Introduction
Glioma is one of the most common primary
intracranial tumors of the central nervous system, with an annual
incidence of ~6/100,000 individuals in the USA (1), accounting for 40–50% of brain tumors.
The median survival time of patients with glioblastoma multiforme
(GBM), the most aggressive subtype of malignant glioma, is 12–16
months (2). Despite the advances made
in multimodal treatment, including surgery, radiotherapy and
chemotherapy, the prognosis for the majority of patients with
malignant glioma remains poor, with a median survival time of 14.6
months (3). Therefore, the
development of novel therapeutic strategies is required.
A potential lifespan-prolonging approach for
patients with glioma is to administer immunotherapy during the
course of treatment (4). This
approach requires suitable tumor antigens with specific
characteristics, including high expression levels in glioma
tissues, restricted expression levels in normal tissues and
inherent immunogenicity. The expression of a potential tumor
antigen, cancer/testis (CT) antigen, is restricted to adult
testicular germ cells under normal conditions, but aberrantly
activated and expressed in a range of tumor types such as melanoma,
bladder, breast, prostate, liver, ovarian, colon and non-small cell
lung cancer (5–9). As the testes do not express major
histocompatibility complex class I, cytotoxic T lymphocytes do not
target the CT antigens expressed in the testis (10). At present, CT antigens are considered
to be novel targets for immunotherapy in various tumor types,
including glioma (11,12).
OY-TES-1, a member of the CT antigen family, is the
human homolog of proacrosin binding protein sp32, which was
originally identified in pigs, guinea pigs and mice (13,14).
OY-TES-1 has been the subject of numerous recent studies (15–17) due to
its expression in various cancerous tissues and restricted
expression in normal tissues. Furthermore, OY-TES-1 is able to
increase the humoral immune response across a broad spectrum of
cancer types, including that of the bladder, prostate, liver,
colon, lung and ovaries (13,15,18). To
the best of our knowledge there is no data available at present
regarding the expression profile and immunogenicity of OY-TES-1 in
patients with brain tumors. Whether OY-TES-1 is expressed in glioma
tissue, and induces an immune response in patients with glioma,
requires further investigation. Therefore, the present study was
performed to examine the expression levels and serum
immunoreactivity of OY-TES-1 in human glioma.
Materials and methods
Patients and specimens
The use of human tissue specimens in the present
retrospective study was approved by the Ethics Committee of the
First Affiliated Hospital of Guangxi Medical University (Nanning,
China) and the written informed consent was obtained from all
patients included in this study. A total of 36 tumor tissue samples
(11 GBM, 7 anaplastic astrocytoma, 11 diffuse astrocytoma, 4
pilocytic astrocytoma, 1 anaplastic ependymoma, 1 pleomorphic
xanthoastrocytoma and 1 ependymoma) and preoperative serum samples
were obtained from in-patients at the Department of Neurosurgery at
the First Affiliated Hospital of Guangxi Medical University between
March 2013 and March 2014. Their clinical data were retrospectively
reviewed. All patients underwent resection of the primary tumor and
did not receive radiotherapy or chemotherapy prior to surgery. The
mean patient age was 36.4 years (range, 3–65) and the gender
distribution was 21 males and 15 females. All tumor specimens were
classified according to the World Health Organization (WHO)
criteria (19), with 17 cases of WHO
grade I–II (low-grade) and 19 cases of WHO grade III–IV
(high-grade) identified. Serum samples from 107 healthy donors (54
male; 53 female) and 7 types of normal tissue samples (3 testis, 3
kidney, 3 thyroid, 3 appendix, 3 spleen, 3 tonsil and 3 normal
brain) were collected between May 2013 and March 2014 and were used
as controls.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from frozen tumor tissues
using the RNAsimple Total RNA kit (TianGen Biotech Co., Ltd.,
Beijing, China), according to the protocol of the manufacturer. The
concentration of isolated RNA was determined by spectrophotometer
(SmartSpec™ plus; Bio-Rad Laboratories, Inc., Hercules, CA, USA),
then 4 µg total RNA was reverse-transcribed into single-stranded
cDNA with PrimeScript II First Strand cDNA Synthesis kit (Takara
Biotechnology Co., Dalian, China). The integrity of obtained cDNA
was tested by amplification of p53 transcripts in a 30-cycle PCR
reaction as previously described (20). The OY-TES-1 gene was amplified using
established primers (21). The PCR
reactions were in a total volume of 25 µl containing 0.125 µl of
Takara Ex Taq HS (Takara Biotechnology Co.), 0.5 µl of 10 µm/l
forward primer, 0.5 µl of 10 µm/l reverse primer, 5 µl of 5X
buffer, 18.375 µl of RNase-free H2O and 0.5 µl of cDNA.
