Introduction
Numerous advanced malignant abdominal tumors have
been reported to lead to malignant ascites (1,2), which can
cause gastrointestinal dysmotility. The symptoms of
gastrointestinal dysmotility consist of abdominal pain, distention,
nausea, vomiting and constipation (3). It is important to identify the reason
for gastrointestinal dysmotility caused by malignant ascites.
Changes in the interstitial cells of Cajal (ICCs) are known to be
partially responsible for malignant ascites-induced
gastrointestinal dysmotility (4), but
the mechanisms are not completely understood.
The excitation and contraction of gastrointestinal
smooth muscle cells are regulated by slow-wave activity, which is a
basic electrical rhythm of the gastrointestinal system (5). The ICCs act as pacemakers, and this
mechanism is attributed to the activation of ion channels (6,7), including
Ca2+-activated chloride channels, non-selective cation
channels and sodium channels (8–10). ICCs
exhibit slow-wave activity and mediate basal electrical rhythms in
the gastrointestinal tract (11,12).
However, little is known about the association between malignant
ascites and ICCs. It was previously reported that malignant ascites
reduces the number of ICCs, and it was demonstrated that
gastrointestinal dysmotility induced by gastric cancer peritoneal
metastasis was relevant to decreases in ICCs and disrupted the
electrical rhythm (3).
Previously, numerous studies demonstrated that
hyperpolarization-activated cyclic nucleotide-gated potassium
channels (HCNs) exist in ICCs, and that these channels may be
important regulators of the excitability and pacemaker activity of
ICCs (13,14). HCN channels are particular cation
channels that participate in cell autonomy and excitability
(15). These channels are activated
by hyperpolarization and intracellular cyclic adenosine
monophosphate (cAMP) (16). The HCN
channel family comprises 4 members, designated HCN1-4, which are
permeable to Na+ and K+ (17). HCN channels activate T-type
voltage-dependent Ca2+ channels via membrane
depolarization, which alters intracellular Ca2+
concentrations (18).
The present study hypothesized that the small
intestinal dysmotility caused by malignant ascites may be
associated with changes in HCN2 channels on ICCs. Significant
changes were identified in ICCs and HCN2 channels under the
conditions of malignant ascites, which revealed a potential
mechanism for malignant ascites-induced gastrointestinal motility
dysfunction.
Materials and methods
Malignant ascites mouse model
Animal experiments were performed under the Rules
and Regulations of the Animal Care and Use Committee at Harbin
Medical University (Harbin, China; approval no., HMUIRB20140022).
C57BL/6 mice (age, 4–6 weeks old; gender, male and female; weight,
16–20 g) were purchased from Biological Technology Development Co.,
Ltd. (Liaoning, China). Mice were group-housed under 12-h
light/dark cycles and raised at a constant temperature of 26–28°C,
and they were given ad libitum access to food and water.
In total, 39 of 46 C57BL/6 mice were used to
generate a model of malignant ascites via the administration of a
0.2 ml intraperitoneal injection of mouse fore-stomach carcinoma
(MFC) cells, MFC cells were obtained from the Type Culture
Collection of the Chinese Academy of Science (Shanghai, China).
Malignant ascites was successfully generated in 37 of the 39 mice
on the ninth day subsequent to intraperitoneal injection. The
control group (7 of 46 C57BL/6 mice) was treated with the same
volume of physiological saline.
ICC isolation and culture
To isolate ICCs, a total of, 100 C57BL/6 wild-type
mice (8–13 days old) were anesthetized with 3.5–5% diethyl ether
(Shanghai Heyi Chemical, Co., Ltd., Shanghai, China) and sacrificed
through cervical dislocation. The intestines from 1 cm below the
pyloric ring to the cecum were resected and opened along the
mesenteric border. The intestinal mucosa was removed, and strips of
muscle were collected. Muscle cells were dispersed via incubation
in an enzyme solution composed of 1.3 mg/ml collagenase type II, 2
mg/ml bovine serum albumin (Roche Applied Science, Penzberg,
Germany), 2 mg/ml trypsin inhibitor and 0.27 mg/ml adenosine
triphosphate at 37°C for 15 min. The cells were spun down at 1,249
× g for 10 min at room temperature and suspensions were then plated
onto sterile glass coverslips coated with murine collagen in M199
medium (HyClone, GE Healthcare Life Sciences, Logan, UT, USA).
These isolated cells were subsequently co-cultured with malignant
ascites in order to investigate the effect of malignant ascites on
ICCs.
Intestinal myoelectrical activity
In total, 10 C57BL/6 mice (7 from malignant ascites
group and 3 from control group) were anesthetized with 1% sodium
amobarbital (40 mg/kg, New Asia Pharmaceutical, Shanghai, China)
subsequent to a 12 h fasting period. A platinum electrode was
placed on the muscular layer under the serosa through a 2-cm
midline abdominal incision. The following parameters were set: 200
µV voltage; 1.0 sec/div time; and 30 Hz frequency. Recordings were
performed for 20 min and saved. Groups of various electrical
activities, including frequency, and maximum, minimum and average
amplitude (µV), were selected randomly and analyzed using RM6240
B/C Multi-Channel Physiological Signal Acquisition and Recorder
System software version usb2.0Z(I) (Chengdu Instrument Factory,
Chengdu, China).
Hematoxylin and eosin (H&E)
staining and electron microscopy analysis
A total of 14 C57BL/6 mice intestinal samples (10
from malignant ascites group and 4 from control group) were
collected immediately following sacrifice. The tissues were fixed
for 24 h using 4% paraformaldehyde, and stained with H&E
subsequent to dehydration, embedding and slicing (3–4 µm
thickness). Structural and morphological changes were observed
under a light microscope (Nikon Corporation, Tokyo, Japan). Images
were captured using a conventional optical camera.
Samples were fixed with 3% glutaraldehyde in PBS (pH
7.2) for 2 h, and then rinsed with PBS, post-fixed in 1% osmium
tetroxide for 2 h at 4°C, dehydrated in a graded series of acetone
and embedded in Epon 812 (EMS, Connecticut, USA). Ultrathin
sections were cut at a thickness of 50–70 nm, which were
subsequently double stained with uranyl acetate and lead citrate,
and examined using an electron microscope (H-7650, Hitachi, Tokyo,
Japan).
Immunofluorescence
ICCs and mice intestinal tissue frozen sections
(including 10 C57BL/6 malignant ascites mice and 4 control mice)
were fixed in 4% paraformaldehyde for 30 min and blocked with 2%
goat serum (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) in PBS containing 0.1% Triton-X for 1 h. The
primary antibodies consisted of anti-c-kit (dilution, 1:50; cat.
no. B0813; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
anti-HCN2 (dilution, 1:50; cat. no. ab84817; Abcam, Cambridge, UK).
