Introduction
Cervical cancer is the most common female genital
tract tumor in developing countries, with patients eventually
succumbing to tumor invasion and metastasis. Lymph node (LN)
metastasis is the most frequent route of metastasis in cervical
cancer, and this is also a vital indicator in predicting the
prognosis of patients and identifying patients requiring
postoperative adjuvant therapy with radiation therapy (RT) and/or
chemotherapy. Studies have revealed that the pelvic LN metastasis
rate of early stage cervical cancer [International Federation of
Gynecology and Obstetrics (FIGO) stage IA1-IB1] is 0%-29.3%,
whereas the rate of locally advanced patients (FIGO stage IB2-IIB)
is as high as 12%-61.8% (1,2). The 5-year survival rate of patients with
LN involvement decreases from 85% to 50% (3). The number of metastatic pelvic and
abdominal LNs is associated with patients' long-term survival
(4). Radical hysterectomy and LN
dissection is the main treatment of early cervical cancer. However,
LNs dissected in this process are mostly non-metastatic, and thus
may result in unnecessary intraoperative and postoperative
complications. Conversely, certain micro-metastatic LNs are not
correctly diagnosed or do not receive proper postoperative adjuvant
treatment, and in consequence may be the main cause of rapid
relapse. Even when LNs are negative, the recurrence rate of
surgically treated patients reaches 10–15%, among which the
majority suffer from pelvic relapse (1,5). The
malignant transformation and lymphatic drainage of human
papillomavirus (HPV)-infected atypical cells most probably account
for the relapse of patients with negative LNs (2,6,7).
HPV infection is the most commonly observed sexually
transmitted disease (8,9), and is responsible for 99% cases of
cervical cancer. More than 200 types of HPV have been identified
currently, of which over 40 types have been reported to be
associated with reproductive tract infection, including 18 types
which are directly connected with the occurrence of cervical
cancer, 15 types that have been confirmed to be high-risk types
that cause the disease, another 3 clinically suspected high-risk
types and 13 identified low-risk types (10–12). Other
than cervical cancer, HPV also leads to cancer of the anus, oral
cavity and esophagus. Although the mechanism by which HPV causes
cancer is not fully understand, evidence has indicated that
heparanase (HPA) is a significant molecule in this mechanism, and
that the high-risk HPV E6 oncogene is capable of inducing
overexpression of HPA through a p53-dependent mechanism (13,14).
HPA is a single mammalian endoglycosidase, whose
activity is observed in white blood cells, mast cells, placenta
tissues, neutrophilic granulocytes and macrophagocytes. It is
associated with cancer progression and aggressive behavior
(15–17). A previous study demonstrated that the
expression of HPA is associated with cancer formation and
progression in acute leukemia, bladder cancer, brain tumor, breast
cancer, colon cancer, gastric cancer, esophagus cancer among
others. For this reason, HPA has become a promising molecule of
tumor-targeted therapy, and a variety of anti-heparanase inhibitors
have been developed for clinical trials, among which P188 is in
phase III clinical trials (18).
Research has identified that high expression of HPA is involved in
lymphatic transfer, distant metastasis and poor clinical prognosis
of diverse malignant carcinomas including cervical cancer. In 2003,
Shinyo et al (19) verified
for the first time that the expression of HPA mRNA was promoted in
advanced cervical cancer, and patients with vascular and LN
involvement demonstrated an extremely high level of HPA, which was
due to the close correlation between HPA expression and tumor
microvascular density. These authors also confirmed that
disease-free survival (DFS) and overall survival in HPA-positive
patients was notably lower than in HPA-negative patients, and
multiple analysis indicated that HPA expression was an independent
prognostic factor. It was affirmed though immunohistochemistry that
the rate of positive HPA protein expression in cervical cancer
patients was 63.3%, and that the expression level is correlated
with tumor size and clinical stage. Overexpression of HPA inhibited
the apoptosis of cervical cancer cell lines in vitro and
promoted their proliferation and growth (20).
On the basis of the above findings, we may conclude
that HPA has a close connection with the occurrence, progression
and LN metastasis of cervical cancer. However, to date, no research
on HPA expression in cervical cancer metastatic LNs has been
identified, and the effect of LN metastasis of cervical cancer
patients caused by abnormal HPA expression still lacks evidentiary
support. To explore the role of HPA in lymphatic metastasis and
patients' clinical prognosis, we study the expression of HPA in
sentinel LNs of cervical cancer and investigate clinicopathological
features of the tumor and patient prognosis. We reveal that the
rate of HPA-positive expression in pathologically confirmed
metastatic LNs is equivalent to that in the primary lesion, and a
significant reduction in the recurrence rate and long-term survival
rate is identified in patients with positive HPA expression in LNs.