The cycling parameters were as follows: Initial denaturation at
94°C, 5 min; denaturation at 94°C, 1 min; annealing at 58°C, 1 min;
extension at 72°C, 2 min, for 35 cycles; and final extension at
72°C, 8 min. Tumor protein (p)53 served as an internal control for
normalization, as previously described (20). The quality of the RNAs and the PCR
product was examined by electrophoresis on 1% agarose gel and
observed under Gel Documentation and Analysis system (Uvipro 7600Z;
UVItec Ltd., Cambridge, UK).
Reverse transcription quantitative PCR
(RT-qPCR)
The presence of OY-TES-1 mRNA was quantitatively
detected using the iCycler iQ™ Multi-Color Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.) with the following primer
sequences: Sense, 5′-GCGACACCTCCCACAAGAC-3′ and antisense,
5′-GCCCACCGTACAAATCCAG-3′. The following 6-carboxyfluorescin
(FAM)-labeled TaqMan probe was also used: 5′-FAM
CAACCAGGTAGGGTCC TAMRA-3′. The amplified product consisted
of a 123 bp segment of the OY-TES-1 gene. The PCR reactions were in
a total volume of 25 µl containing 2.5 µl 10xbuffer, 5 µl 25 mM
MgCl2, 1 µl 2.5 mM dNTP, 0.75 µl 10 µM forward primer,
0.75 µl 10 µM reverse primer, 1 µl 10 µM probe primer, 12 µl
RNase-free H2O and 2 µl cDNA template. Thermocycling
conditions were as follows: 95°C for 2 min, followed by 40 cycles
of 95°C for 5 sec and 60°C for 20 sec. The target OY-TES-1 mRNA was
quantified by measuring the Cq value (22). The Cq value was defined as the
threshold cycle number at which the fluorescence generated by
cleavage of the probe passed above the baseline value. The value of
the target OY-TES-1 mRNA in each sample was normalized to
hypoxanthine phosphoribosyl transferase (HPRT) amplification
(23). All samples were run in
triplicate.
ELISA analysis
Serum antibody against OY-TES-1 was detected by
ELISA, as described previously (15).
The recombinant OY-TES-1 protein (15) and maltose binding protein (MBP; blank
control) (15) were diluted serially
from 1:100 to 1:3,200, coated onto 96-well plates and incubated at
4°C overnight. Subsequently, the plates were blocked with 5% nonfat
milk and incubated with serum (1:400; 100 µl/well) at 37°C for 1 h,
followed by incubation with horseradish peroxidase (HRP)-conjugated
sheep anti human IgG (cat. no. 109-035-003; dilution, 1:5,000;
Jackson ImmunoResearch, West Grove, PA, USA). Finally, the plates
were incubated with 3,3′,5,5′-tetramethylbenzidine at room
temperature for 20 min, and 2 mol/l sulfuric acid was added to
terminate the reaction. The absorbance was measured at a wavelength
of 450 nm using a microplate reader. The healthy donor serum
samples were used as negative controls. All the serum samples were
evaluated ≥2 times. A positive reaction was defined as an optical
density (OD) value that exceeded the mean OD of the healthy donor
sera by three standard deviations.
Immunohistochemistry (IHC)
IHC was performed using the tissue samples from
patients with glioma who were anti-OY-TES-1 antibody seropositive.
The testis and normal brain tissues were used as positive and
negative controls, respectively. The IHC procedure was performed
according to a previous protocol (15). In brief, deparaffinized tissue
sections underwent heat-based antigen retrieval in citrate buffer
(pH 6.0, 10 mM). Following the inactivation of endogenous
peroxidase, the tissue sections were incubated with an
anti-OY-TES-1 primary antibody (cat. no. ab64809; dilution,
1:1,000; Abcam, Cambridge, UK) or rabbit pre-immune serum (negative
control) (15) at 4°C overnight.
Subsequently, the tissue sections were washed and incubated with a
HRP-labeled goat anti-rabbit IgG (cat. no. D-3004; dilution, 1:500;
Shanghai Long Island Biotec, Shanghai, China) at room temperature
for 1 h, labeled with 3,3′-diaminobenzidine and counterstained with
hematoxylin. Then they were viewed under an optical microscopy
(Olympus BX53; Olympus Corporation, Tokyo, Japan).
Sequencing analysis
The open reading frame (ORF) of OY-TES-1 was
amplified from the cDNA of tumor tissues using PCR with specific
primers as follows: Sense, 5′-GCGGCGGATCTTCTCCGGCCATG-3′ and
antisense, 5′-ACGGGATCCTTATCAGTTGGGCTGGGGTGT-3′. A total of 35 PCR
amplification cycles were performed, each consisting of
denaturation at 98°C for 10 sec, followed by annealing at 63°C for
15 sec and extension at 72°C for 2 min. The final extension step
was performed at 72°C for 10 min. PCR products were purified and
ligated into pMD8-T vectors (Takara Biotechnology Co.), which were
transformed into DH5α competent cells (Beijing TransGen Biotech
Co., Ltd., Beijing, China) (24). The
transformed cells were smeared on LB-ampicillin agar plates
containing X-gal. White colonies were screened and then inoculated
into 5 ml bacterial culture medium overnight. Plasimid was
extracted by EZ Spine Column Plasimid Mini-Preps kit (Sangon
Biotechnology Co., Shanghai, China) and verified by PCR, as
previously described (21). Clones
with the correct insertion were identified via Sanger sequencing in
3730XL DNA Analyzer (Sino Genomax Co., Ltd., Beijing, China).
