Introduction
In several clinical trials, first-line combination
chemotherapies containing bevacizumab (Bev) were revealed to
improve clinical outcomes in patients with advanced non-squamous
non-small cell lung cancer (non-sq NSCLC) (1–3). Sandler
et al (3) reported significant
survival benefits, such as overall survival (OS) of >1 year
(12.3 months), with addition of Bev to paclitaxel plus carboplatin
in the treatment of non-sq NSCLC. Although Bev was approved for
NSCLC in 2009 in Japan, there are insufficient data regarding the
efficacy, toxicity and predictive markers for Bev treatment. The
present study evaluated the efficacy and safety of Bev-containing
combination chemotherapy in patients with non-sq NSCLC in clinical
settings in Japan. Landmark survival analysis, or disease control
at 8 weeks, was reported to be a more powerful predictor of
subsequent survival compared with the traditional tumor response
rate in advanced NSCLC (4). This may
provide an early assessment of subsequent outcome. Since treatment
with bevacizumab occasionally results in cavitary lesion without
tumor shrinkage (5), stable disease
may also be important for understanding drug efficacy. Therefore,
landmark analysis was utilized in the present study. In addition,
the identification of predictive markers for Bev-containing
chemotherapy efficacy was attempted. The mutation of epidermal
growth factor receptor (EGFR) is a key factor in predicting
the response and survival rate following EGFR-tyrosine kinase
inhibitor (EGFR-TKI) treatment (6,7), but no
data have demonstrated the importance of EGFR mutations in
predicting the effect of Bev treatment. In the present study,
multivariate analysis using Cox's regression model revealed that
specific EGFR mutations are predictive markers for Bev
treatment efficacy.
Patients and methods
Patient eligibility
Patients scheduled for Bev treatment between August
2010 to July 2012 were prospectively enrolled in the present study.
Eligible patients had histologically or cytologically confirmed
inoperable advanced, stage IIIB-IV, or recurrent non-sq NSCLC, an
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
of 0–2, and adequate organ function for cytotoxic chemotherapy. The
main exclusion criteria were diagnoses of squamous cell carcinoma
or symptomatic brain metastasis, although patients with controlled
brain metastases were eligible, surgery or surgical biopsy within 4
weeks, a history of significant hemoptysis, of >2.5 ml per
episode, coagulation treatment, bleeding tendency, active
concomitant malignancy, presence of significant comorbidities such
as uncontrolled hypertension, interstitial pneumonia, active
gastrointestinal ulcer, angina pectoris, pregnancy or lactation or
other factors as judged by a medical oncologist. Protocol-specified
demographics, disease characteristics including EGFR
mutation status and patient medical history were collected at
baseline. Patients were treated at four major hospitals
participating in the Shinjuku Thoracic Oncology Group (STOG), Keio
University School of Medicine (Tokyo, Japan), National Center for
Global Health and Medicine (Tokyo, Japan), Tokyo Medical University
(Tokyo, Japan), Tokyo Women's Medical University (Tokyo, Japan).
The study protocol was approved by the institutional review board
at each institution. All patients provided written informed consent
prior to inclusion in the present study.
Study design and treatment
The primary endpoints were progression-free survival
(PFS) and safety. The secondary endpoints were the response rate
(RR), time to response and landmark survival (4). Patients received 15 mg/kg Bev every 3
weeks in conjunction with the chemotherapy prescribed by attending
physicians, followed by 15 mg/kg Bev every 3 weeks with or without
chemotherapy as maintenance. Any line of chemotherapy was
permitted. Bev-containing regimens were as follows: Carboplatin
(CBDCA) + pemetrexed (PEM); cisplatin (CDDP) + PEM; CBDCA +
paclitaxel (PTX); PEM; docetaxel (DTX); CBDCA + DTX; and CBDCA +
PEM + erlotinib (n=41, n=21, n=18, n=9, n=9, n=3 and n=1,
respectively). All chemotherapy regimens contained either PEM
(n=72) or a taxane (DTX or PTX; n=30). Treatment was continued
until tumor progression or the patient experienced unacceptable
toxicities, such as grade 2 or severe hemoptysis, grade 3 or severe
bleeding, or by the decision of attending physician.