Our study proposes HPA as a significant marker for the diagnosis of
micro-metastasis of LN in cervical cancer and a theoretical basis
for HPA-targeted therapy of cervical cancer and metastatic LNs
simultaneously.
Materials and methods
Patients
We retrospectively reviewed 102 consecutive patients
with histologically confirmed cervical squamous cell cancer and
well-documented clinical reports, who received standard surgery in
the Second Affiliated Hospital of Zhengzhou University, China,
between January 2007 and December 2012. Among the patients, there
were 53 cases with positive LNs (group A) and 49 negative cases
(group B). In group A, the primary lesion and positive LNs were
selected, while the primary and all LNs were selected in group B.
Slices were secondly confirmed by experienced pathologists through
routine pathological methods and no patients had undergone
RT/chemotherapy or immunotherapy. The tumor stage was determined
according to the 2011 FIGO clinical classification system for
cervical cancer (21). Tumor
differentiation was graded according to the World Health
Organization (WHO) classification (22).
Of all cases, 29 were stage IA-IB and 73 were stage
IIA, while 38 were well-differentiated and 64 were moderately to
poorly differentiated. The complete follow-up data were obtained
and the longest maturity was 60 months. Of all cases, 19 suffered a
relapse, 12 succumbed to the disease, and the shortest survival
period was 7 months. The survival period was calculated from the
date of surgery, and the date of mortality or the last follow-up
was recorded as the follow-up termination date. The follow-up
deadline was December 30, 2012 and the median follow-up time was
56.5 months.
The study was conducted in accordance with the
declaration of Helsinki, and with approval from the Ethics
Committee of Zhengzhou University. Written informed consent was
obtained from all participants.
Reagents and sample processing
Consecutive 4-µm-thick tissue sections were cut from
formalin-fixed and paraffin-embedded tissue samples for routine
hematoxylin and eosin (HE) staining and HPA and cytokeratin (CK)19
immunohistochemical staining, respectively. CK19 is a squamous
epithelial marker. Concentrated rabbit anti-human HPA1 antibody
(1:150, sc-25825) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). A ready-to-use mouse anti-human CK19 monoclonal
antibody (MAB-0056), DAB kit, UltraSensitive™ SP IHC kit, citrate
antigen repair fluid, and hematoxylin and eosin were purchased from
Maixin.bio (Fuzhou, China).
Immunohistochemistry
Slices were cleaned, dried and wiped with
Poly-L-Lysine solution, then set aside after heating at 55–60°C for
2 h in the oven. Sample sections measuring 4 µm were boiled in
citrate buffer liquid (pH 6.0) for antigen repair. The endogenous
peroxidases were blocked by rinsing the slides in 10 vol hydrogen
peroxide (3%). Slices were incubated with rabbit anti-human HPA
monoclonal antibody (1:150, sc-25825) or mouse anti-human CK19
monoclonal antibody (1:150, MAB-0056) overnight at 4°C followed by
a streptavidin-biotin-peroxidase complex compound at room
temperature for 10 min and finally washed three times with
phosphate-buffered saline (PBS) for 3 min each time. The peroxidase
reaction was visualized with a diaminobenzidine (DAB) buffer. The
slides were rinsed clean under running water and then
counterstained in hematoxylin, dehydrated in 70, 95 and 100%
ethanol and xylene, and then mounted with a coverslip by neutral
balsam. A negative control sample was obtained by replacing the
primary antibody with PBS, while the positive control sample
contained confirmed positive specimens.
Determination of positive
immunohistochemical results
Subsequently, the stained and coded sections were
assessed by two pathologists blinded to the groups according to the
combination of staining density and percentage of positive cells.
Staining results were distributed through a 0 to 3 intensity
scoring scale: 3, brown/yellow granules in the cytoplasm; 2, yellow
granules; 1 or 0, faint yellow granules or no granules,
respectively. The positive ratio of cells (number of positive cells
/ number of total cells × 100%) was calculated at high
magnification: 6–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points;
≥75%, 4 points. The final score was the product of the staining
density score and the positive cell ratio score, and ≥3 points was
considered positive.