Statistical analysis
Results are presented as the mean ± (SD). All
statistical analyses were performed using SPSS version 15.0 (SPSS
Inc., Chicago, IL, USA). The association between gene expression
levels, the presence of antibodies in the sera and the
clinicopathological characteristics of patients with glioma was
evaluated using Fisher's exact test. The expression levels of
OY-TES-1, relative to HPRT, in glioma samples of different WHO
grades and normal tissues were compared using the Mann-Whitney U
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
OY-TES-1 mRNA expression is
upregulated in glioma
To examine the presence of OY-TES-1 mRNA in glioma,
conventional RT-PCR was initially performed to detect OY-TES-1
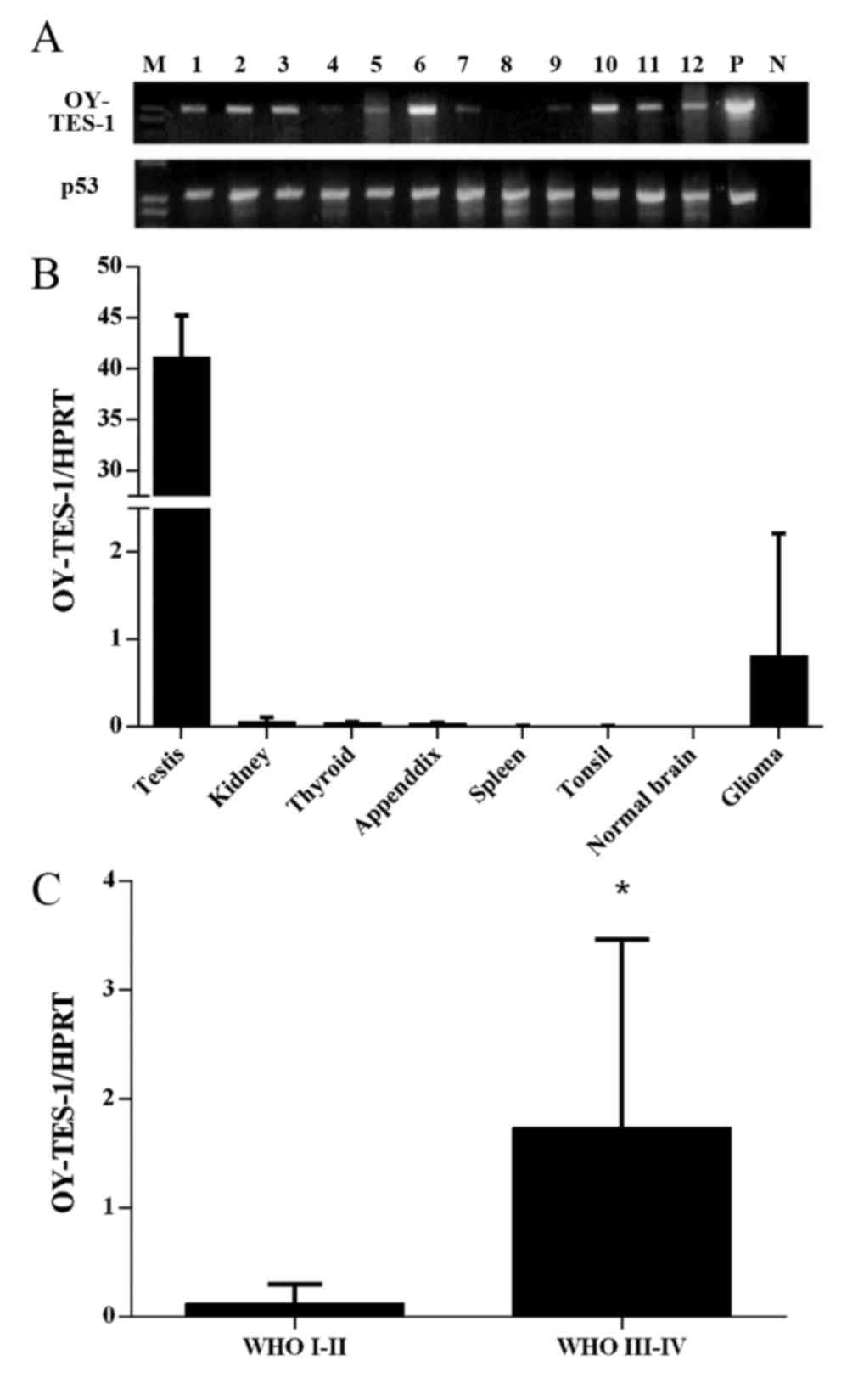
transcription. A total of 78% (28/36) of the glioma tissue samples
were OY-TES-1 mRNA positive (Fig.