The samples were incubated with the primary antibodies at 4°C
overnight, washed twice with PBS and incubated with the fluorescein
isothiocyanate (FITC)-labeled anti-rabbit secondary antibody
(dilution, 1:100; cat. no. M0808; Vector Laboratories, Inc.,
Burlingame, CA, USA). The samples were then subsequently washed
with PBS, counterstained with DAPI (Beyotime Institue of
Biotechnology, Beijing, China) and finally examined using
fluorescence microscopy (Nikon E800, Japan) at excitation
wavelengths of 488 and 594 nm.
Gene expression analysis
Total RNA, extracted from 15 C57BL/6 malignant
ascites mice and 4 control mice, was isolated in the Super clean
workbench, using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Upon mixing, RNA was extracted using
chloroform. RNA samples were diluted in diethylpyrocarbonate (DEPC)
-treated water subsequent to precipitation, dissolution and
centrifugation at 6,288 × g for 5 min, at 4°C. In total, 1 mg of
DNase-treated RNA was reverse transcribed into complementary DNA
(cDNA) using First-Strand Synthesis kit (Takara Bio, Inc., Otsu,
Japan). Subsequently, ~3.5 µl oligo deoxythymidine, 3.5 µl
deoxynucleotides (dNTP), 2 µg RNA and 19.25 µl DEPC-treated water
were added to a 35 µl buffer solution (10 mmol/l dNTP, RNA
Polymerase, 25 mmol/l Mgcl2). Following pre-denaturation
at 70°C for 3 min, the mixture was placed on ice, and 7 µl 5X
buffer and 1.75 µl Moloney murine leukemia virus reverse
transcriptase was added. Primers were used to amplify the products
under the following conditions: 42°C 1 h, 95°C 5 min and 4°C until
use. The cDNA samples were stored at −20°C until use.
Quantitative polymerase chain reaction (qPCR)
analysis was performed in triplicate with Platinum SYBR Green qPCR
Super Mix-UDG (Takara Bio, Inc.). The 20 µl reaction solution
contained 10 µl SYBR Premix Ex Taq (Takara Bio, Inc.), 1 µl PCR
forward primer, 1 µl PCR reverse primer, 0.5 µl template DNA and 6
µl DEPC-treated water. The reactions were conducted for 40 cycles
under the following conditions: 95°C for 30 sec, 95°C for 5 sec and
60°C for 34 sec, on the LightCycler® 480 Real-Time PCR
System (Roche Applied Science). For the quantification of gene
expression, GAPDH mRNA was used as the endogenous control. The
relative expression levels of HCN2 and cAMP mRNA, normalized to
GAPDH mRNA, were calucated using the 2−∆∆Cq value
(19).
The primer sequences used in the present study are
shown in Table I.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction.
| Transcript
name | Primer | Sequence
(5′-3′) |
|---|
| HCN2 | F |
CTGCGTGAGGAGATTGTGAA |
|
| R |
TTTGAGCTTTGTCAGCATGG |
| cAMP | F |
GGTGCCAAGGATTGAAGAAG |
|
| R |
CTGCCCACTGCTAGTTTGGT |
| GAPDH | F |
AGAAGGTGGTGAAGCAGGCATC |
|
| R |
CGAAGGTGGAAGAGTGGGAGTTG |
Flow cytometry analysis
ICCs isolated from C57BL/6 mice subsequent to being
co-cultured with malignant ascites were cultured with Fluo-4 and
acetoxymethyl/dimethyl sulfoxide (Beijing, China) at 37°C for 20
min, and then incubated with Hanks' balanced salt solution
supplemented with 1% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at room temperature for 40 min. Cells were washed
with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered
saline and resuspended. Ca2+ ions were detected and
analyzed after 10 min using flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) with an excitation wavelength of 494 nm
and an emission wavelength of 516 nm.
Statistical analysis
Comparisons between the control and malignant
ascites groups were analyzed using a Student's t-test in SPSS
version 17.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Generation of malignant ascites
model
C57BL/6 mice were used to generate a malignant
ascites model via the administration of an intraperitoneal
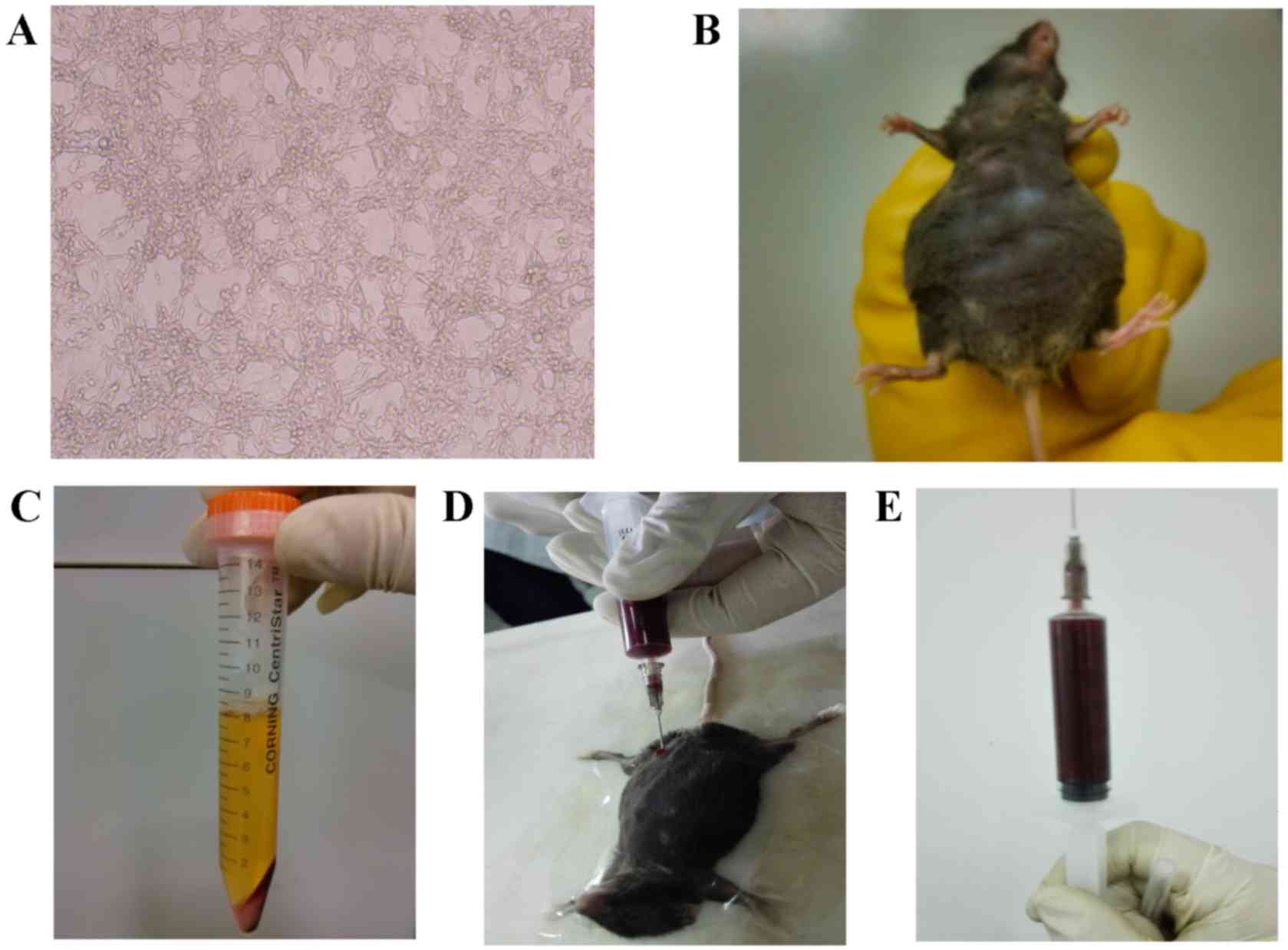
injection of MFC cells (Fig. 1A). The
animals in the control group were treated with physiological
saline. Ascites was generated on the ninth day subsequent to
intraperitoneal injection. The mice were sacrificed between the
14th and 21st day subsequent to ascites generation according to
their survival status (Fig. 1B). The
fluid in the ascites was generally light pink (Fig. 1C) at the first extraction and
gradually became bloody at later time-points (Fig. 1D and E).