Statistical analysis
The results of immunohistochemical staining were
expressed as the means ± standard deviation. The expression of HPA
was examined by the χ2 test. Survival rates were
calculated using the Kaplan-Meier method and differences were
examined using the log-rank test. Furthermore, the multivariate
analysis was determined by the Cox proportional hazards model.
These analyses were performed using SPSS version 11.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HPA in primary lesions
and LNs of cervical squamous cancer
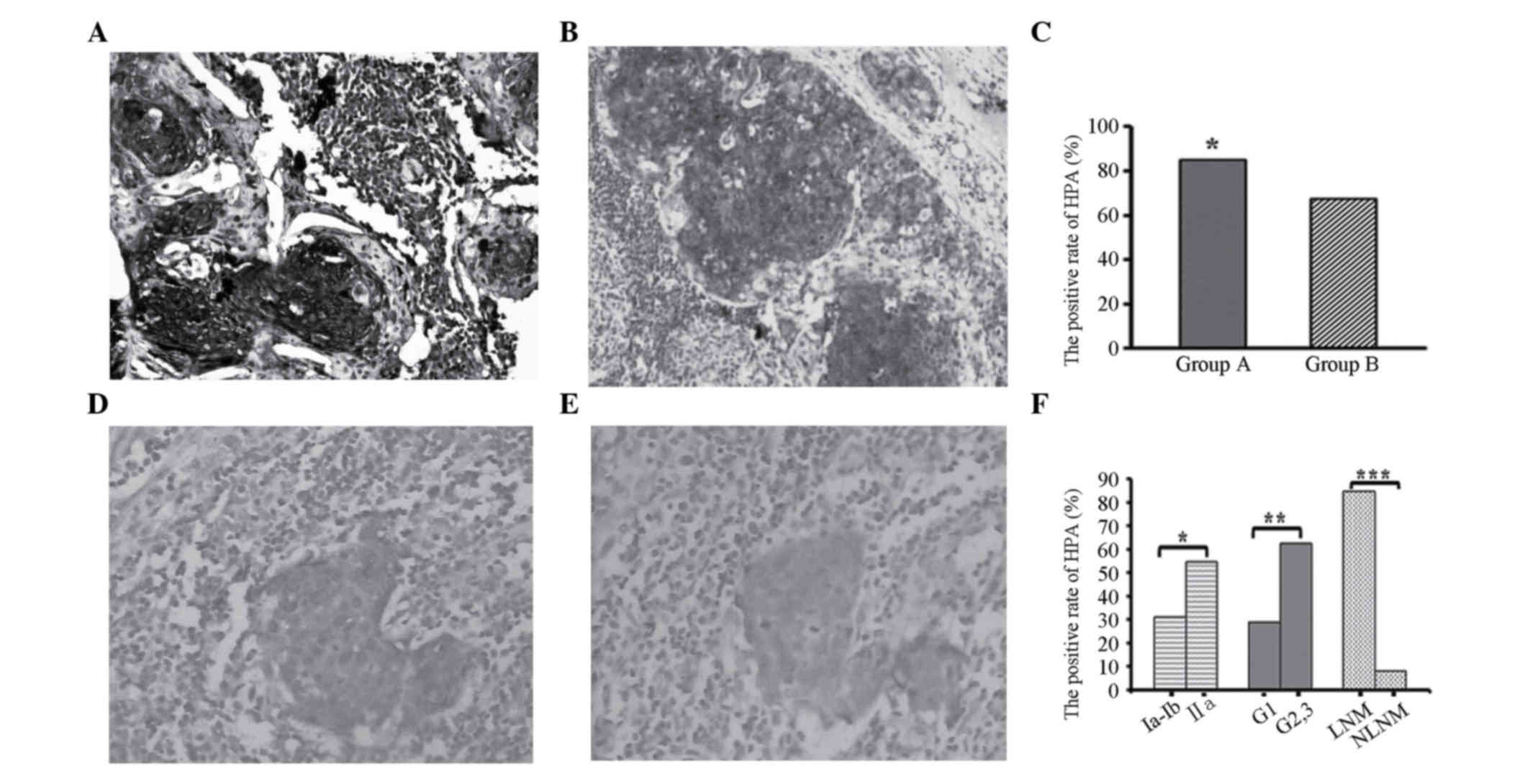
Positive HPA expression was detected in primary
lesions and metastatic LNs as browny yellow or brown particles,
which were located in the cytoplasm and membrane of cancer cells.