1A), with 76% (13/17) of low-grade tumors and 79% (15/19) of
high-grade tumors positive for OY-TES-1 mRNA. Subsequently, the
association between OY-TES-1 mRNA expression and the
clinicopathological characteristic of patients with glioma,
including gender, age and WHO tumor grade, were examined. As
presented in Table I, no significant
association was observed between the expression of OY-TES-1 mRNA
and the clinicopathological parameters.
 | Table I.Association between OY-TES-1 mRNA
expression and serum anti-OY-TES-1 antibody and the
clinicopathological characteristics of patients with glioma. |
Table I.
Association between OY-TES-1 mRNA
expression and serum anti-OY-TES-1 antibody and the
clinicopathological characteristics of patients with glioma.
| Clinicopathological
characteristic | No. of mRNA positive
patients/total (%) | P-valuea | No. of serum
anti-OY-TES-1 antibody positive patients/total (%) | P-valuea |
|---|
| Gender |
|
|
|
|
| Male | 16/21 (76.2) | 1.00 | 3/21 (14.3) | 1.00 |
|
Female | 12/15 (80.0) |
| 2/15 (13.3) |
|
| Age, years |
|
|
|
|
|
<30 | 9/13 (69.2) | 0.42 | 3/13 (23.1) | 0.34 |
|
≥30 | 19/23 (82.6) |
| 2/23 (8.67) |
|
| WHO tumor
grade |
|
|
|
|
|
I–II | 13/17 (76.5) | 1.00 | 3/17 (17.6) | 0.65 |
|
III–IV | 15/19 (78.9) |
| 2/19 (10.5) |
|
As a high frequency of OY-TES-1 mRNA expression was
present in the glioma tumor samples, the expression pattern of
OY-TES-1 mRNA was further examined. RT-qPCR analysis demonstrated
that the OY-TES-1 mRNA expression levels in glioma tissues were
markedly elevated compared with normal tissues (with the exception
of testis tissue); but significantly higher than normal brain
tissues (P=0.0015; Fig. 1B). The
RT-qPCR results also revealed that high-grade tumors expressed
significantly higher levels of OY-TES-1 mRNA compared with
low-grade tumors (P=0.0002; Fig.
1C).
Anti-OY-TES-1 antibody is present in
the patient serum samples
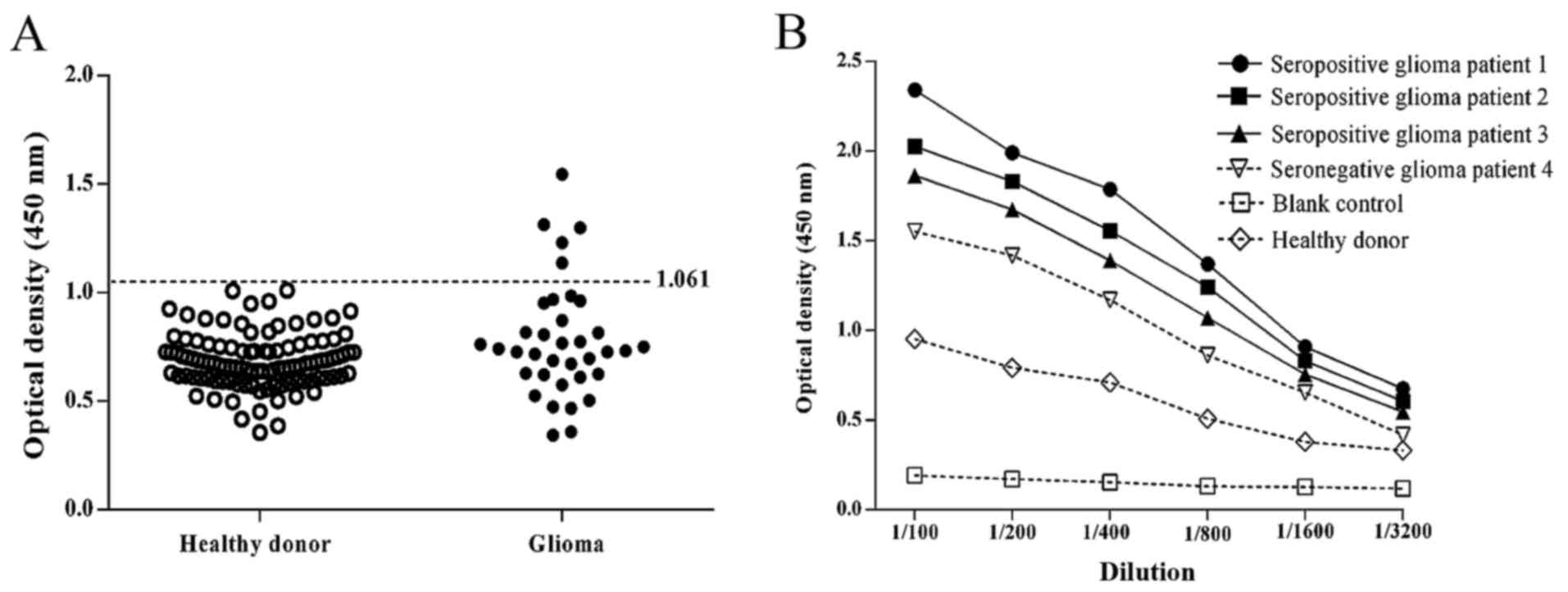
The expression of serum antibodies directed against
OY-TES-1 was analyzed in 36 patients with glioma and 107 healthy
donors by ELISA analysis. The OY-TES-1 antibody was detected in the
serum of 5/36 (14%) patients with glioma, whereas the sera of all
healthy donors were negative for the anti-OY-TES-1 antibody
(Fig. 2A). Titration curves were
produced for anti-OY-TES-1 antibody-positive and -negative sera
from representative patients with glioma, in addition to a healthy
donor control, using the recombinant OY-TES-1 protein (Fig. 2B). The possibility of an association
between the presence of antiOY-TES-1 antibodies in the sera and the
clinicopathological characteristics of patients with glioma was
evaluated, but no statistically significant association was
identified (Table I).
