Introduction
Angiogenesis plays a vital role in the growth and
metastasis of lung cancer. It has been reported that vascular
endothelial growth factor (VEGF), as the strongest angiogenesic
factor, not only acts on the proliferation and differentiation of
endothelial cells but also as a chemotactic factor for directional
movement of activated monocytes to a site of inflammation and tumor
growth (1,2).
It is clear that hypoxia-inducible factor (HIF)-1α
regulates VEGF protein synthesis through the PI3K pathway and the
hypoxia-activated PI3K/Akt/mTOR pathway (3). Transforming growth factor (TGF)-β1 can
induce EMT and enhance tumor metastasis (4). There are many reports on the PI3K/Akt
signaling pathway and angiogenesis in A549 lung cancer cells,
whereas reports on regulation of angiogenic factors [i.e.,
angiotensin II (ANG-II), TGF-β1 or tumor necrosis factor (TNF)-α]
by PI3K/Akt signaling and the effect of PI3K inhibition is limited.
Thus, in the present study, we used the PI3K inhibitor LY294002 on
A549 cells under normoxic and hypoxic conditions. The migratory
ability of A549 cells was determined by scratch assay. The levels
of HIF-1α and AKT1 mRNA were determined by reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR) and
concentrations of VEGF, ANG-II, TGF-α/β1 and TNF-α in the culture
supernatant were measured by double-antibody sandwich enzyme-linked
immunosorbent assay (ELISA). The findings of the present study
provide a theoretical basis for the prevention and treatment of
lung cancer in the hypoxic environment.
Materials and methods
Cell lines and cell culture
The human non-small cell lung cancer A549 cell line,
was a gift from the Central Laboratory of Basic Medical Sciences,
Fourth Military Medical University (Xi'an, China). The cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (Sijiqing Biotechnology Co., Hangzhou, China) and
penicillin/streptomycin (Ybiotech, Shanghai, China). The cells were
maintained at 37°C in an incubator with 5% CO2 and
medium was changed every 2–3 days, then divided into 2–3 culture
flasks. Logarithmic growth phase cells were used for subsequent
experiments. For hypoxic exposure, the cells were placed in an
incubator chamber that was tightly sealed and thoroughly flushed
with 1% O2/5% CO2, and balance N2
and incubated at 37°C. PI3K inhibitor, LY294002 (Beyotime Biotech,
Jiangsu, China) was added to the medium at a final concentration of
30 µM (5). Untreated cells were taken
as the control group and cells treated by the inhibitor as the
suppression group. The cells were then cultured under normoxic or
hypoxic conditions and termed normoxic control group, normoxic
suppression group, hypoxic control group and hypoxic suppression
group, respectively. The cells were collected after 48 h culture in
the incubator. Experiments were repeated 3 times.
Observation of morphology and
migration
A549 cell morphology was observed by Wright-Giemsa
stain. The migratory ability of A549 cells was determined by
scratch assay at the 0, 6 and 20 h time-points, when cultured under
normoxic and hypoxic conditions, under an inverted fluorescence
microscope (Olympus, Tokyo, Japan).
RNA extraction, reverse transcription
and quantitative PCR
Total RNA was extracted from cell samples with
TRIzol reagent (Ambion Life Technologies, Carlsbad, CA, USA) and
quantified with a NanoDrop 2000 Spectrophotometer (Thermo
Scientific Inc., Bremen, Germany). The first strand cDNA was
synthesized by M-MLV reverse transcriptase (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The target mRNAs in cultured A549 cells were
quantified by RT-qPCR using TransScript First-Strand cDNA Synthesis
Super Mix (TransGen Biotech Co., Ltd., Beijing, China) using the
AB/Applied Biosystems 7500 Real-Time PCR Detection System (Applied
Biosystems Life Technologies, Foster City, CA, USA). Each PCR
reaction was performed in triplex tubes, and GAPDH was used as an
endogenous control to standardize the amount of sample mRNA. The
total reaction volume was 20 µl and thermal profile was as follows;
two-step PCR amplification, pre-denaturing: 95°C for 30 sec; 95°C
for 5 sec, and 60°C annealing for 31 sec, for a total of 40 cycles.
The raw data were analyzed with iQ5 software (Bio-Rad, Berkeley,
CA, USA) (6). The primers [Sangon
Biotech (Shanghai) Co., Ltd. (Shanghai, China)] used for qPCR were:
HIF-1α forward, 5′-ATACATGGTACCCACGAAGTGTTCCTTTG-3′ and reverse,
5′-ATACATCTCGAGAAAGAGACAAGTCCA-3′; AKT1 forward,
5′-ATGAGCGACGTGGCTATTGT-3′ and reverse, 5′-TGAAGGTGCCATCATTCTTG-3′;
GAPDH forward, 5′-ATCAAGAAGGTGGTGAAGCA-3′ and reverse,
5′-CAAAGGTGGAGGAGTGGGT-3′.