Histological and dynamic changes in
the small intestine induced by malignant ascites
Electrophysiology was performed to observe
peristaltic changes in the intestine caused by malignant ascites.
Compared with those in the control mice (Fig. 2Aa), the amplitude was lower and the
frequency was slower in mice with malignant ascites (Fig. 2Ab), which suggests that malignant
ascites led to a decline in the peristaltic function of the
intestines. The histological characteristics of the intestines were
compared between the malignant ascites and control groups. The
intestinal villi were shorter and fewer in number under light
microscopy, and the thickness of the intestinal muscularis was in
homogeneous and hydropic in mice with malignant ascites (Fig. 2Ba). The morphology of the intestinal
villi was intact, and the thickness of the intestinal muscularis
was uniform in the control groups (Fig.
2Bb). These results suggest that the motility dysfunction
induced by malignant ascites was associated with changes in the
intestines.
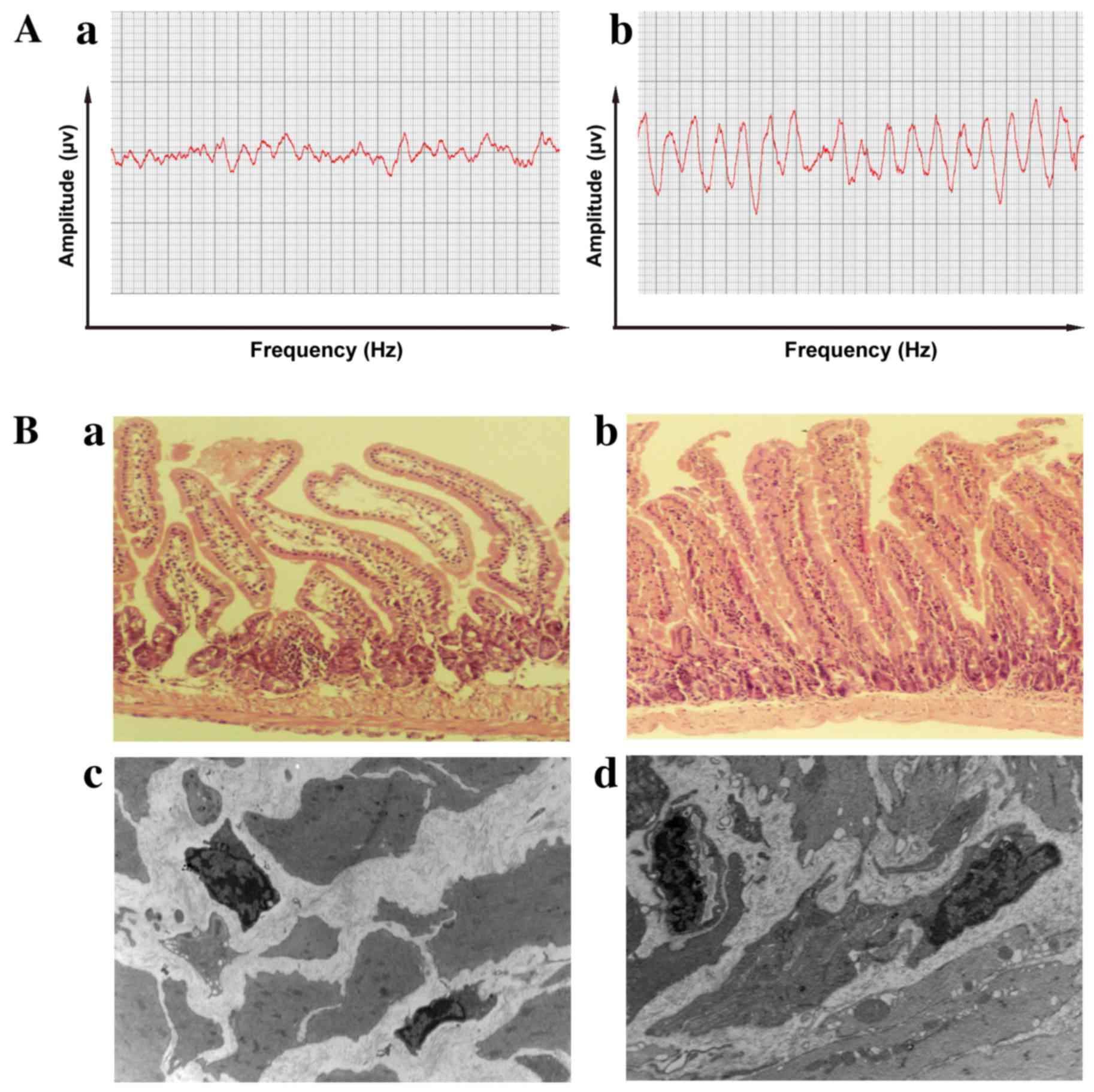 | Figure 2.Electrophysiological and pathological
characteristics of the intestine. (A)(a) The peristaltic amplitude
in the intestine was reduced and irregular in mice with malignant
ascites. (b) The control mice exhibited regular waves and stable
frequencies. (B)(a) Light microscopy revealed that the intestinal
villi were shorter and fewer in number, and the thickness of the
intestinal muscularis was inhomogeneous and hydropic, in mice with
malignant ascites compared with control mice (magnification, ×200).