OY-TES-1 protein is detectable in
glioma tissues from anti-OY-TES-1 antibody seropositive
patients
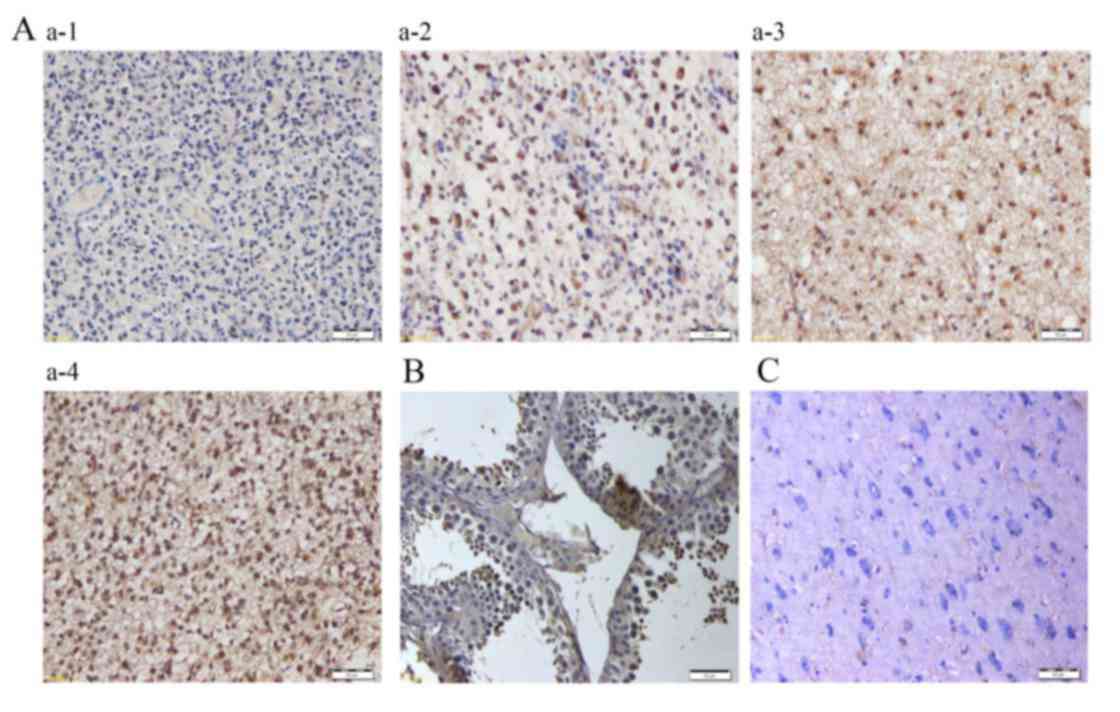
As the glioma tissues from the 5 anti-OY-TES-1
antibody seropositive patients were all positive for the presence
of OY-TES-1 mRNA, the expression of the OY-TES-1 protein in glioma
tissues was examined (Fig. 3). IHC
detected the OY-TES-1 protein in all the glioma tissue.
Furthermore, it was observed that OY-TES-1 protein was primarily
localized in the cytoplasm of the tumor cells, with occasional
positive staining in the nuclei. In certain cases, heterogeneity of
OY-TES-1 protein expression was observed in tumor tissues.
No aberrance is observed in the ORF of
OY-TES-1 in glioma tissues from anti-OY-TES-1 antibody seropositive
patients
To determine whether the humoral immune response
against OY-TES-1 in the patients with glioma was due to an
aberrance in this gene, the full-length ORF of the OY-TES-1 gene
was amplified from the tumor tissues of anti-OY-TES-1 antibody
seropositive patients and sequenced. No aberrant changes, including
mutations, deletions or insertions, were detected in the ORF of the
OY-TES-1 gene (data not shown).