Evaluation
Tumor response was assessed according to the
Response Evaluation Criteria in Solid Tumors version 1.1 (8). Tumors were assessed at baseline by
computed tomography, magnetic resonance imaging, bone scintigraphy
and/or fluorodeoxyglucose positron emission tomography. During
treatment, a radiographic evaluation was performed subsequent to at
least every two courses of treatment and/or at the time of
suspected disease progression. To confirm response, a partial
response (PR) or complete response (CR), radiographic evaluations
were recommended 4 weeks subsequent to the original evaluations.
The disease control rate (DCR) was defined as [CR + PR + stable
disease (SD)]/total patients. Toxicities were graded according to
the National Cancer Institute Common Toxicity Criteria version 4.0
(9).
Statistical analysis
The primary endpoints were PFS and safety. Using the
13-week PFS rates of 80.1% in the chemotherapy plus Bev arm and
67.7% in the chemotherapy-alone arm from the ECOG 4599 study
(3), the sample size was calculated
to achieve a power of 80% with a two-sided α of 0.05, and the
expected and threshold values for PFS were 6.2 and 4.5 months,
respectively. The estimated minimum sample size was 77, which was
calculated by SWOG statistical tools using a one-arm binomial
setup. Allowing for a maximum dropout rate of 30% and considering
that the present study was an observational cohort study with a
minimum of 12 months of follow-up, 100 patients were sought for
enrollment. PFS and OS subsequent to the initiation of the
Bev-containing chemotherapy were estimated by the Kaplan-Meier
method. PFS was defined as the time from the initiation of Bev
therapy to investigator-assessed disease progression or mortality
from any cause. OS was defined as the time from the initiation of
the Bev therapy to mortality from any cause. OS, PFS and responses
were assessed in all eligible patients on an intent-to-treat basis.
Patients without an event (progression or mortality) were censored
at the last follow-up or data cutoff date, whichever occurred
first. Univariate and multivariate analyses were performed using
Cox's proportional hazards model to assess the independent effects
of patient and disease characteristics on PFS and OS. To avoid
possible confounding effects of treatment with EGFR-TKIs prior to
accrual of the present study (‘pre-treatment with EGFR-TKIs’)
multivariate analyses of PFS and OS were adjusted for
‘pre-treatment with EGFR-TKIs’. Landmark survival analyses
(4) of PFS and OS were performed by
comparing the patients who achieved PR or SD 8 weeks subsequent to
Bev administration. All P-values in the present study are
two-sided. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was conducted using
IBM SPSS Statistics version 19.0 software (IBM SPSS, Armonk, NY,
USA). The present study is registered with the University Hospital
Medical Information Network (UMIN) Clinical Trials Registry
(www.umin.ac.jp/ctr) as trial number
UMIN000004609.