(b) The morphology of the intestinal villi was intact and the
thickness of the intestinal muscularis was uniform in the control
group (magnification, ×200). (c) Ultrastructurally, ICC volume was
reduced, their nuclei were clearly condensed and the number of
cytoplasmic processes in ICCs were decreased, with few connections
with other cells being observed (magnification, ×4,000). (d) In the
control group, the ICCs exhibited well-centered nuclei and numerous
processes that connected with other cells (magnification, ×4,000).
ICC, interstitial cell of Cajal. |
Ultrastructural examinations revealed that ICC
volume was reduced, their nuclei were clearly condensed and the
number of cytoplasmic processes in ICCs was decreased, with few
connections with other cells being observed in the malignant
ascites group (Fig. 2Bc). In the
control group, the ICCs exhibited well-centered nuclei and numerous
cellular processes of ICCs that connected with other cells
(Fig. 2Bd).
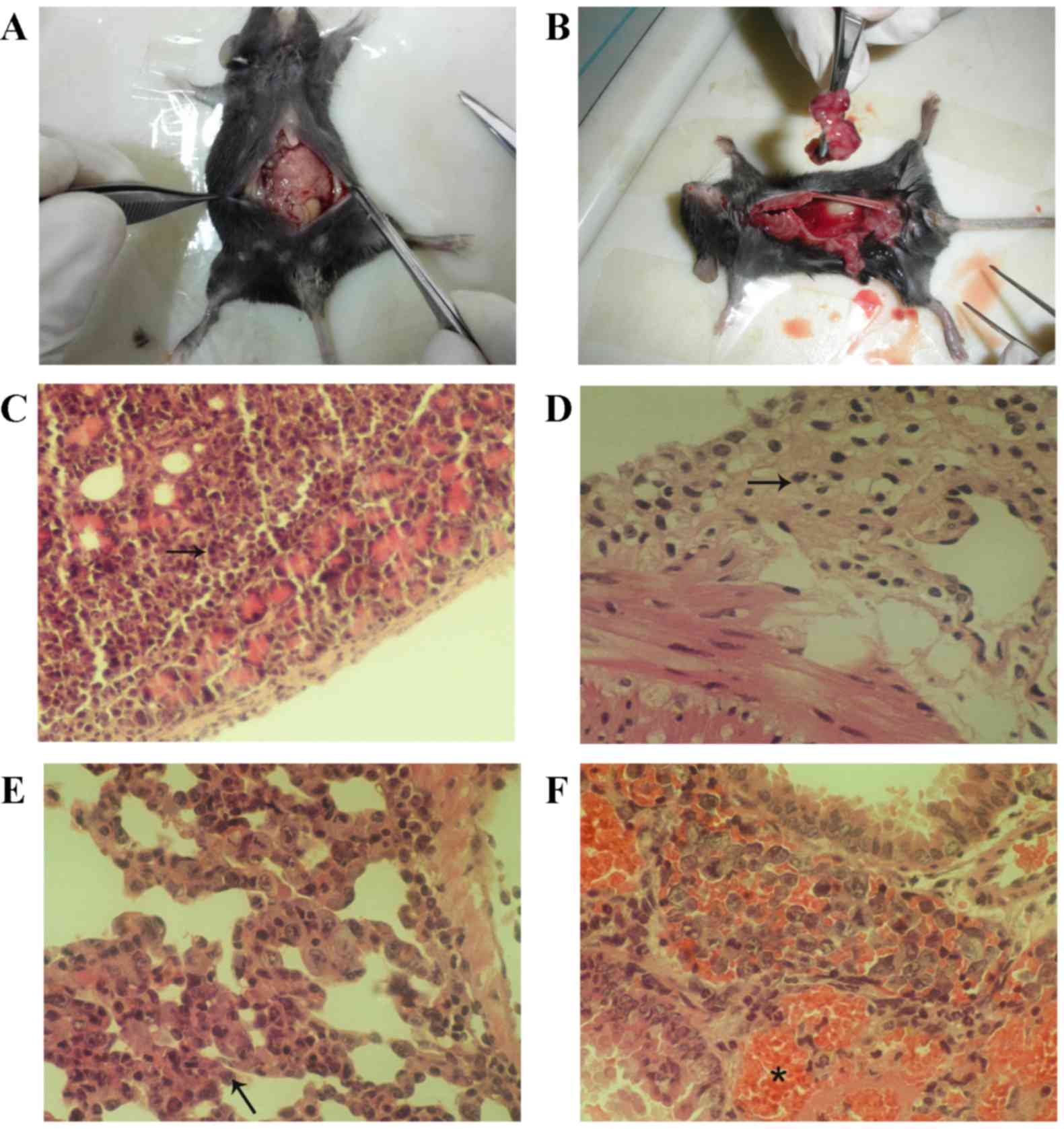
Generation of an animal model of
peritoneal metastasis
A total of 28 out of the 39 mice underwent ascites
extraction and pathological examinations. Tumors were identified in
the abdominal cavities of the malignant ascites mice. The size of
the tumors was similar to a grain of rice (~1 mm3;
Fig. 3A and B). Light microscopy
revealed that tumor cells (indicated by an arrow in the figure)
invaded in the serosa of the intestine, omentum and pancreas and
destroyed the surrounding tissues (Fig.
3C and D), which indicates successful generation of the
malignant ascites model. In total, 11 of the 28 mice (39.28%)
exhibited invasive tumor cells, and 22 of the 28 mice (78.57%)
exhibited pulmonary metastases. Lung tissues with invasive tumor
cells (Fig. 3E, arrow) were
associated with a large hemorrhage (Fig.
3F, asterisk).
Effect of malignant ascites on ICCs
and HCN2 in the intestines
Pacemaker cells and pacemaker channels are involved
in changes in the intestinal peristaltic function (14). ICCs are key pacemaker cells in the
small intestine (14). Therefore,
immunofluorescence was used to investigate the ICCs in the
intestines of the two groups examined in the present study. In
control group, c-kit expression was shown as a bold line in the
control group (Fig. 4A and B, arrow),
but in the malignant ascites groups, the expression of c-kit was
low, weak, spotty and granular in the muscularis layer in the
malignant ascites groups (Fig. 4C and
D, arrow). These changes in ICCs are consistent with a previous
study by our group (4).