The HPA level was significantly increased in primary lesions and
metastatic LNs of cervical cancer (Fig.
1A and B). Pathologically diagnosed non-metastatic LNs also
demonstrated positive staining in single cell or focal spots. For
these samples, consecutive section CK19 immunohistochemical
staining was conducted. Then pathologists determined whether there
was LN involvement or not according to the HE and CK19 staining
status. The location of positive CK19 staining was in accordance
with HPA expression sites in HPA-positive LNs (Fig. 1D and E).
The expression rate of HPA in primary lesions of
cervical cancer was 76.5% (78/102). Forty-five cases (84.9%, 45/53)
in group A demonstrated positive HPA expression in primary lesions
and metastatic LNs, while in group B 33 cases (67.3%, 33/49) were
positive among the primary lesions and 4 cases (8.2%, 4/49) among
LNs. The positive expression of group A notably exceeded that in
group B (P<0.05, Fig. 1C).
Following immunohistochemical staining of HPA and CK19, the number
of LN metastasis cases rose to 57.
Correlation between HPA expression and
clinicopathological features of cervical squamous cancer
In terms of clinicopathological features, the
expression rate of HPA in the LNs of stage IIA patients was
distinctly higher that in stage IA-IB patients. In addition, the
expression rate of LNs was higher in the moderate and
low-differentiated tumors compared with that in well-differentiated
tumors. Finally, patients with positive LN metastasis expressed
higher levels of HPA than non-metastatic cases. All of these
differences were statistically significant (P<0.05, Fig. 1F).
Correlation between expression of HPA
in LNs and survival of cervical squamous cancer patients
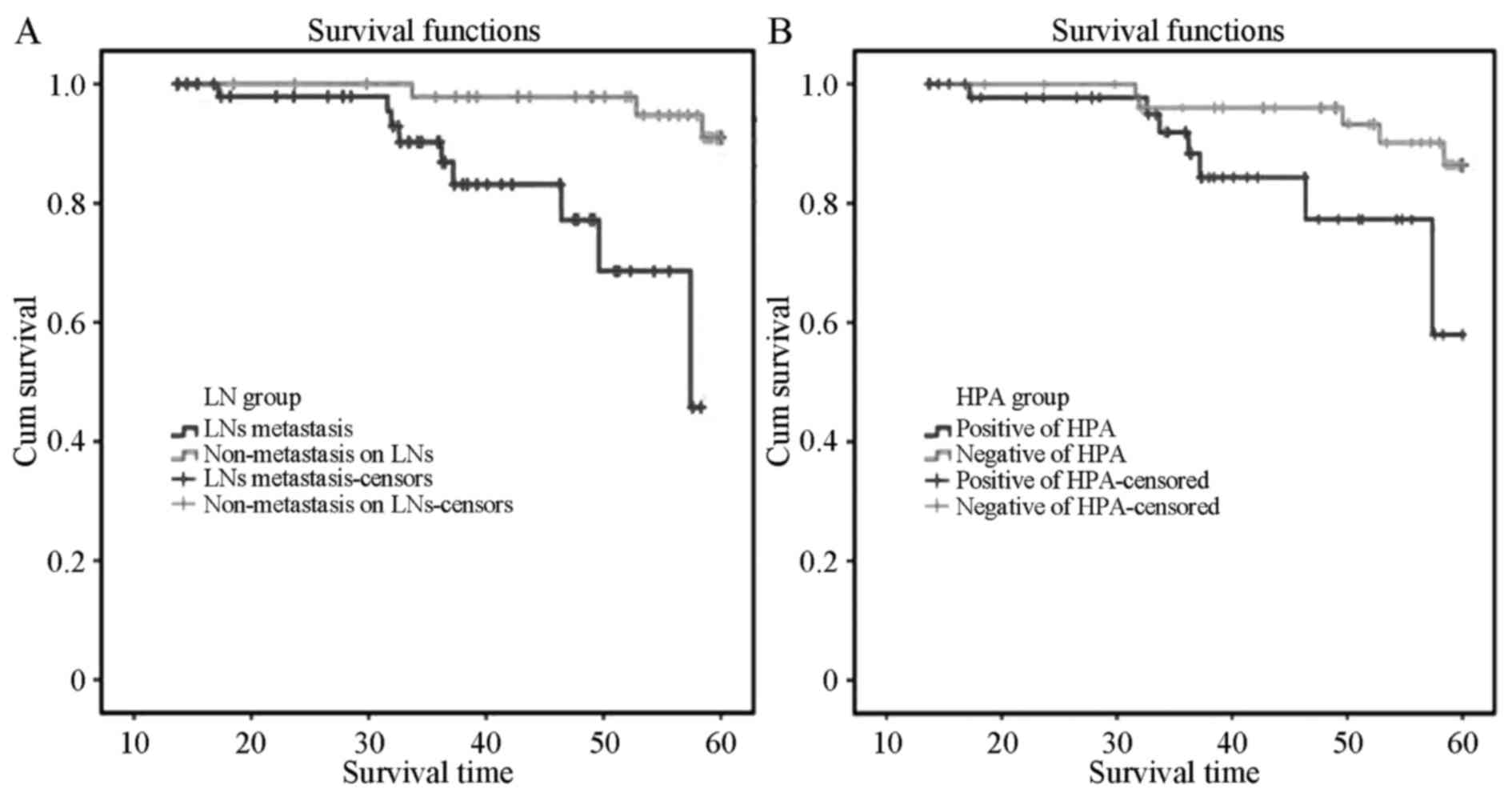
The 5-year overall survival rate was 73.3% and the
median overall survival (MOS) was 49.0 months. The MOS of the 53
patients with positive LN metastasis in group A was 36.0 months,
while in group B, the MOS was 58.5 months. Kaplan-Meier survival
analysis indicated that the MOS of the positive metastasis group
was distinctly lower than that of the non-metastatic patients, and
the difference was statistically significant (P=0.023) by log-rank
test (Fig. 2A). The MOS of the 49
patients with positive HPA expression was 38.5 months, while the