Results
Patients characteristics and
treatment
Between August 2010 and July 2012, a total of 102
patients were enrolled in the present study, with a data cutoff
date of September 30, 2014. The duration of follow-up subsequent to
final registration was 14 months. The median follow-up time was
1,021 days (33.6 months). Patient characteristics are summarized in
Table I. Histological analysis
revealed that 98 patients (96.1%) exhibited adenocarcinoma, three
patients exhibited NSCLC that was not otherwise specified, and one
patient exhibited pleomorphic carcinoma. The majority of the
patients were receiving first-line treatment (n=57, 56%), but
patients receiving second-line (n=22, 22%), third-line (n=12, 12%)
and later lines of treatment, fourth, fifth, sixth and seventh
line, n=5, n=4, n=1, and n=1, respectively, were also included. At
the data cutoff date, the median numbers of cycles of Bev
administration were 9, 6, and 7 for the first-, second-, and third-
and later lines of treatment, respectively. EGFR mutation
status was available for the majority of the patients (98%), with
mutations identified in 44.1% of the patients. Amongst the patients
with EGFR mutations, mutation types were characterized for
39 patients. The most common type of mutation was an exon 19
deletion (n=28, including one case with T790M), followed by the
exon 21 mutations L858R, L858R and T790M, and L861Q (n=11 total;
n=8; n=2; n=1, respectively). Amongst the patients with major
EGFR mutations, 14 of 28 patients (50%) with the exon 19
deletion and 8 of 11 patients (73%) with the exon 21 mutations
received ‘pre-treatment with EGFR-TKIs’. In total, 12 of 28
patients (43%) with the exon 19 deletion and 8 of 11 patients (73%)
with the exon 21 mutations were treated with EGFR-TKIs subsequent
to completion of the present study, that is, ‘post-treatment with
EGFR-TKIs’. Only five patients with the exon 19 deletion lacked
information regarding EGFR-TKI treatment, but all patients with the
exon 21 mutations received EGFR-TKIs prior and/or subsequent to the
present study.
 | Table I.Patient characteristics (n=102). |
Table I.
Patient characteristics (n=102).
| Characteristic | Classification | n (%) |
|---|
| Age, years | (median, range) | 64 |
(36–85) |
| Sex | Male | 60 | (58.8) |
|
| Female | 42 | (41.2) |
| Smoking history | + | 61 | (59.8) |
|
| − | 41 | (40.2) |
| Performance
status | 0 | 66 | (64.7) |
|
| 1 | 34 | (33.3) |
|
| 2 | 2 |
(2.0) |
| Histology | Adenocarcinoma | 98 | (96.1) |
|
| Others | 4 |
(3.9) |
| Stage | IIIA | 2 |
(2.0) |
|
| IIIB | 8 |
(7.8) |
|
| IV | 77 | (75.5) |
|
| Recurrent | 15 | (14.7) |
| Brain metastasis | + | 11 | (10.8) |
|
| − | 91 | (89.2) |
| Platinum
combination | + | 84 | (82.4) |
|
| − | 18 | (17.6) |
| Treatment line | First | 57 | (55.9) |
|
| Second | 22 | (21.6) |
|
| Third and later | 23 | (22.5) |
| EGFR mutation | + | 45 | (44.1)a |
|
| − | 55 | (53.9) |
|
| Unknown | 2 |
(2.0) |
Toxicity
The toxicity profile is summarized in Table II. The most frequent grade 3 or 4
adverse events were neutropenia and hypertension. Severe grade 3 or
4 hematological toxicities included leukopenia (25.5%), neutropenia
(42.2%), anemia (6.9%), thrombocytopenia (4.9%) and febrile
neutropenia (3.9%). Severe grade 3 or 4 Bev-associated adverse
events included hypertension (30.4%), proteinuria (5.9%),
thromboembolism (4.9%) and epistaxis (1.0%). Overall, the majority
of adverse events were manageable. No treatment-associated
mortality was observed.
 | Table II.Toxicities observed. |
Table II.
Toxicities observed.