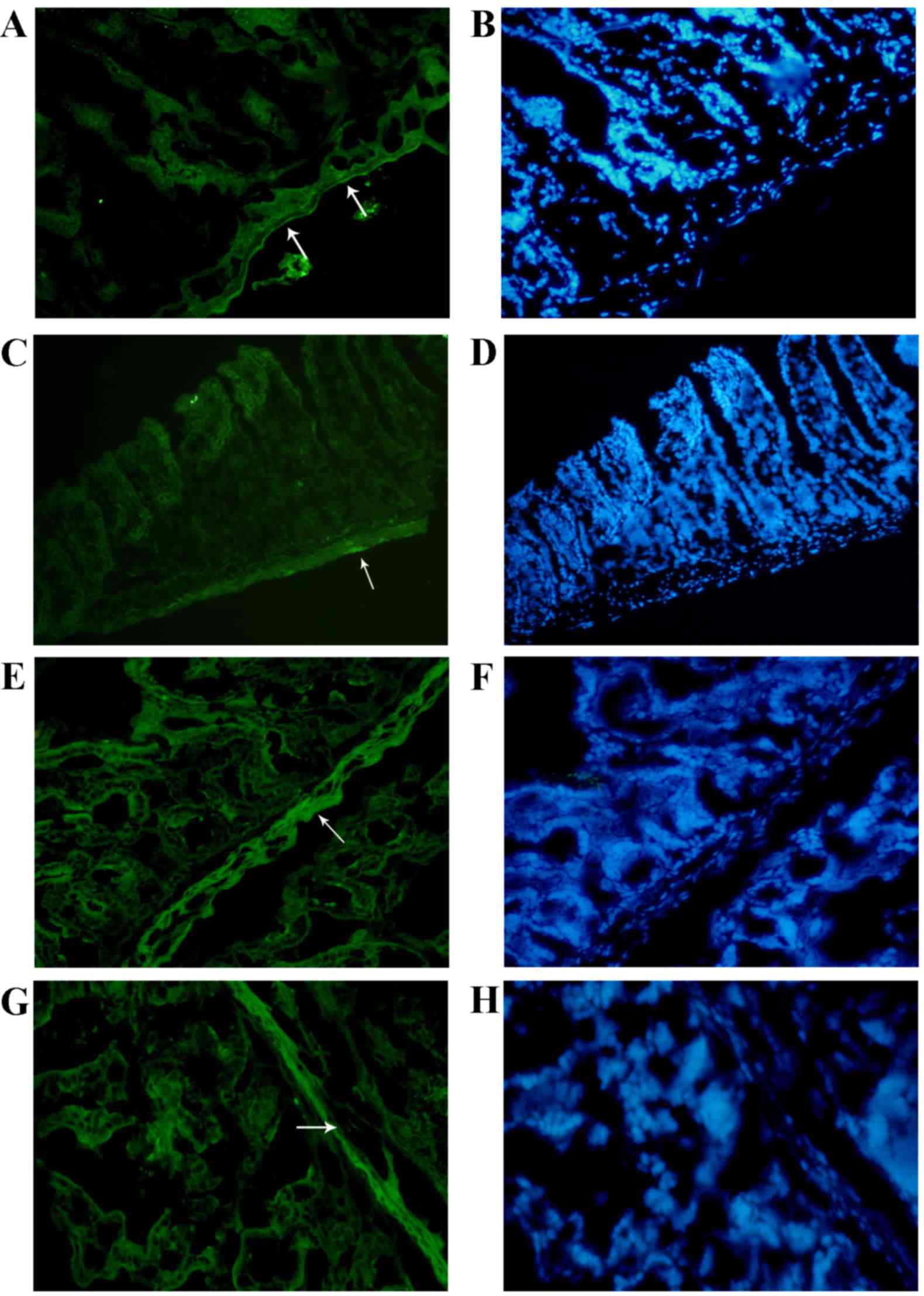 | Figure 4.Immunofluorescence detection of c-kit
and HCN2 in the intestine. (A) In the immunofluorescence analysis,
c-kit expression in ICCs was shown as a bold line in the control
groups (magnification, ×400), and (C) c-kit expression was reduced
in the mice with malignant ascites (magnification, ×400). (G)
Immunofluorescence detection revealed no evident changes in HCN2
expression in the malignant ascites group (magnification, ×400)
compared with (E) the control group (magnification, ×400). (B, H)
However, the muscularis propria was significantly thinner in the
malignant ascites group compared with that in the control group.
(magnification, ×400). HCN2, hyperpolarization-activated cyclic
nucleotide-gated potassium channel 2. |
These results suggested that the gastrointestinal
dysmotility induced by malignant ascites was relevant to the
decreased expression and morphological changes observed in ICCs.
Furthermore, the HCN2 channel is a pacemaker channel that is
involved in gastrointestinal peristalsis (20). Compared with the control group
(Fig. 4E and F, arrow),
immunofluorescence analysis indicated that there were no evident
changes in HCN2 in the malignant ascites group (Fig. 4G and H, arrow). By contrast, the
muscularis propria was significantly thinner in the malignant
ascites group compared with the control group.
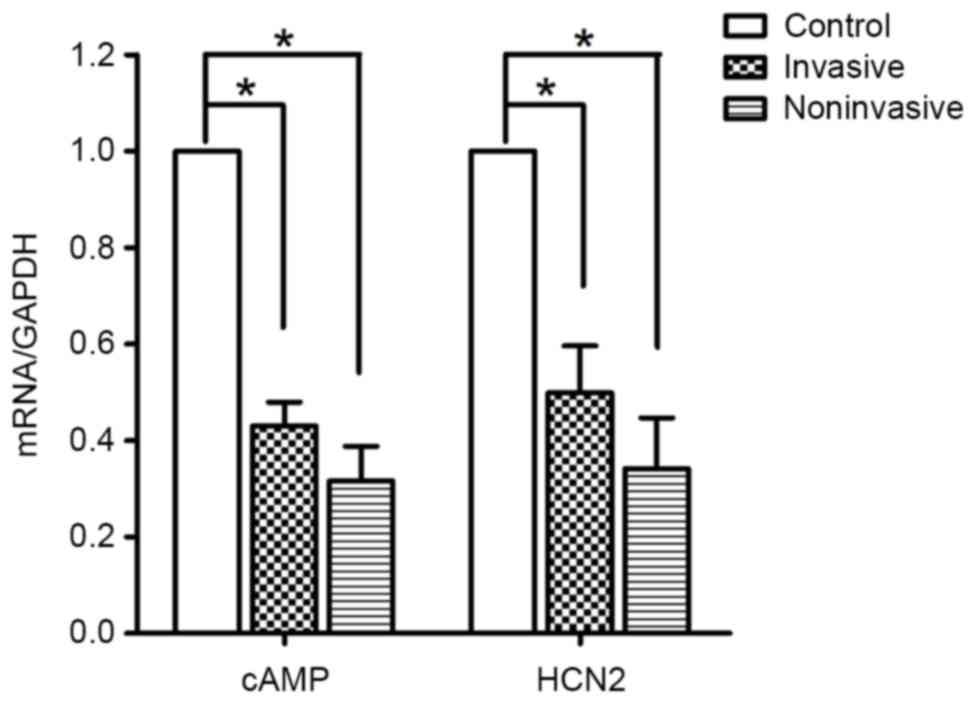
qPCR detection of HCN2 expression
The present study performed qPCR to additionally
elucidate changes in HCN2 expression. The qPCR analyses detected
messenger RNA (mRNA) transcripts for HCN2 in the model and control
groups. However, the mRNA transcript levels for HCN2 in the
malignant ascites group were significantly lower compared with
those in the control group (P=0.01; Fig.