MOS of the 53 negative patients was 57.0 months, indicating that
the MOS of cervical squamous cancer patients with positive HPA
expression was distinctly lower than that of negative patients
(P=0.04, Fig. 2B). Single-factor Cox
regression analysis suggested that clinical staging,
differentiation degree, LN metastasis and expression of HPA notably
affected patient prognosis (P<0.05); moreover, multiple-factor
Cox model analysis indicated that LN metastasis and the expression
of HPA were independent risk factors affecting the prognosis of
cervical cancer patients (P<0.05, Table I).
 | Table I.Cox regression analysis of prognostic
factors in patients with cervical squamous carcinoma. |
Table I.
Cox regression analysis of prognostic
factors in patients with cervical squamous carcinoma.
| Features | B | SE | RR | P-value | 95% CI |
|---|
| Clinical stage
(IA-IB, IIA) | 1.132 | 0.378 | 1.323 | 0.067 | 0860-2.676 |
| Degree of
differentiation | 1.027 | 0.339 | 1.218 | 0.073 | 0.854–2.854 |
| Lymph node
involvement (negative, positive) | 1.942 | 0.451 | 1.636 | 0.039 | 1.203–3.203 |
| Expression of HPA
(negative, positive) | 1.561 | 0.362 | 1.473 | 0.047 | 1.188–2.188 |
Discussion
Our study demonstrated that the level of HPA
expression in pathologically diagnosed metastatic retroperitoneal
LNs was as high as that in primary lesions. The expression rate of
HPA in LNs of cervical squamous cancer patients was much higher in
patients of stage IIA than in those of stage IA-IB. In addition,
moderate or poorly differentiated cases expressed more HPA than
well-differentiated cases. The long-term survival rate of patients
with positive HPA expression was notably lower, which indicated
that the expression of HPA was an independent risk factor affecting
the prognosis of cervical cancer patients. Hence, our results
further confirmed that HPA was abnormally highly expressed in
cervical cancer patients and that metastatic retroperitoneal LNs
were homologous with primary lesions of cervical squamous cancer,
indicating that the abnormal expression of HPA played a significant
role in LN metastasis of cervical cancer and was involved in the
course of retroperitoneal LN metastasis, thereby facilitating
distant metastasis in cervical cancer patients and affecting their
long-term survival.