|
| All n (%) | G3 or more n
(%) |
|---|
| Anemia | 58(56.9) | 7(6.9) |
|
Thrombocytopenia | 47(46.1) | 5(4.9) |
| Leukopenia | 69(67.6) | 26(25.5) |
| Neutropenia | 65(63.7) | 43(42.2) |
| Febrile
neutropenia | 4(3.9) | 4(3.9) |
|
Hypoalbuminemia | 42(41.2) | 1(1.0) |
| Increased AST | 45(44.1) | 3(2.9) |
| Increased ALT | 40(39.2) | 2(2.0) |
| Increased ALP | 19(18.6) |
|
| Increased
creatinine | 15(14.7) |
|
| Proteinuria | 49(48.0) | 6(5.9) |
| Declining PS | 56(54.9) | 10(9.8) |
| Nausea | 42(41.2) | 2(2.0) |
| Vomiting | 16(15.7) | 1(1.0) |
| Appetite loss | 52(51.0) | 2(2.0) |
| Diarrhea | 10(9.8) |
|
| Constipation | 40(39.2) |
|
| Mucositis oral | 11(10.8) |
|
| Fatigue | 27(26.5) | 1(1.0) |
| Malaise | 39(38.2) |
|
| Macular rash | 14(13.7) |
|
| Fever up | 18(17.6) |
|
| Dyspnea | 10(9.8) |
|
| Peripheral motor
neuropathy | 3(2.9) | 1(1.0) |
| Peripheral sensory
neuropathy | 27(26.5) | 4(3.9) |
| Dysgeusia | 15(14.7) |
|
| Alopecia | 13(12.7) |
|
| Pain | 11(10.8) |
|
| Headache | 5(4.9) | 1(1.0) |
|
Hypertensiona | 79(77.5) | 31(30.4) |
| Epistaxis | 16(15.7) | 1(1.0) |
| Thromboembolic
event | 7(6.9) | 5(4.9) |
| Bleeding | 5(4.9) | 1(1.0) |
| Infection | 7(6.9) | 2(2.0) |
| Duodenal ulcer | 1(1.0) | 1(1.0) |
| Gait
disturbance | 1(1.0) | 1(1.0) |
| Meningitis | 1(1.0) | 1(1.0) |
Efficacy
The objective response to Bev-containing
chemotherapies is summarized in Table
III. With 102 evaluable patients, the RR was 44.1% and the DCR
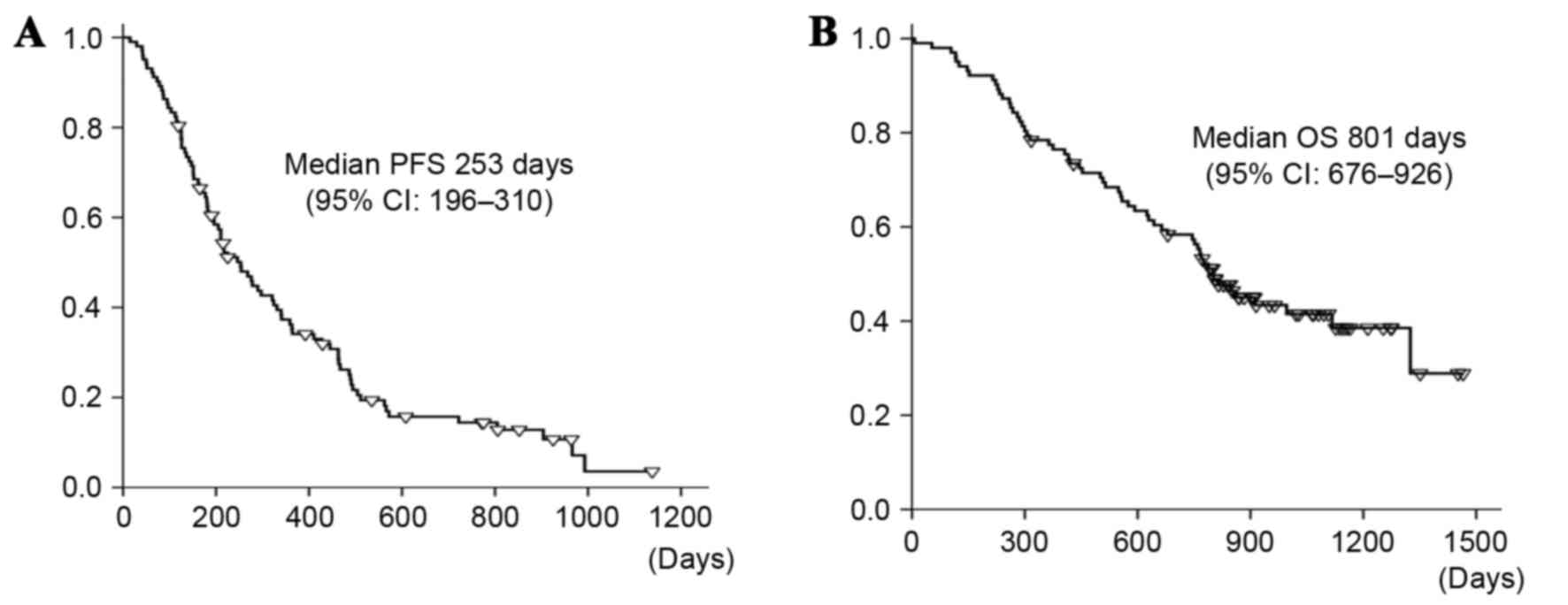
was 92.2%. Only 1 patient achieved a CR. The median PFS was 8.3
months (95% CI=6.4–10.2 months), which is longer compared with the
predefined expected and threshold values for PFS (6.2 and 4.5
months, respectively), as demonstrated in Fig. 1A. The primary PFS endpoint was met.
The median OS was 26.3 months (95% CI=22.2–30.4 months), as
illustrated in Fig. 1B. There was no
significant difference in the time to response to BEV between
chemotherapy-naïve: Median, 73 days; range, 25–519 days, and
previously treated patients: Median, 40 days; range, 23–535 days
(P=0.265). No significant difference was observed in PFS between
patients with SD, 268 days (95% CI=196–416 days) and those who