5). The cAMP-dependent regulation of pacemaker activity may be
mediated through HCN channels (21).
Therefore, the expression of cAMP was also detected using qPCR. The
mRNA transcript levels for cAMP in the malignant ascites group were
lower compared with those in the control group (P=0.01; Fig. 5), similar to the results of HCN2 mRNA
expression. These results suggest that the expression of HCN2 in
ICCs is inhibited by malignant ascites, and that malignant
ascites-induced gastrointestinal dysmotility is associated with
decreased ICC number and reduced HCN2 expression.
Morphological analyses of ICCs and
HCN2-mediated regulation of Ca2+ concentration in
ICCs
The mechanism of gastrointestinal dysmotility
mediated by ICCs and HCN2 was additionally examined to determine
the effect of malignant ascites on ICCs. ICCs isolated from C57BL/6
mice subsequent to being co-cultured with malignant ascites were
detected using immunofluorescence and divided into 2 groups. One
group was cultured with malignant ascites, while the other group
served as the control group. Changes in c-kit and HCN2 expression
on ICCs were observed. In the control group, c-kit was highly
expressed in the cytoplasm and membranes of ICCs, which exhibited a
slender morphology and evident connections with other cells
(Fig. 6Aa). However, the ICCs were
evidently smaller in the malignant ascites group, and c-kit
expression appeared markedly decreased (Fig. 6Aa and b). Electron microscopy revealed
that the ICCs were oval or circular and exhibited numerous slender
processes on the surface. In the control group, the nuclei were
large, and numerous mitochondria were observed in the cytoplasm
(Fig. 6Ac). However, in the malignant
ascites group the nuclei of ICCs were pyknotic, the number of
cellular processes were reduced and the size of the ICCs was
markedly reduced (Fig. 6Ad). HCN2
expression was also decreased in the malignant ascites group
(Fig. 6Af) compared with that in the
control group (Fig. 6Ae). These
results suggested that malignant ascites decreased the expression
of HCN2 on ICCs, thereby inducing gastrointestinal motility
dysfunction. HCN2 is a cation channel that exhibits pacing
functions, and the release and reuptake of Ca2+ are
linked to the generation of pacemaker activity (14). Therefore, the present study
investigated Ca2+ concentrations using flow cytometry.
The Ca2+ concentrations detected in the malignant
ascites group (82.60±2.21%) were significantly lower compared with
those in the control group (95.51±1.09%; P=0.01, P<0.05;
Fig. 6B). These results suggested
that malignant ascites reduced the Ca2+ concentration in
ICCs, and that HCN2 regulated Ca2+ concentrations in
ICCs and altered intestinal peristalsis in the malignant ascites
group (We set a gate as M1 to represent FITC positive cells.).
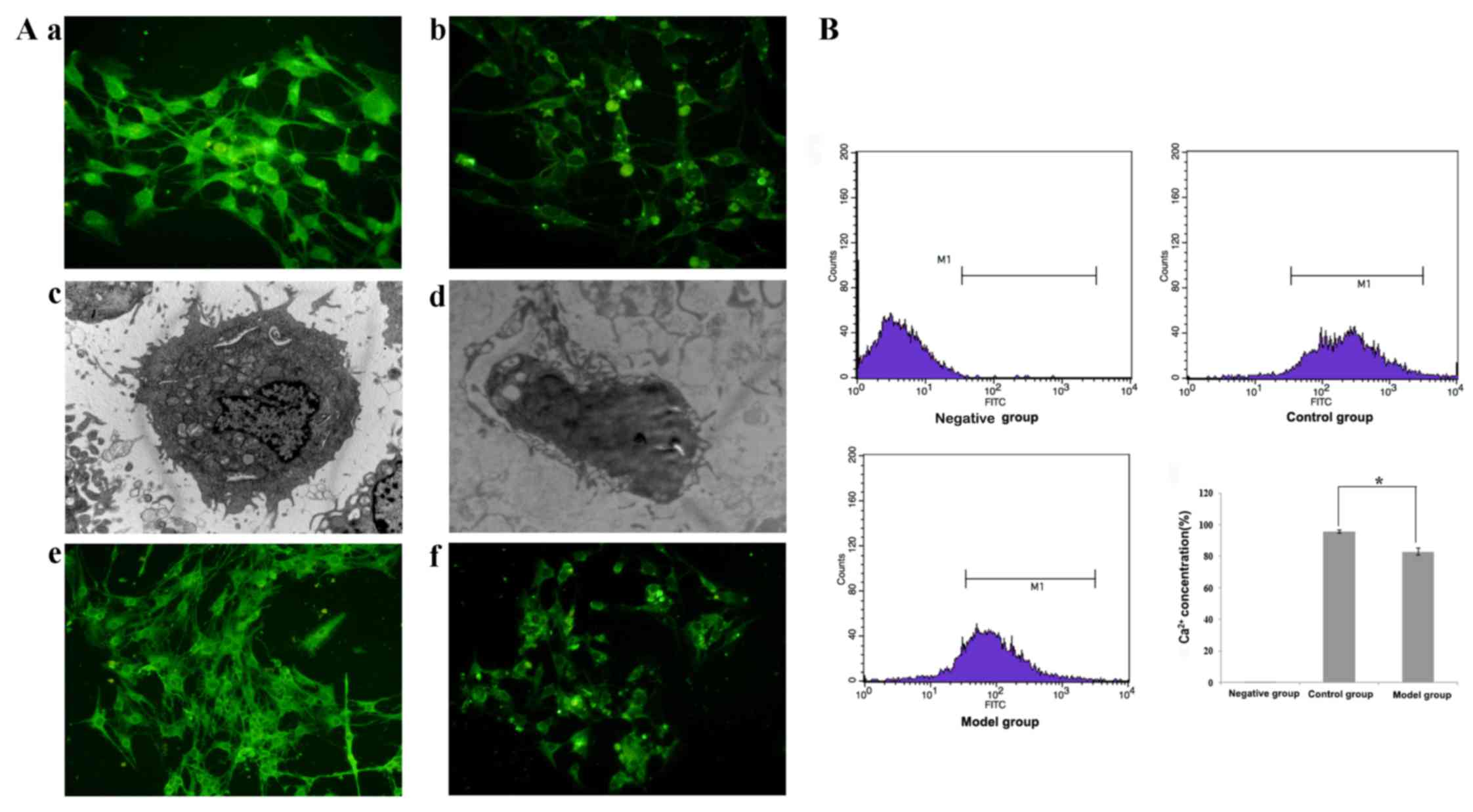 | Figure 6.Morphological analyses of ICCs and
HCN2 regulation of the Ca2+ concentration in ICCs.