Discussion
As a member of the CT antigen family, OY-TES-1 is
listed in the database of the Ludwig Institute for Cancer Research
(Brussels, Belgium) (25), in which
it is also named as CT23. OY-TES-1 is frequently expressed at the
mRNA level in various types of cancer (13,15,18).
OY-TES-1 protein is expressed in ~60% of ovarian (15) and ~43% of colorectal (18) tumors. To the best of our knowledge,
OY-TES-1 expression patterns at the mRNA and protein level, and its
immunogenicity in brain tumors, including glioma, have yet to be
elucidated.
The present study demonstrated that 78% of the
glioma tissue samples expressed OY-TES-1 mRNA, which was detected
via conventional RT-PCR. The levels of OY-TES-1 expression were
high compared with those observed in previous studies of OY-TES-1
mRNA expression using the same primers in other types of cancer,
including bladder (11/39, 28%), breast (2/5, 40%), colon (2/13,
15%), liver (2/5, 40%), ovarian (23/100, 23%) and colorectal
(44/60, 73%) cancer (13,15,18). Due
to the high proportion of glioma tissues in the present study that
expressed OY-TES-1, the quantity of OY-TES-1 mRNA was investigated
using RT-qPCR. The results revealed that OY-TES-1 mRNA expression
was elevated in glioma, compared with a panel of normal tissues
(with the exception of the testis) but significantly higher than
normal brain tissues (P=0.0015). The data suggest that OY-TES-1 is
a novel target for the treatment of glioma, from an
immunotherapeutic standpoint.
The present study investigated the association
between OY-TES-1 mRNA expression and the clinicopathological
characteristics of patients with glioma. The data suggested that
there is no significant association between the presence of
OY-TES-1 mRNA and clinicopathological characteristics in glioma;
however, the level of OY-TES-1 mRNA was significantly higher in
high-grade compared with low-grade glioma samples. It has been
established that a higher grade of glioma is correlated with
greater malignancy and a poorer prognosis (26). Therefore, the expression of OY-TES-1
mRNA may be used as a novel prognostic marker for glioma. Follow-up
studies are required to further investigate the association between
the quantity of OY-TES-1 mRNA and patient outcome.
Although the brain is located in an
immune-privileged anatomical site, a humoral immune response to
several tumor antigens has been detected in patients with glioma
(27–29), suggesting that the brain is not
completely immunoprivileged. The presence of antibodies against a
number of tumor antigens has been examined in association with the
survival of patients with glioma (30). The current study examined OY-TES-1
seroreactivity in patients with glioma, in addition to healthy
individuals. The results demonstrated that 5/36 (14%) of patients
with glioma had the anti-OY-TES-1 antibody present in their serum,
whereas this antibody was not expressed in any of the serum samples
from healthy donors 0/107 (0%). The anti-OY-TES-1 antibody has
previously been observed in patients with other types of cancer,
with a frequency of 4.8–40% (13,15). A
previous study detected the presence of the anti-OY-TES-1 antibody
in 9.6% of patients with colorectal cancer (15), a result concordant with data from the
current study in patients with glioma. However, no significant
association was identified between the presence of the
anti-OY-TES-1 antibody in the serum and the clinicopathological
characteristics of patients with glioma in the present study.
The molecular mechanisms underlying the immune
response against OY-TES-1 in patients with glioma requires further
investigation, as our serum immunoreactive result demonstrated that
OY-TES-1 may possess immunogenic potential in patients with glioma.
The present study utilized IHC to examine glioma tissues from
anti-OY-TES-1 antibody seropositive patients. The results revealed
that the OY-TES-1 protein was detectable in all the glioma tissue
samples, and was primarily localized to the cytoplasm of tumor
cells. As OY-TES-1 is not localized to the cell surface, it may be
hypothesized that a mutation in the gene encoding OY-TES-1 may
trigger a humoral immune response against OY-TES-1 in patients with
brain tumors, in a similar manner to the mutated version of the p53
tumor suppressor gene detected in colon cancer and glioma, which
raises the levels of the corresponding antibodies in patient's sera
(31–32). However, the sequence analysis
performed in the present study revealed no significant variation in
the ORF of the OY-TES-1 gene, providing no support for this
hypothesis.
Therefore, enhanced levels of OY-TES-1 expression
may occur as a result of the immune response to cancer, as in the
case of human epidermal growth receptor 2, an oncogene that is
amplified in certain types of cancer (33). A previous study suggested that large
quantities of immunocompetent cells, including B lymphocytes, are
able to invade the tissue of malignant gliomas with a large
necrotic area (34), increasing the
possibility of an interaction between immunocompetent cells and
amplified gene products, which are able to function as antigens. An
alternative hypothesis is that the humoral response against
OY-TES-1 may be induced by the products of post-translational
modifications of the OY-TES-1 protein. Previous studies have
reported that sumoylated or hyperphosphorylated proteins may serve
as autoimmunogenic targets, including sumoylated heat shock protein
90 (35) and hyperphosphorylated
paratarg-7 (36). Thus, the presence
of a humoral response against OY-TES-1 in patients with glioma may
be a predictor of the cellular immune response, as is the case with
NY-ESO-1, a CT antigen defined using serological analysis of
recombinant expression libraries, in esophageal cancer (37).