achieved PR, 266 days (95% CI=125–431 days) by landmark analysis
(P=0.97). There was also no significant difference observed in OS
between patients with SD, 860 days (95% CI=640–1079 days) and those
who achieved PR, 664 days (95% CI=329–999 days) by landmark
analysis (P=0.35). Univariate and multivariate analyses were
performed to identify the variables significantly associated with
PFS and OS, as summarized in Tables
IV and V. Univariate analysis
revealed that several clinical factors were associated with PFS
(P<0.15), as illustrated in Table
IV. In the crude model, multivariate analysis indicated that a
higher T factor, EGFR exon 21 mutations, and poor PS were
linked to significantly shorter PFS. Subsequent to adjustment for
pretreatment with EGFR-TKIs, multivariate analysis revealed that a
higher T factor [adjusted hazard ratio (HR)=1.33] and poor PS
(adjusted HR=1.63) were associated with significantly shorter PFS,
whilst EGFR exon 19 deletion (adjusted HR=0.47) was
associated with prolonged PFS (P<0.05), as demonstrated in
Table V. In the crude model for OS,
multivariate analysis indicated that a lower T factor and
EGFR exon 19 deletion were significant favorable prognostic
factors. Subsequent to adjustment for pretreatment with EGFR-TKIs,
multivariate analysis revealed that EGFR mutations (adjusted
HR=0.20), better PS (adjusted HR=0.45), and the absence of primary
site lesions (adjusted HR=0.29) were significant favorable
prognostic factors (P<0.05). The Kaplan-Meier estimation of OS
was significantly higher for patients with the EGFR exon 19
mutations compared with the estimation for patients with the
EGFR exon 21 mutations or wild-type EGFR
(P=0.037).
 | Table III.Response to intervention. |
Table III.
Response to intervention.
| Response | n (%) |
|---|
| Complete
response | 1(1.0) |
| Partial
response | 44(43.1) |
| Response rate | (44.1) |
| Stable disease | 48(47.1) |
| Non-CR/non-PD | 1(1.0) |
| Disease
control | (92.2) |
| Progressive
disease | 5(4.9) |
| Not evaluable | 3(2.9) |
 | Table IV.Progression-free survival by
univariate Cox's regression analyses using an adjusted model for
pretreatment of EGFR-TKI (P<0.15). |
Table IV.
