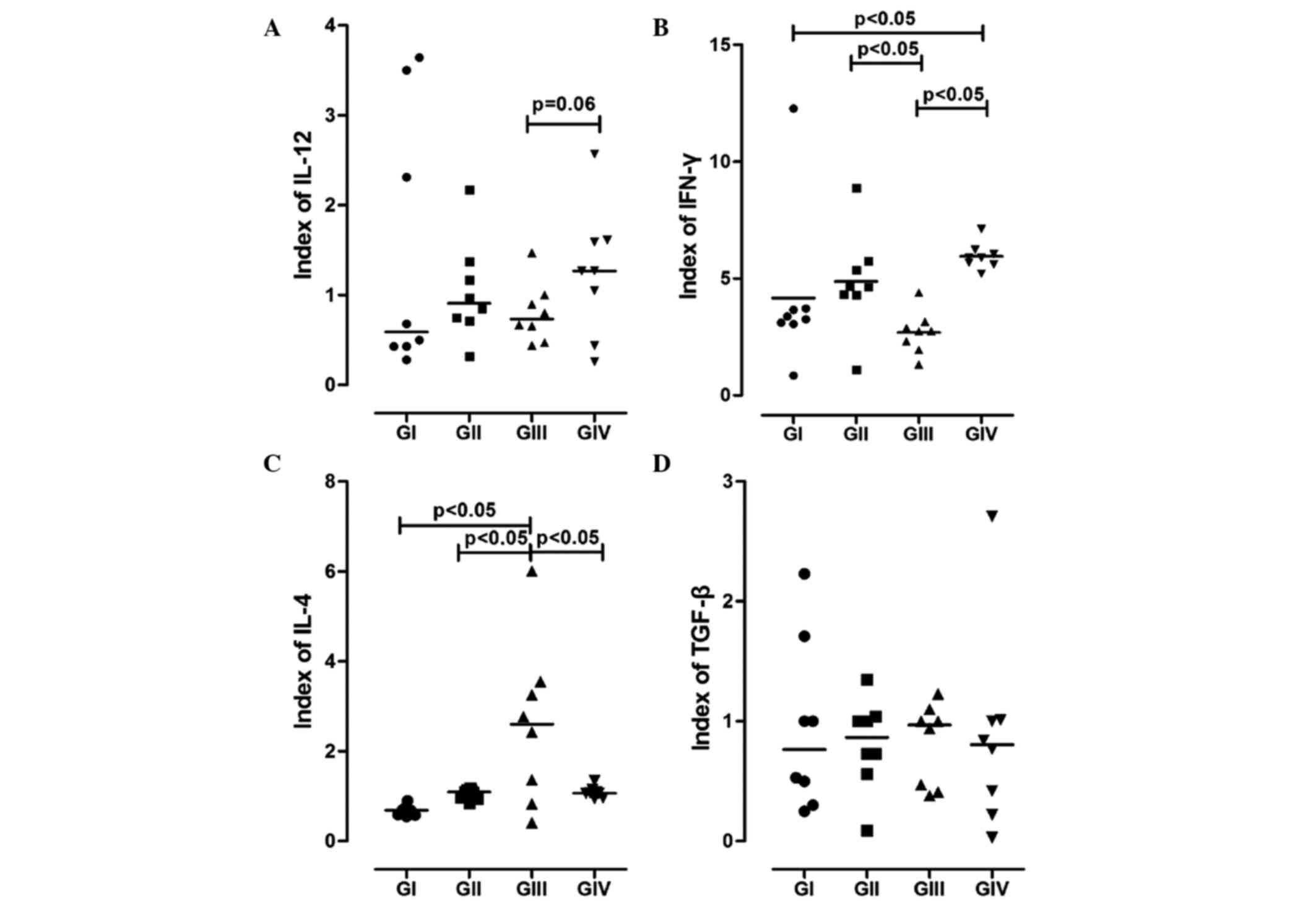 | Figure 2.Representation of cytokines
synthesised by bone marrow-derived dendritic cells (BMDCs). The
levels of (A) IL-12, (B) IFN-γ, (C) IL-4 and (D) TGF-β in the
supernatant of BMDCs from the various groups were determined using
ELISAs. GI, non-7,12-dimethyl-benzanthracene (DMBA)/non-physical
activity (PA) group; GII, non-DMBA/PA group; GIII, DMBA/non-PA
group; GIV, DMBA/PA group. IL, interleukin; IFN-γ, interferon-γ;
TGF-β, transforming growth factor-β. |
When analysing IFN-γ synthesis, the groups that
experienced PA (GII and GIV) showed higher expression levels, and a
significant difference was observed for GIV compared with GI and
GIII (P<0.05; Fig. 2).