(A)(a) ICCs were analyzed using fluorescence and electron
microscopy. Fluorescence microscopy revealed that c-kit was highly
expressed in the ICC cytoplasm and membrane (magnification, ×400).
(c) Ultrastructural examination demonstrated that the ICCs were
oval or circular and exhibited numerous slender processes on the
surface. The nuclei were large, and numerous mitochondria were
observed in the cytoplasm in the control group (magnification,
×5,000). (b) Fluorescence (magnification, ×400) and (d) electron
microscopy (magnification, ×5,000) revealed apoptotic ICCs with a
shrunken cell volume and condensed nuclei subsequent toco-culture
with malignant ascites. Changes in HCN2 expression on ICCs were
observed. (e) HCN2 expression was decreased in the malignant
ascites group (magnification, ×400) compared with (f) the control
group (magnification, ×400). (B) Flow cytometry analysis of
Ca2+ concentrations. Flow cytometry revealed a
significantly lower Ca2+ concentration in the malignant
ascites group compared with that in the control group. The ratio of
the Ca2+ concentration in the malignant ascites group to
that in the control group is expressed as the mean ± standard error
of the mean. *P<0.05, significant difference between the control
and malignant ascites groups. The present study set a gate as M1 to
represent FITC positive cells. ICC, interstitial cell of Cajal;
HCN2, hyperpolarization-activated cyclic nucleotide-gated potassium
channel 2; FITC, fluorescein isothiocyanate. |
Discussion
Patients with malignant ascites exhibit abdominal
pain and abdominal distention caused by gastrointestinal
dysmotility (22). The present data
suggest that gastrointestinal dysmotility caused by malignant
ascites is associated with HCN2 channels and Ca2+
concentrations in ICCs. The major findings of the present study
were that malignant ascites reduced HCN2 expression and
Ca2+ concentrations in ICCs and affected intestinal
peristalsis.
The mechanisms underlying malignant ascites-induced
gastrointestinal dysmotility are not clear, but our previous study
suggests that pathological changes in ICCs are involved in this
process (3). ICCs generate slow-wave
activity and regulate gastrointestinal peristalsis (23). The stem cell factor (SCF)/c-kit
signaling pathway is important in the loss of ICCs (24). A previous study indicated that SCF
improves the function of ICCs and promotes gastrointestinal
peristalsis (25). The present study
additionally examined the mechanisms of malignant ascites-induced
gastrointestinal dysmotility. The present data demonstrated that
malignant ascites altered ICC morphology and triggered apoptosis.
ICC nuclei were pyknotic, and cell processes were reduced in these
cells. The pacemaker function of ICCs was also decreased. ICCs are
pacemaker cells that generate electrical activity to drive
contractility in the gastrointestinal tract via Ca2+
transients (26). Intracellular
Ca2+ serves a critical role in the generation of
pacemaker activity in ICCs and electrical rhythmicity in the
gastrointestinal tract (27–29). Previous studies have suggested that
slow-wave generation depends on T-type Ca2+conductance,
and that this T-type current is involved in the pacemaker activity
of ICCs (30). Flow cytometry data
demonstrated that Ca2+ concentrations were significantly
reduced in malignant ascites, which suggests that malignant ascites
reduce Ca2+concentrations in ICCs in the intestine, and
additionally inhibit action potential and slow waves in the
intestine, as well as decelerating intestinal peristalsis.
HCN2 channels are cation channels that generate
hyperpolarization-activated cation currents, which contribute to
various physiological properties and functions, including pacemaker
activity (31,32). HCN channels are associated with
spontaneous rhythmic activities, and regulate the excitability of
vagal and spinal afferents, which participate in the generation of
abdominal distention and abdominal pain (33). HCN channels are pacemaker channels
that regulate gastrointestinal peristalsis (14). The present study demonstrated that
malignant ascites reduced HCN2 expression. The HCN channel family
consists of four homologous members (HCN1-HCN4). HCN2-positive
neurons and their terminal endings are in physical proximity to the
cellular network of ICCs (34). HCN
channels are expressed in the heart and nervous system (35). HCN channels exist in the bladder, and
affect bladder excitation via bladder ICCs (36). HCN channels also exist in the
digestive system, including the stomach and colon of mice (14,37). The
present study isolated ICCs from intestines and observed HCN2
expression in the ICCs. Previous studies have demonstrated the
importance of cAMP binding for HCN channel function (15,37,38). The
present study revealed that cAMP and HCN2 mRNA levels were reduced
in the malignant ascites group. Therefore, the present study
hypothesized that malignant ascites would reduce HCN2 expression,
and that certain substances present in malignant ascites would
reduce cAMP expression and HCN2 channels activity. Our previous
study (4) revealed abundant
inflammatory cells in malignant ascites, primarily consisting of
mononuclear cells and T lymphocytes, which may affect cAMP and HCN2
expression.
In conclusion, the findings of the present study
suggested that the small intestinal dysmotility caused by malignant
ascites is associated with changes in HCN2 channels in ICCs, which
offers a potential therapeutic target for gastrointestinal
dysmotility in advanced malignant ascites.
Acknowledgements
The present study was supported by the National
Natural Science Fund (grant no. 81372611), the National Natural
Science Youth Fund (grant no. 81301750) and the Education
Department of Heilongjiang Province key project (grant no.
12521z017).
References
|
1
|
Becker G, Galandi D and Blum HE: Malignant
ascites: Systematic review and guideline for treatment. Eur J
Cancer. 42:589–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sangisetty SL and Miner TJ: Malignant
ascites: A review of prognostic factors, pathophysiology and
therapeutic measures. World J Gastrointest Surg. 4:87–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng H, He Y, Tong J, Sun L, Yang D, Li
H, Ao N, Jin X and Zhang Q: Is gastrointestinal dysfunction induced
by gastric cancer peritoneal metastasis relevant to impairment of
interstitial cells of Cajal? Clin Exp Metastasis. 28:291–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Kong D, He Y, Wang X, Gao L, Li J,
Yan M, Liu D, Wang Y, Zhang L and Jin X: The impact of inflammatory
cells in malignant ascites on small intestinal ICCs' morphology and
function. J Cell Mol Med. 19:2118–2127. 2015.PubMed/NCBI
|
|
5
|
Sanders KM, Ward SM and Koh SD:
Interstitial cells: Regulators of smooth muscle function. Physiol
Rev. 94:859–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR,
Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG,
Kendrick ML, et al: Ano1 is a selective marker of interstitial
cells of Cajal in the human and mouse gastrointestinal tract. Am J
Physiol Gastrointest Liver Physiol. 296:G1370–G1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh SD, Sanders KM and Ward SM:
Spontaneous electrical rhythmicity in cultured interstitial cells
of cajal from the murine small intestine. J Physiol. 513:203–213.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
d'antonio C, Wang B, McKay C and Huizinga
JD: Substance P activates a non-selective cation channel in murine
pacemaker ICC. Neurogastroenterol Motil. 21:985-e79. 2009.
|
|
9
|
Huizinga JD, Zarate N and Farrugia G:
Physiology, injury, and recovery of interstitial cells of Cajal:
Basic and clinical science. Gastroenterology. 137:1548–1556. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strege PR, Ou Y, Sha L, Rich A, Gibbons
SJ, Szurszewski JH, Sarr MG and Farrugia G: Sodium current in human
intestinal interstitial cells of Cajal. Am J Physiol Gastrointest
Liver Physiol. 285:G1111–G1121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ward SM and Sanders KM: Involvement of
intramuscular interstitial cells of Cajal in neuroeffector
transmission in the gastrointestinal tract. J Physiol. 576:675–682.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanders KM and Ward SM: Interstitial cells
of Cajal: A new perspective on smooth muscle function. J Physiol.