Progression-free survival by
univariate Cox's regression analyses using an adjusted model for
pretreatment of EGFR-TKI (P<0.15).
| Variable | HR | 95% CI | P-value |
|---|
| Performance status
(0, 1, 2) | 1.94 | 1.26–2.26 | 0.003 |
| T factor (0, 1, 2,
3, 4) | 1.30 | 0.08–1.08 | 0.005 |
| N factor (0, 1, 2,
3) | 1.25 | 1.05–1.05 | 0.01 |
| Brain metastasis
(no/yes) | 2.22 | 1.16–4.16 | 0.02 |
| Target lesion
(no/yes) | 2.62 | 1.19–5.19 | 0.02 |
| Recurrence
subsequent to surgery vs. IIIA-IV | 2.17 | 1.12–4.12 | 0.02 |
| History of surgery
(no/yes) | 0.58 | 0.35–0.35 | 0.03 |
| Primary site
(no/yes) | 2.01 | 1.03–3.03 | 0.04 |
| EGFR exon 19
deletion (no/yes) | 0.57 | 0.33–0.33 | 0.04 |
| Combined treatment
(taxane/PEM) | 0.61 | 0.38–1.38 | 0.05 |
| Chemotherapy
regimen (PEM/docetaxel/Pt + PEM/Pt + taxane) | 1.28 | 0.96–1.96 | 0.09 |
 | Table V.Progression-free survival by
multivariate Cox's regression analyses using an adjusted model for
pretreatment of EGFR-TKI. |
Table V.
Progression-free survival by
multivariate Cox's regression analyses using an adjusted model for
pretreatment of EGFR-TKI.
| Variable | HR | 95% CI | P-value |
|---|
| T factor (0, 1, 2,
3, 4) | 1.33 | 1.10–1.10 | 0.003 |
| EGFR exon 19
deletion (no/yes) | 0.47 | 0.25–0.25 | 0.02 |
| Performance status
(0, 1, 2, 3, 4) | 1.63 | 1.02–2.02 | 0.04 |
| N factor (0, 1, 2,
3) | 1.17 | 0.97–1.97 | 0.11 |
| Combined treatment
(taxane/PEM) | 0.73 | 0.41–1.41 | 0.28 |
| Brain metastasis
(no/yes) | 0.87 | 0.39–1.39 | 0.74 |
Discussion
The present study evaluated the efficacy and safety
of Bev-containing chemotherapy regimens in clinical practice in
Japan, as first and later lines of chemotherapy. The RR of the
present cohort study was 44.1%, and the median PFS was 8.3 months,
data that demonstrates better efficacy for patients compared with
those of previous reports (E4599, RR 35%, PFS 6.2 months; ARIES, RR
49%, PFS 6.6 months) (2,3). The primary endpoint of the present
study, PFS, was met, suggesting that Bev-containing chemotherapy is
effective in clinical settings in Japan. The median OS, 26.3
months, was also improved compared with those of previous trials
(E4599, 12.3 months; ARIES, 13.0 months; SAiL, 14.6 months)
(1–3).
The effect of Bev was similar to that observed in a Japanese phase
II trial that used Bev as first-line chemotherapy (JO19907)
(10), which reported an RR of 60.7%,
a median PFS of 6.9 months and a median OS of 22.8 months, although
the present study included patients receiving first-line and
subsequent Bev treatment.
With respect to the severe adverse events of special
interest due to Bev treatment, grade III or higher hypertension,
proteinuria, thromboembolism and epistaxis occurred in 30.4, 5.9,
4.9 and 1.0% of patients, respectively, in the present study
compared with rates of 7, 3.1, 0.2 and 0.7%, respectively, in the
E4599 trial (3) and 6, 3, 8 and 1%,
respectively, in the SAiL study (1).