In conclusion, the results of the present study
indicated that OY-TES-1 mRNA is expressed in a high proportion of
glioma tissues and possesses inherent immunogenicity. Therefore, it
may present a novel target for specific immunotherapy in the
treatment of brain tumors, particularly glioma. At present,
determination of the prognosis using follow-up data and the
cell-mediated immune response to OY-TES-1 is under investigation,
which may aid understanding of the role of OY-TES-1 in
tumorigenesis.
Acknowledgements
The present was supported by the National Natural
Science Foundation of China (grant nos. 81360371 and 81360374),
Guangxi Nature Science Foundation (grant nos. 0728148, 0832144 and
2011GXNSFA 018275) and the Guangxi Educational Bureau (grant no.
BGXZ2007010). The authors would like to thank Ms. Fang Chen for her
technical assistance.
References
|
1
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol.
16:(Suppl 4). iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naydenov E, Tzekov C, Minkin K, Nachev S,
Romansky K and Bussarsky V: Long-term survival with primary
glioblastoma multiforme: A clinical study in bulgarian patients.
Case Rep Oncol. 4:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oike T, Suzuki Y, Sugawara K, Shirai K,
Noda SE, Tamaki T, Nagaishi M, Yokoo H, Nakazato Y and Nakano T:
Radiotherapy plus concomitant adjuvant temozolomide for
glioblastoma: Japanese mono-institutional results. PLoS One.
8:789432013. View Article : Google Scholar
|
|
4
|
Ehtesham M, Black KL and Yu JS: Recent
progress in immunotherapy for malignant glioma: Treatment
strategies and results from clinical trials. Cancer control.
11:192–207. 2004.PubMed/NCBI
|
|
5
|
Zendman AJ, Ruiter DJ and Van Muijen GN:
Cancer/testis-associated genes: Identification, expression profile,
and putative function. J Cell Physiol. 194:272–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
7
|
Kalejs M and Erenpreisa J: Cancer/testis
antigens and gametogenesis: A review and ‘brain-storming’ session.
Cancer Cell Int. 5:42005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: Potential targets for immunotherapy. Cancer sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fratta E, Coral S, Covre A, Parisi G,
Colizzi F, Danielli R, Nicolay HJ, Sigalotti L and Maio M: The
biology of cancer testis antigens: Putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okumura H, Noguchi Y, Uenaka A, Aji T, Ono
T, Nakagawa K, Aoe M, Shimizu N and Nakayama E: Identification of
an HLA-A24-restricted OY-TES-1 epitope recognized by cytotoxic
T-cells. Microbiol Immunol. 49:1009–1016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao J, Caballero OL, Yung WK, Weinstein
JN, Riggins GJ, Strausberg RL and Zhao Q: Tumor subtype-specific
cancer-testis antigens as potential biomarkers and
immunotherapeutic targets for cancers. Cancer Immunol Res.
2:371–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freitas M, Malheiros S, Stávale JN, Biassi
TP, Zamunér FT, de Souza Begnami M, Soares FA and Vettore AL:
Expression of cancer/testis antigens is correlated with improved
survival in glioblastoma. Oncotarget. 4:636–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono T, Kurashige T, Harada N, Noguchi Y,
Saika T, Niikawa N, Aoe M, Nakamura S, Higashi T, Hiraki A, et al:
Identification of proacrosin binding protein sp32 precursor as a
human cancer/testis antigen. Proc Natl Acad Sci USA. 98:3282–3287.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baba T, Niida Y, Michikawa Y, Kashiwabara
S, Kodaira K, Takenaka M, Kohno N, Gerton GL and Arai Y: An
acrosomal protein, sp32, in mammalian sperm is a binding protein
specific for two proacrosins and an acrosin intermediate. J Biol
Chem. 269:10133–10140. 1994.PubMed/NCBI
|
|
15
|
Luo B, Yun X, Fan R, Lin YD, He SJ, Zhang
QM, Mo FR, Chen F, Xiao SW and Xie XX: Cancer testis antigen
OY-TES-1 expression and serum immunogenicity in colorectal cancer:
Its relationship to clinicopathological parameters. Int J Clin Exp
Pathol. 6:2835–2845. 2013.PubMed/NCBI
|
|
16
|
Fu J, Luo B, Guo WW, Zhang QM, Shi L, Hu
QP, Chen F, Xiao SW and Xie XX: Down-regulation of cancer/testis
antigen OY-TES-1 attenuates malignant behaviors of hepatocellular
carcinoma cells in vitro. Int J Clin Exp Pathol. 8:7786–7797.