Conversely, the synthesis levels of IL-4 showed an
antagonistic behaviour to IL-12 and IFN-γ; they were significantly
increased in GIII compared with the other groups (P<0.05).
There were no significant changes in the secretion
of TGF-β among the groups (P>0.05; Fig. 2D). However, GIII showed a discreet
increase in the production of TGF-β, as compared with the other
groups, and GIV showed attenuated expression of this cytokine.
Discussion
Physical activity has been shown to promote
anti-tumour responses (20), and this
effect may occur via the regulation of genes that have been
implicated in cancer prevention (21), or through favourable effects on immune
responses (20).
In our previous studies, we investigated the effect
of PA on immunological behaviour, including T-cell and macrophage
profiles (17,18). It was demonstrated that PA induced a
decrease in the number of regulatory T-cells (Treg) in animal
spleens, and there was an increase in the number of CD4+
T-cells expressing IFN-γ, IL-12 and TNF-α, which are associated
with a Th1 profile (17).
Furthermore, the levels of IL-12 and IFN-γ, which are associated
with an M1 macrophage profile, were increased under the influence
of PA (18). Based on these findings,
the objective of this study was to assess the influence of PA in
the maturation process of BMDCs under immunosuppressive conditions,
in order to improve our understanding of the behaviour of BMDCs
under these conditions.
Although the clinical effects of PA under
immunosuppressive conditions, such as in cancer patients, are not
yet fully understood, the use of a prolonged exercise protocol in
this study was justified, given the previously described benefits
of PA. In the present study, female Balb/c mice served as
experimental models and carcinogenesis was induced using DMBA, a
traditional carcinogen that also has immunosuppressive properties
(17,18).
In the present study, mice that performed PA and
received DMBA (GIV) showed external tumour masses, while mice that
received DMBA but did not perform PA (GIII) had a frequency of
external tumour masses of 35%. Similar findings were observed in a
study by Lane et al (22),
wherein animals that performed PA and consumed a balanced diet had
lower levels of tumours compared with animals that did not perform
exercise. Furthermore, in a previous study, the body composition of
animals that performed PA was not altered by the exercise regimen,
although trained animals showed greater tumor masses compared with
the sedentary group (23).
In order for an anti-tumour immune response to be
effective, its various components must work synergistically; the
components of the innate immune response, including macrophages,
natural killer cells and DCs (3),
must act together with those of the acquired immune response,
including T and B lymphocytes (4).
Previous studies demonstrated that mature DCs were
able to induce a potent and specific anti-tumour response by
stimulating T-cells, in particular CD8 T-cells, and inhibiting
metastasis to the lungs (3,24).
In the present study, bone marrow cells were shown
to differentiate into DCs that underwent maturation following
culturing under the influence of stimulatory factors, in spite of
the immunosuppressive effects of DMBA. BMDCs from GIII mice (DMBA
induction without PA) showed a higher synthesis level of
suppressive cytokines. However, the number of differentiated BMDCs
was significantly higher in the groups that performed PA, and the
expression pattern of cytokines was associated with Th1 and M1
profiles.