A total of 11% of patients exhibited severe hypertension in the
JO19907 study conducted in Japan (10). The higher incidence of hypertension in
the current study may reflect the baseline characteristics of the
patients, such as the higher incidence of comorbid hypertension at
the time of Bev-containing chemotherapy initiation: 46.1% of
patients exhibited hypertension at baseline. Although the present
study revealed a higher incidence of hypertension, all toxicity was
manageable.
The EGFR mutation type was a predictive
marker for Bev-containing chemotherapy efficacy in the present
study, with the EGFR exon 19 mutation being a favorable
predictor of PFS. Previously, several studies suggested that the
effects of EGFR-TKI treatment differ according to the type of
EGFR mutation (11–13). Regarding treatment with afatinib, a
second-generation irreversible EGFR-TKI, the EGFR mutation
type was associated with OS in patients with EGFR
mutation-positive lung adenocarcinoma (11). Patients with the EGFR exon 19
deletion experienced a significant survival benefit with afatinib
treatment compared to chemotherapy, although no such benefit was
observed in patients with the EGFR exon 21 mutations
(11). In two phase II studies of
EGFR-TKIs, erlotinib or gefitinib, plus Bev, improved PFS was seen
upon addition of Bev to the EGFR-TKI regimens in the patients with
the EGFR exon 19 deletion, but not for the patients with the
exon 21 mutations (12,13). The reason for the Bev-mediated
survival benefit in patients with the EGFR exon 19 deletions
remains unclear. Several studies have reported distinct biochemical
properties of different EGFR mutations that may explain the
different responses to EGFR-TKIs (14,15). An
association between EGFR and vascular endothelial growth factor
(VEGF) has also been reported. EGFR-mutated tumors display
higher VEGF expression levels than wild-type EGFR tumors
(16). EGFR-TKI-resistant tumors also
produce greater levels of VEGF, and the amount of VEGF production
varies according to the mutation type (17,18). These
data may explain the favorable efficacy of Bev-containing
chemotherapy in patients with EGFR exon 19 deletions.
There were several limitations to the present study.
Firstly, the sample size was small compared with those in previous
studies (SAiL, n=2212; ARIES, n=1967) (1–2). Secondly,
the treatment was not restricted to first-line chemotherapy,
although Bev-containing chemotherapy was used for first-line
treatment. Concerning the OS, the higher frequency of EGFR
mutations in the Japanese population may favorably affect patient
outcomes compared with previous reports (SAiL and ARIES). In a
Japanese phase II study (10), OS was
22.8 months and PFS was 6.9 months for first-line treatment with
CBDCA + PTX + Bev. In that trial, the EGFR mutation data
were not available, but 41% of patients received EGFR-TKIs as
post-protocol therapy. In the present study, EGFR mutations
were exhibited in 43.1% of patients. A considerable percentage of
patients may have benefited from post-progression treatment with
EGFR-TKIs. A major strength of the present study was that
EGFR mutation status data were available for the majority of
patients. This allowed the importance of EGFR exon 19
mutation as a possible predictive marker for Bev treatment efficacy
to be elucidated.
In conclusion, Bev-containing combination
chemotherapy was effective in treating patients with non-sq NSCLC
in clinical settings in Japan. Adverse events were well-tolerated
and acceptable. Even though these are the results of ad hoc
analyses, multivariate analysis revealed that a lower T factor,
better PS, and the EGFR exon 19 mutations were associated
with prolonged PFS.