576:721–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Donnell AM, Coyle D and Puri P:
Decreased expression of hyperpolarisation-activated cyclic
nucletide-gated channel 3 in Hirschsprung's disease. World J
Gastroenterol. 21:5635–5640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shahi PK, Choi S, Zuo DC, Kim MY, Park CG,
Kim YD, Lee J, Park KJ, So I and Jun JY: The possible roles of
hyperpolatization-activated cyclic nucleotide channels in
regulating pacemaker activity in colonic interstitial cells of
Cajal. J Gastroenterol. 49:1001–1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harzheim D, Pfeiffer KH, Fabritz L,
Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB and Seifert R:
Cardiac pacemaker function of HCN4 channels in mice is confined to
embryonic development and requires cyclic AMP. EMBO J. 27:692–703.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zong X, Krause S, Chen CC, Krüger J,
Gruner C, Cao-Ehlker X, Fenske S, Wahl-Schott C and Biel M:
Regulation of hyperpolarization-activated cyclic nucleotide-gated
(HCN) channel activity by cCMP. J Biol Chem. 287:26506–26512. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DiFrancesco JC and DiFrancesco D:
Dysfunctional HCN ion channels in neurological diseases. Front Cell
Neurosci. 6:1742015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernard M, Dejos C, Berges T, Regnacq M
and Voisin P: Activation of rhodopsin gene transcription in
cultured retinal precursors of chicken embryo: Role of Ca(2+)
signaling and hyperpolarization-activated cation channels. J
Neurochem. 129:85–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pape HC: Queer current and pacemaker: The
hyperpolarization-actived cation current in neurons. Annu Rev
Physiol. 58:299–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DiFrancesco D and Tortora P: Direct
activation of cardiac pacemaker channels by intracellular cyclic
AMP. Nature. 351:145–147. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chasen M and Bhargava R: Gastrointestinal
symptoms, electrogastrography, inflammatory makers, and PG-SGA in
patients with advanced cancer. Support Care Cancer. 20:1283–1290.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JC, Thuneberg L, Berezin I and
Huizinga JD: Generation of slow waves in membrane potential is an
intrinsic property of interstitial cells of Cajal. Am J Physiol.
277:G409–G423. 1999.PubMed/NCBI
|
|
24
|
Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D
and Wang JM: Decreased SCF/c-kit signaling pathway contributes to
loss of interstitial cells of Cajal in gallstone disease. Int J
Clin Exp Med. 7:4099–4106. 2014.PubMed/NCBI
|
|
25
|
Kong D, Li J, Zhao B, Xia B, Zhang L, He
Y, Wang X, Gao L, Wang Y, Jin X and Lou G: The effect of SCF and
ouabain on small intestinal motility dysfunction induced by gastric
cancer peritoneal metastasis. Clin Exp Metastasis. 32:267–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh RD, Gibbons SJ, Saravanaperumal SA,
Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz
GJ, et al: Ano1, a Ca2+-activated Cl- channel,
coordinates contractility in mouse intestine by Ca2+
transient coordination between interstitial cells of Cajal. J
Physiol. 592:4051–4068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ward SM, Ordog T, Koh SD, Baker SA, Jun
JY, Amberg G, Monaghan K and Sanders KM: Pacemaking in interstitial
cells of Cajal depends upon calcium handling by endoplasmic
reticulum and mitochondria. J Physiol. 525:355–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drumm BT, Sergeant GP, Hollywood MA,
Thornbury KT, Matsuda TT, Baba A, Harvey BJ and McHale NG: The
effect of high [K(+)]o on spontaneous Ca(2+) waves in freshly
isolated interstitial cells of Cajal from the rabbit urethra.
Physiol Rep. 2:e002032014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu MH, Sung TS, O'Driscoll K, Koh SD and
Sanders KM: Intracellular Ca(2+) release from endoplasmic reticulum
regulates slow wave currents and pacemaker activity of interstitial
cells of Cajal. Am J Physiol Cell Physiol. 308:C608–C620. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng H, Park KS, Koh SD and Sanders KM:
Expression and function of a T-type Ca2+ conductance in
interstitial cells of Cajal of the murine small intestine. Am J
Physiol Cell Physiol. 306:C705–C713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Biel M, Schneider A and Wahl C: Cardiac
HCN channels: Structure, function, and modulation. Trends
Cardiovasc Med. 12:206–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Notomi T and Shigemoto R:
Immunohistochemical localization of Ih channel subunits, HCN1-4, in
the rat brain. J Comp Neurol. 471:241–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YP, Sun BY, Li Q, Dong L, Zhang GH,
Grundy D and Rong WF: Hperpolarization-activated cyclic
nucleotide-gated cation channel subtypes differentially modulate
the excitability of murine small intestinal afferents. World J
Gastroenterol. 18:522–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang S, Xiong CJ, Sun HM, Li XS, Zhang GQ,
Wu B and Zhou DS: The distribution of HCN2-positive cells in the
gastrointestinal tract of mice. J Anat. 221:303–310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biel M, Wahl-Schott C, Michalakis S and
Zong X: Hyperpolarization-activated cation channels: From genes to
function. Physiol Rev. 89:847–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He P, Deng J, Zhong X, Zhou Z, Song B and
Li L: Identification of a hyperpolarization-activated cyclic
nucleotide-gated channel and its subtypes in the urinary bladder of
the rat. Urology. 79:1411.e7-e13. 2012. View Article : Google Scholar
|
|
37
|
Herrmann S, Schnorr S and Ludwig A: HCN
channels-modulators of cardiac and neuronal excitability. Int J Mol
Sci. 16:1429–1447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wainger BJ, DeGennaro M, Santoro B,
Siegelbaum SA and Tibbs GR: Molecular mechanism of cAMP modulation
of HCN pacemaker channels. Nature. 411:805–810. 2001. View Article : Google Scholar : PubMed/NCBI
|