The expression levels of costimulatory molecules
were also increased in BMDCs from the mice that were subjected to
PA. These results were consistent with those reported in previous
studies (25,26), wherein PA was associated with an
increase in the number of DCs, as well as increases in the
expression of MHC class II, CD80 and CD86. However, Ru and Peijie
(27) reported that over-training
could induce immunosuppression by reducing the number of DCs and
costimulatory molecule expression. Chiang et al (25) demonstrated that the groups subjected
to PA produced higher levels of IL-12 compared with sedentary
groups, mainly when comparing groups under DMBA induction (GIII vs.
GIV). In the present study, the groups that underwent a training
protocol showed increased expression levels of IL-12 and IFN-γ, and
reduced expression levels of suppressive cytokines in the
supernatant of cultured BMDCs.
In conclusion, the present study demonstrated that
PA stimulated the maturation of BMDCs and increased the expression
of IL-12 and IFN-γ, and decreased the synthesis of IL-4 and TGF-β,
by these cells. These results suggested a potential polarization of
the immune response towards a Th1 profile, which is thought to
exert anti-tumour effects. Therefore, the authors of the present
study suggest that a combination of DC-based immunotherapy and PA
should be investigated in further studies, in particular with
regard to the activation of tumour-specific T-cells by DCs and
tumour-infiltration by immune cells. Furthermore, patients with
cancer should consider performing regular PA to complement
conventional therapies, including chemotherapy, radiotherapy and
surgery, as well as newer therapies, such as immunotherapy.
Acknowledgements
The present study was supported by grants from the
Foundation for Research Assistance of the State of Minas Gerais
(grant no. FAPEMIG-RED-00011-14), the National Council for
Scientific and Technological Development (grant no. CAPES-PDSE
0592/13-7) and the Uberaba Foundation for Teaching and
Research.
References
|
1
|
Ashley DM, Faiola B, Nair S, Hale LP,
Bigner DD and Gilboa E: Bone marrow-generated dendritic cells
pulsed with tumor extracts or tumor RNA induced antitumor immunity
against central nervous system tumors. J Exp Med. 186:1177–1182.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fields RC, Shimizu K and Mulé JJ: Murine
dendritic cells pulsed with whole tumor lysates mediate potent
antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci
USA. 95:9482–9487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zitvogel LZ, Mayordomo JI, Tjandrawan T,
DeLeo AB, Clarke MR, Lotze MT and Storkus WJ: Therapy of murine
tumors with tumor peptidepulsed dendritic cells: Dependence on T
cells, B7 costimulation, and T helper cell 1-associated cytokines.
J Exp Med. 183:87–97. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabrilovich DI, Chen HL, Girgis KR,
Cunningham HT, Meny GM, Nadaf S, Kavanaugh D and Carbone DP:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gastl GA, Abrams JS, Nanus DM, Oosterkamp
R, Silver J, Liu F, Chen M, Albino AP and Bander NH: Interleukin-10
production by human carcinoma cell lines and its relationship to
interleukin-6 expression. Int J Cancer. 55:96–101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kavanaugh DY and Carbone DP: Immunologic
dysfunction in cancer. Hematol Oncol Clin North Am. 10:927–951.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wojtowicz-Praga S: Reversal of
tumor-induced immunosuppression: A new approach to cancer therapy.
J Immunother. 20:165–177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaVoy EC, Bollard CM, Hanley PJ, O'Connor
DP, Lowder TW, Bosch JA and Simpson RJ: A single bout of dynamic
exercise by healthy adults enhances the generation of
monocyte-derived-dendritic cells. Cell Immunol. 295:52–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blannin AK, Gleeson M and Brooks S: Effect
of lactacidosis on human leucocyte adherence: A possible
explanation of why the leucocyte count continues to rise after
cessation of very high intensity exerciseGleeson M: Immune function
in sport and exercise. Philadelphia: Elsevier; pp. 67–89. 2006,
View Article : Google Scholar
|
|
11
|
Fairey AS, Courneya KS, Field CJ and
Mackey JR: Physical exercise and immune system function in cancer
survivors. Cancer. 94:539–551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woods JA: Physical activity, exercise, and
immune function. Brain Behav Immun. 19:369–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petersen AM and Pedersen BK: The
anti-inflammation effect of exercise. J Appl Physiol (1985).