HPA plays its biological role via its glycosidase
activity, which is involved in degrading heparan sulphate (HS) in
the extracellular matrix (ECM), and in an enzymatic
activity-independent manner (23). HS
is a primary component at the interface between virtually every
eukaryotic cell and its ECM, and is vital in maintaining biological
processes in sick and healthy individuals. HS combines with
proteoglycan to form heparan sulfate proteoglycans as
three-dimensional structures of the matrix to maintain the
connection of normal cells. In certain cases, they not only provide
a storage depot for heparin-binding molecules in the cell
microenvironment, but also decisively regulate their accessibility,
function and mode of action by connecting with receptors as signal
molecules (24,25). Overexpression of HPA in cervical
cancer tissues degrades the side chain of heparan sulfate
glycosaminoglycan (HS-GAG) connected on perlecan located on the
surface of the ECM (26,27), which results in the release of
multiple cytokines and growth factors that bond to HS-GAG, thus
facilitating the transfer of cervical cancer cells to the lymph
vessels.
HPA plays a significant role in promoting the
formation of lymph vessels, which has been reported to be one of
the main transfer pathways of malignant cells. Tumor cells produce
and release growth factors that are associated with the formation
of lymph vessels, including vascular endothelial growth factor
(VEGF)-C and VEGF-D, hence inducing formation and transfer
(28). High levels of VEGF-C may be
detected in the serum of cervical cancer patients, and the
expression of VEGF-C in samples of patients with positive LN
metastasis is extraordinarily high in comparison with that of
non-metastatic patients (29,30). By inhibiting the function of the
upstream regulatory factors of VEGF-C, the expression of VEGF-C and
its effect of inducing angiogenesis is suppressed (31). VEGF-C and VEGF, together with the
essential lymphangiogenesis factor Proxl, may be involved in the
formation of early lymph vessels during the progression of cervical
neoplasia, which explains the reason why LN involvement may even be
detected in certain early-stage cervical cancers. Although
experimental studies that identify the function of HPA in promoting
the secretion of VEGF are poorly reported, melanoma, breast and
prostate cancer cells with overexpression of HPA cause the level of
VEGF-C to increase by 3–5 times, and promote angiogenesis of
transplant tumors. Conversely, the silencing of HPA genes reduces
the VEGF-C level (32). The present
study revealed that the expression level of HPA in primary lesions
of cervical cancer patients with LN metastasis was notably higher
than that in non-metastatic cases, which supports the hypothesis
that HPA promotes LN metastasis from the point of view of
clinicopathology.
When evaluating the role HPA plays in tumor sentinel
LN dissemination, Dafni et al (33) observed that Eb tumors with high
expression of HPA increased extravasation, interstitial convection
and lymphatic drain of the contrast material using dynamic
contrast-enhanced magnetic resonance imaging (MRI), and changes in
MR contrast enhancement were detectable when only a few Eb cells
were identified near and within the nodes. These authors
demonstrated that HPA of the tumor cells could promote the release
of vascular endothelial growth factor, which triggered secondary
angiogenesis during tumor cell cloning in LNs. Our study identified
that metastatic LNs have the same HPA expression level as the
original tumor site, which theoretically supported their theory.
Our results revealed that the prognosis of patients with positive
HPA in LNs was poorer than that of patients without HPA expression;
this may be due to the promotion of angiogenesis by HPA, which
facilitated the formation of tumor cloning and shortened the
formation time of the secondary tumor, and ultimately constitutes a
threat to long-term survival in patients (33).
Notably, we observed that out of the 49
clinicopathologically diagnosed patients without metastatic LNs, 4
cases were positive for immunostaining of HPA and CK19, and these
cases were identified by an experienced pathologist to have
micro-metastasis of the LNs. CK19 is the most common diagnostic
marker of LN micro-metastasis in gynecological malignant tumors
(7,34,35). Our
study revealed that, in the primary lesion and metastatic LNs, the
positive expression rate of HPA was over 80% and no less than the
rate of CK19, which supported the theory that HPA could be a
promising biomarker of LN metastasis in cervical cancer.
For the first time, we confirm that overexpression
of HPA may be detected in metastatic retroperitoneal LNs of
cervical cancer, and may be involved in lymphatic transfer of
cervical cancer and further affect patient prognosis. Our findings
support the theory that HPA could be a biomarker for the diagnosis
of LN micro-metastasis in cervical cancer, offer a significant
basis for the development of positron emission tomography tracers,
and provide a promising target marker for the treatment of patients
with LN metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81341065), the Henan International Cooperation Project (Zhengzhou,
China; grant no. 134300510047) and the Natural Science Research
Program of the Education Department of Henan Province (Zhengzhou,
China; grant no. 12A320024).