2015.PubMed/NCBI
|
|
17
|
Fan R, Huang W, Luo B, Zhang QM, Xiao SW
and Xie XX: Cancer testis antigen OY-TES-1: Analysis of protein
expression in ovarian cancer with tissue microarrays. Eur J
Gynaecol Oncol. 36:298–303. 2015.PubMed/NCBI
|
|
18
|
Tammela J, Uenaka A, Ono T, Noguchi Y,
Jungbluth AA, Mhawech-Fauceglia P, Qian F, Schneider S, Sharma S,
Driscoll D, et al: OY-TES-1 expression and serum immunoreactivity
in epithelial ovarian cancer. Int J Oncol. 29:903–910.
2006.PubMed/NCBI
|
|
19
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gure AO, Chua R, Williamson B, Gonen M,
Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ and
Altorki NK: Cancer-testis genes are coordinately expressed and are
markers of poor outcome in non-small cell lung cancer. Clin Cancer
Res. 11:8055–8062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cen YH, Guo WW, Luo B, Lin YD, Zhang QM,
Zhou SF, Luo GR, Xiao SW and Xie XX: Knockdown of OY-TES-1 by RNAi
causes cell cycle arrest and migration decrease in bone
marrow-derived mesenchymal stem cells. Cell Biol Int. 36:917–922.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He SJ, Gu YY, Yu L, Luo B, Fan R, Lin WZ,
Lan XW, Lin YD, Zhang QM, Xiao SW and Xie XX: High expression and
frequently humoral immune response of melanoma-associated antigen
D4 in glioma. Int J Clin Exp Pathol. 7:2350–2360. 2014.PubMed/NCBI
|
|
24
|
Froger A and Hall JE: Transformation of
plasmid DNA into E. coli using the heat shock method. J Vis
Exp. 253:2007.
|
|
25
|
Almeida LG, Sakabe NJ, deOliveira AR,
Silva MC, Mundstein AS, Cohen T, Chen YT, Chua R, Gurung S, Gnjatic
S, et al: CTdatabase: A knowledge-base of high-throughput and
curated data on cancer-testis antigens. Nucleic Acids Res.
37:D816–D819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang P, Wang Y, Peng X, You G, Zhang W,
Yan W, Bao Z, Wang Y, Qiu X and Jiang T: Management and survival
rates in patients with glioma in China (2004–2010): A retrospective
study from a single-institution. J Neurooncol. 113:259–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sahin U, Türeci O, Schmitt H, Cochlovius
B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I and
Pfreundschuh M: Human neoplasms elicit multiple specific immune
responses in the autologous host. Proc Natl Acad Sci USA.
92:11810–11813. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischer U, Struss AK, Hemmer D, Pallasch
CP, Steudel WI and Meese E: Glioma-expressed antigen 2 (GLEA2): A
novel protein that can elicit immune responses in glioblastoma
patients and some controls. Clin Exp Immunol. 126:206–213. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Struss AK, Romeike BF, Munnia A,
Nastainczyk W, Steudel WI, König J, Ohgaki H, Feiden W, Fischer U
and Meese E: PHF3-specific antibody responses in over 60% of
patients with glioblastoma multiforme. Oncogene. 20:4107–4114.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pallasch CP, Struss AK, Munnia A, König J,
Steudel WI, Fischer U and Meese E: Autoantibodies against
GLEA2 and PHF3 in glioblastoma:
Tumor-associated autoantibodies correlated with prolonged survival.
Int J Cancer. 117:456–459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scanlan MJ, Chen YT, Williamson B, Gure
AO, Stockert E, Gordan JD, Türeci O, Sahin U, Pfreundschuh M and
Old LJ: Characterization of human colon cancer antigens recognized
by autologous antibodies. Int J Cancer. 76:652–658. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weller M, Bornemann A, Ständer M, Schabet
M, Dichgans J and Meyermann R: Humoral immune response to p53 in
malignant glioma. J Neurol. 245:169–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheever MA, Disis ML, Bernhard H, Gralow
JR, Hand SL, Huseby ES, Qin HL, Takahashi M and Chen W: Immunity to
oncogenic proteins. Immunol Rev. 145:33–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamanaka R, Tanaka R and Saito T:
Immunohistochemical analysis of tumor-infiltrating lymphocytes and
adhesion molecules (ICAM-1, NCAM) in human gliomas. Neurol Med Chir
(Tokyo). 34:583–587. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Preuss KD, Pfreundschuh M, Weigert M,
Fadle N, Regitz E and Kubuschok B: Sumoylated HSP90 is a dominantly
inherited plasma cell dyscrasias risk factor. J Clin Invest.
125:21792015. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grass S, Preuss KD, Wikowicz A, Terpos E,
Ziepert M, Nikolaus D, Yang Y, Fadle N, Regitz E, Dimopoulos MA, et
al: Hyperphosphorylated paratarg-7: A new molecularly defined risk
factor for monoclonal gammopathy of undetermined significance of
the IgM type and Waldenstrom macroglobulinemia. Blood.
117:2918–2923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen YT, Scanlan MJ, Sahin U, Türeci O,
Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M and Old
LJ: A testicular antigen aberrantly expressed in human cancers
detected by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|