References
|
1
|
Crinò L, Dansin E, Garrido P, Griesinger
F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL
and Zhou C: Safety and efficacy of first-line bevacizumab-based
therapy in advanced non-squamous non-small-cell lung cancer (SAiL,
MO19390): A phase 4 study. Lancet Oncol. 11:733–740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch TJ Jr, Spigel DR, Brahmer J,
Fischbach N, Garst J, Jahanzeb M, Kumar P, Vidaver RM, Wozniak AJ,
Fish S, et al: Safety and effectiveness of bevacizumab-containing
treatment for non-small-cell lung cancer: Final results of the
ARIES observational cohort study. J Thorac Oncol. 9:1332–1339.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lara PN Jr, Redman MW, Kelly K, Edelman
MJ, Williamson SK, Crowley JJ and Gandara DR: Southwest Oncology
Group: Disease control rate at 8 weeks predicts clinical benefit in
advanced non-small cell lung cancer: Results from Southwest
Oncology Group randomized trials. J Clin Oncol. 26:463–467. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyazaki M, Naoki K, Sato T, Tanaka K,
Tsuzuki K, Yoshida S, Tomomatsu K, Tasaka S, Soejima K, Sayama K
and Asano K: A case of advanced lung adenocarcinoma with cavity
formation shrunken by bevacizumab added on the 3rd course of
6th-line chemotherapy. Gan To Kagaku Ryoho. 39:421–424. 2012.(In
Japanese). PubMed/NCBI
|
|
6
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naoki K, Soejima K, Okamoto H, Hamamoto J,
Hida N, Nakachi I, Yasuda H, Nakayama S, Yoda S, Satomi R, et al:
The PCR-invader method (structure-specific 5′ nuclease-based
method), a sensitive method for detecting EGFR gene mutations in
lung cancer specimens; comparison with direct sequencing. Int J
Clin Oncol. 16:335–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
|
|
10
|
Niho S, Kunitoh H, Nokihara H, Horai T,
Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K,
et al: Randomized phase II study of first-line
carboplatin-paclitaxel with or without bevacizumab in Japanese
patients with advanced non-squamous non-small-cell lung cancer.
Lung Cancer. 76:362–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seto T, Kato T, Nishio M, Goto K, Atagi S,
Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al:
Erlotinib alone or with bevacizumab as first-line therapy in
patients with advanced non-squamous non-small-cell lung cancer
harbouring EGFR mutations (JO25567): An open-label, randomised,
multicentre, phase 2 study. Lancet Oncol. 15:1236–1244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichihara E, Hotta K, Nogami N, Kuyama S,
Kishino D, Fujii M, Kozuki T, Tabata M, Harada D, Chikamori K, et
al: Phase II trial of gefitinib in combination with bevacizumab as
first-line therapy for advanced non-small cell lung cancer with
activating EGFR gene mutations: The Okayama Lung Cancer Study Group
Trial 1001. J Thorac Oncol. 10:486–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okabe T, Okamoto I, Tamura K, Terashima M,
Yoshida T, Satoh T, Takada M, Fukuoka M and Nakagawa K:
Differential constitutive activation of the epidermal growth factor
receptor in non-small cell lung cancer cells bearing EGFR gene
mutation and amplification. Cancer Res. 67:2046–2053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang
XC, Guo AL, Zhang YF, An SJ, Mok TS and Wu YL: Better survival with
EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small
cell lung cancer patients is due to differential inhibition of
downstream signals. Cancer Lett. 265:307–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reinmuth N, Jauch A, Xu EC, Muley T,
Granzow M, Hoffmann H, Dienemann H, Herpel E, Schnabel PA, Herth
FJ, et al: Correlation of EGFR mutations with chromosomal
alterations and expression of EGFR, ErbB3 and VEGF in tumor samples
of lung adenocarcinoma patients. Lung Cancer. 62:193–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Takayama K, Wang S, Shiraishi Y,
Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I and
Nakanishi Y: Addition of bevacizumab enhances antitumor activity of
erlotinib against non-small cell lung cancer xenografts depending
on VEGF expression. Cancer Chemother Pharmacol. 74:1297–1305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakade J, Takeuchi S, Nakagawa T, Ishikawa
D, Sano T, Nanjo S, Yamada T, Ebi H, Zhao L, Yasumoto K, et al:
Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of
HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR
mutation. J Thorac Oncol. 9:775–783. 2014. View Article : Google Scholar : PubMed/NCBI
|