References
|
1
|
Ho CM, Chien TY, Huang SH, Wu CJ, Shih BY
and Chang SC: Multivariate analysis of the prognostic factors and
outcomes in early cervical cancer patients undergoing radical
hysterectomy. Gynecol Oncol. 93:458–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slama J, Dundr P, Dusek L and Cibula D:
High false negative rate of frozen section examination of sentinel
lymph nodes in patients with cervical cancer. Gynecol Oncol.
129:384–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quinn MA, Benedet JL, Odicino F,
Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY and
Pecorelli S: Carcinoma of the cervix uteri. FIGO 26th annual report
on the results of treatment in gynecological cancer. Int J Gynaecol
Obstet. 95:(Suppl 1). S43–S103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ditto A, Martinelli F, Lo Vullo S, Reato
C, Solima E, Carcangiu M, Haeusler E, Mariani L, Lorusso D and
Raspagliesi F: The role of lymphadenectomy in cervical cancer
patients: the significance of the number and the status of lymph
nodes removed in 526 cases treated in a single institution. Ann
Surg Oncol. 20:3948–3954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slama J, Fischerova D, Pinkavova I, Zikan
M and Cibula D: Human papillomavirus DNA presence in pelvic lymph
nodes in cervical cancer. Int J Gynecol Cancer. 20:126–132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JS, Namkoong SE, Han SK, Nha DJ, Lee
HY and Kim SJ: Comparison of L1 consensus primers with E6 type
specific primers for detection of human papillomaviruses in
paraffin sections of cervical neoplasia. J Korean Med Sci. 8:60–67.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noventa M, Ancona E, Cosmi E, Saccardi C,
Litta P, D'Antona D, Nardelli GB and Gizzo S: Usefulness, methods
and rationale of lymph nodes HPV-DNA investigation in estimating
risk of early stage cervical cancer recurrence: a systematic
literature review. Clin Exp Metastasis. 31:853–867. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 30:(Suppl 5).
F12–F23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tjalma WA, Van Waes TR, Van den Eeden LE
and Bogers JJ: Role of human papillomavirus in the carcinogenesis
of squamous cell carcinoma and adenocarcinoma of the cervix. Best
Pract Res Clin Obstet Gynaecol. 19:469–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garland SM: Can cervical cancer be
eradicated by prophylactic HPV vaccination? Challenges to vaccine
implementation. Indian J Med Res. 130:311–321. 2009.PubMed/NCBI
|
|
11
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoste G, Vossaert K and Poppe WA: The
clinical role of HPV testing in primary and secondary cervical
cancer screening. Obstet Gynecol Int. 2013:6103732013.PubMed/NCBI
|
|
13
|
Hirshoren N, Bulvik R, Neuman T,
Rubinstein AM, Meirovitz A and Elkin M: Induction of heparanase by
HPV E6 oncogene in head and neck squamous cell carcinoma. J Cell
Mol Med. 18:181–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baraz L, Haupt Y, Elkin M, Peretz T and
Vlodavsky I: Tumor suppressor p53 regulates heparanase gene
expression. Oncogene. 25:3939–3947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams DH and Shaw S: Leucocyte-endothelial
interactions and regulation of leucocyte migration. Lancet.
343:831–836. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blotnick S, Peoples GE, Freeman MR,
Eberlein TJ and Klagsbrun M: T lymphocytes synthesize and export
heparin-binding epidermal growth factor-like growth factor and
basic fibroblast growth factor, mitogens for vascular cells and
fibroblasts: differential production and release by CD4+ and CD8+ T
cells. Proc Natl Acad Sci USA. 91:2890–2894. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vlodavsky I, Eldor A, Haimovitz-Friedman
A, Matzner Y, Ishai-Michaeli R, Lider O, Naparstek Y, Cohen IR and
Fuks Z: Expression of heparanase by platelets and circulating cells
of the immune system: possible involvement in diapedesis and
extravasation. Invasion Metastasis. 12:112–127. 1992.PubMed/NCBI
|
|
18
|
Pisano C, Vlodavsky I, Ilan N and Zunino
F: The potential of heparanase as a therapeutic target in cancer.