98:1154–1162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cleveland RJ, Eng SM, Stevens J, Bradshaw
PT, Teitelbaum SL, Neugut AI and Gammon MD: Influence of
prediagnostic recreational physical activity on survival from
breast câncer. Eur J Cancer Prev. 21:46–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedenreich CM and Rohan TE: Physical
activity and risk of breast cancer. Eur J Cancer Prev. 4:145–151.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
LaVoy EC, Bollard CM, Hanley PJ, O'Connor
DP, Bosch JA and Simpson RJ: A single bout of dynamic exercise
enhances the expansion of MAGE-A4 and PRAME-specific cytotoxic
t-cells from healthy adults. Exerc Immunol Rev. 21:144–153.
2015.PubMed/NCBI
|
|
17
|
Abdalla DR, Murta EF and Michelin MA: The
influence of physical activity on the profile of immune response
cells and cytokine synthesis in mice with experimental breast
tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev.
22:251–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdalla DR, Aleixo AA, Murta EF and
Michelin MA: Innate immune response adaptation in mice subjected to
administration of DMBA and physical activity. Oncol Lett.
7:886–890. 2014.PubMed/NCBI
|
|
19
|
Terme M, Ullrich E, Aymeric L, Meinhardt
K, Coudert JD, Desbois M, Ghiringhelli F, Viaud S, Ryffel B, Yagita
H, et al: Cancer-induced immunosuppression: IL-18-elicited
immunoablative NK cells. Cancer Res. 72:2757–2767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goh J, Endicott E and Ladiges WC:
Pre-tumor exercise decreases breast cancer in old mice in a
distance-dependent manner. Am J Cancer Res. 4:378–384.
2014.PubMed/NCBI
|
|
21
|
Buehlmeyer K, Doering F, Daniel H,
Kindermann B, Schulz T and Michna H: Alteration of gene expression
in rat colon mucosa after exercise. Ann Anat. 190:71–80. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lane HW, Keith RE, Strahan S and White MT:
The effect of diet, exercise and 7,12-dimethylbenz(a)anthracene on
food intake, body composition and carcass energy levels in female
virgin BALB/c mice. J Nutr. 121:1876–1882. 1991.PubMed/NCBI
|
|
23
|
Thompson HJ, Ronan AM, Ritacco KA,
Tagliaferro AR and Meeker LD: Effect of exercise on the induction
of mammary carcinogenesis. Cancer Res. 48:2720–2723.
1988.PubMed/NCBI
|
|
24
|
Asavaroengchai W, Kotera Y and Mulé JJ:
Tumor lysate-pulsed dendritic cells can elicit an effective
antitumor immune response during early lymphoid recovery. Proc Natl
Acad Sci USA. 99:931–936. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiang LM, Chen YJ, Chiang J, Lai LY, Chen
YY and Liao HF: Modulation of dendritic cells by endurance
training. Int J Sports Med. 28:798–803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao HF, Chiang LM, Yen CC, Chen YY,
Zhuang RR, Lai LY, Chiang J and Chen YJ: Effect of a periodized
exercise training and active recovery program on antitumor activity
and development of dendritic cells. J Sports Med Phys Fitness.
46:307–314. 2006.PubMed/NCBI
|
|
27
|
Ru W and Peijie C: Modulation of dendritic
cells and NKT cells excessive exercise in rats. J Med Biol Eng.
29:190–194. 2009.
|
