Biochem Pharmacol. 89:12–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinyo Y, Kodama J, Hongo A, Yoshinouchi M
and Hiramatsu Y: Heparanase expression is an independent prognostic
factor in patients with invasive cervical cancer. Ann Oncol.
14:1505–1510. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng C, Ke ZF, Luo WR, Yao YH, Hu XR, Jie
W, Yin JB and Sun SJ: Heparanase overexpression participates in
tumor growth of cervical cancer in vitro and in vivo. Med Oncol.
30:4032013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prat J: FIGO Committee on Gynecologic
Oncology: Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of the Female Reproductive
OrgansWHO/IARC Classification of Tumours. 6. 4th. IARC; Lyon:
2014
|
|
23
|
Vlodavsky I, Elkin M and Ilan N: Impact of
heparanase and the tumor microenvironment on cancer metastasis and
angiogenesis: basic aspects and clinical applications. Rambam
Maimonides Med J. 2:e00192011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: the dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbouri D, Afratis N, Gialeli C, Vynios
DH, Theocharis AD and Karamanos NK: Syndecans as modulators and
potential pharmacological targets in cancer progression. Front
Oncol. 4:42014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hasengaowa Kodama J, Kusumoto T, Shinyo Y,
Seki N, Nakamura K, Hongo A and Hiramatsu Y: Loss of basement
membrane heparan sulfate expression is associated with tumor
progression in endometrial cancer. Eur J Gynaecol Oncol.
26:403–406. 2005.PubMed/NCBI
|
|
27
|
Kodama J, Shinyo Y, Hasengaowa Kusumoto T,
Seki N, Nakamura K, Hongo A and Hiramatsu Y: Loss of basement
membrane heparan sulfate expression is associated with pelvic lymph
node metastasis in invasive cervical cancer. Oncol Rep. 14:89–92.
2005.PubMed/NCBI
|
|
28
|
Ozasa R, Ohno J, Iwahashi T and Taniguchi
K: Tumor-induced lymphangiogenesis in cervical lymph nodes in oral
melanoma-bearing mice. J Exp Clin Cancer Res. 31:832012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biedka M, Makarewicz R, Marszałek A, Sir
J, Kardymowicz H and Goralewska A: Labeling of microvessel density,
lymphatic vessel density and potential role of proangiogenic and
lymphangiogenic factors as a predictive/prognostic factors after
radiotherapy in patients with cervical cancer. Eur J Gynaecol
Oncol. 33:399–405. 2012.PubMed/NCBI
|
|
30
|
Liu H, Xiao J, Yang Y, Liu Y, Ma R, Li Y,
Deng F and Zhang Y: COX-2 expression is correlated with VEGF-C,
lymphangiogenesis and lymph node metastasis in human cervical
cancer. Microvasc Res. 82:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu D, Li L, Zhang XX, Wan DY, Xi BX, Hu
Z, Ding WC, Zhu D, Wang XL, Wang W, et al: SIX1 promotes tumor
lymphangiogenesis by coordinating TGFβ signals that increase
expression of VEGF-C. Cancer Res. 74:5597–5607. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen-Kaplan V, Naroditsky I, Zetser A,
Ilan N, Vlodavsky I and Doweck I: Heparanase induces VEGF C and
facilitates tumor lymphangiogenesis. Int J Cancer. 123:2566–2573.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dafni H, Cohen B, Ziv K, Israely T,
Goldshmidt O, Nevo N, Harmelin A, Vlodavsky I and Neeman M: The
role of heparanase in lymph node metastatic dissemination: dynamic
contrast-enhanced MRI of Eb lymphoma in mice. Neoplasia. 7:224–233.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang HY, Sun JM, Lu HF, Shi DR, Ou ZL, Ren
YL and Fu SQ: Micrometastases detected by cytokeratin 19 expression
in sentinel lymph nodes of patients with early-stage cervical
cancer. Int J Gynecol Cancer. 16:643–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagai T, Niikura H, Okamoto S, Nakabayashi
K, Matoda M, Utsunomiya H, Nagase S, Watanabe M, Takeshima N and
Yaegashi N: A new diagnostic method for rapid detection of lymph
node metastases using a one-step nucleic acid amplification (OSNA)
assay in endometrial cancer. Ann Surg Oncol. 22:980–986. 2015.
View Article : Google Scholar : PubMed/NCBI
|