ELISA
The concentrations of VEGF, ANG-II, TGF-α/β1 and
TNF-α in the culture supernatant were determined by ELISA according
to the human ELISA kit instructions (Xinbosheng Biotechnology Co.,
Ltd., Beijing, China).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation and were analyzed with Student's t-test
(two-tailed). P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell morphology and the effect of
hypoxia and LY294002 on the migration of A549 cells
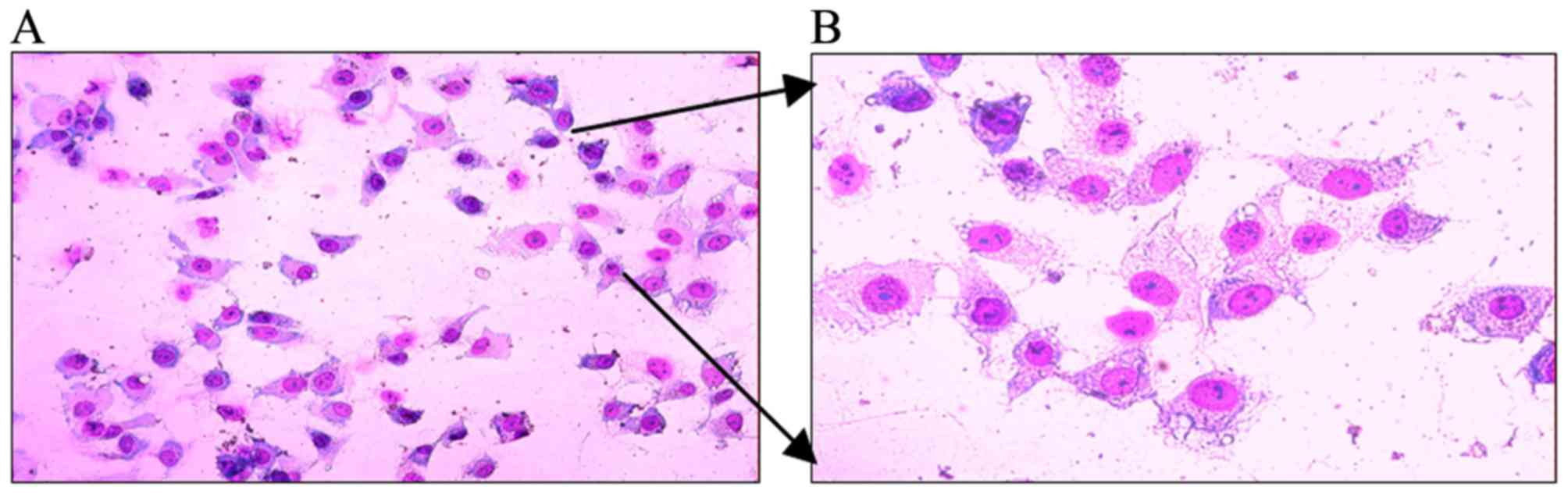
A549 cells were stained with Wright-Giemsa and
appeared as having epithelial cell-like adherent growth [Fig. 1A (x40 magnification) and B (x100
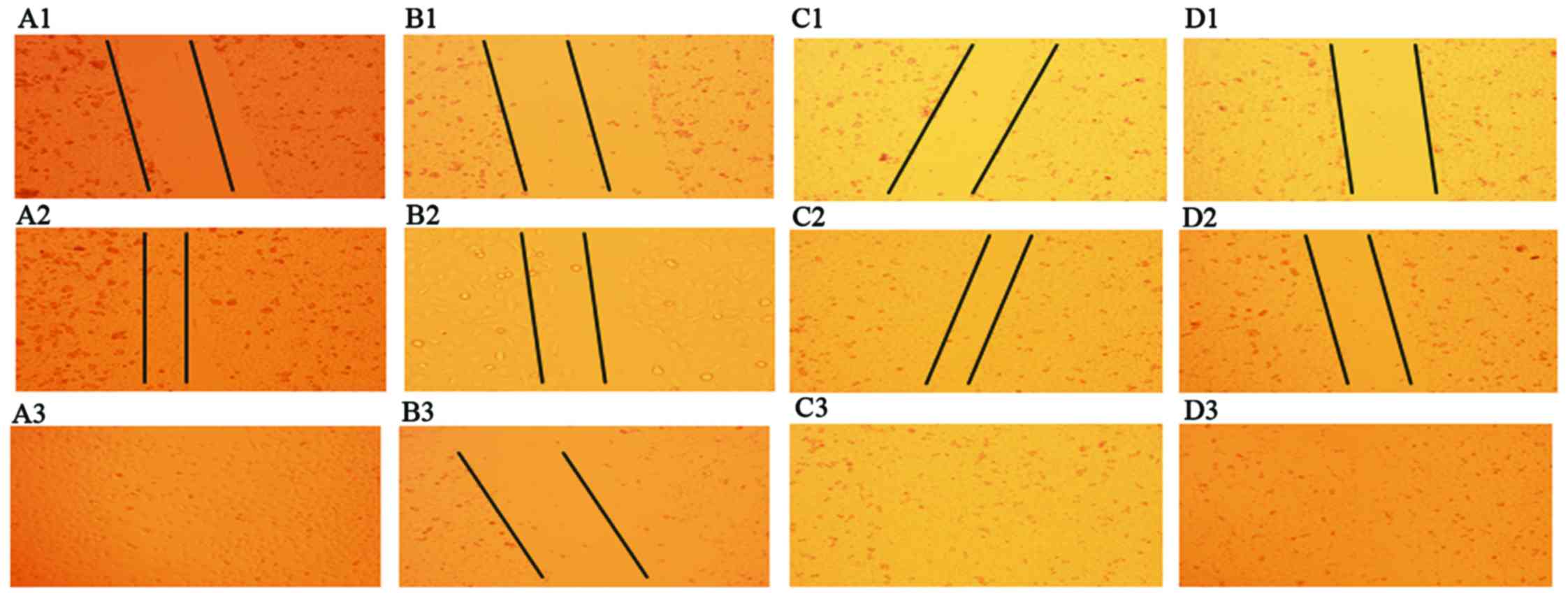
magnification)]. Whether under normoxic or hypoxic conditions,
scratch wounds were completely filled after 20 h in untreated
cells. By contrast, the scratch wounds were not completely filled
after 20 h when the cells were treated with LY294002 (Fig. 2).
Effect of hypoxia and LY294002 on
HIF-1α and AKT1 mRNA expression
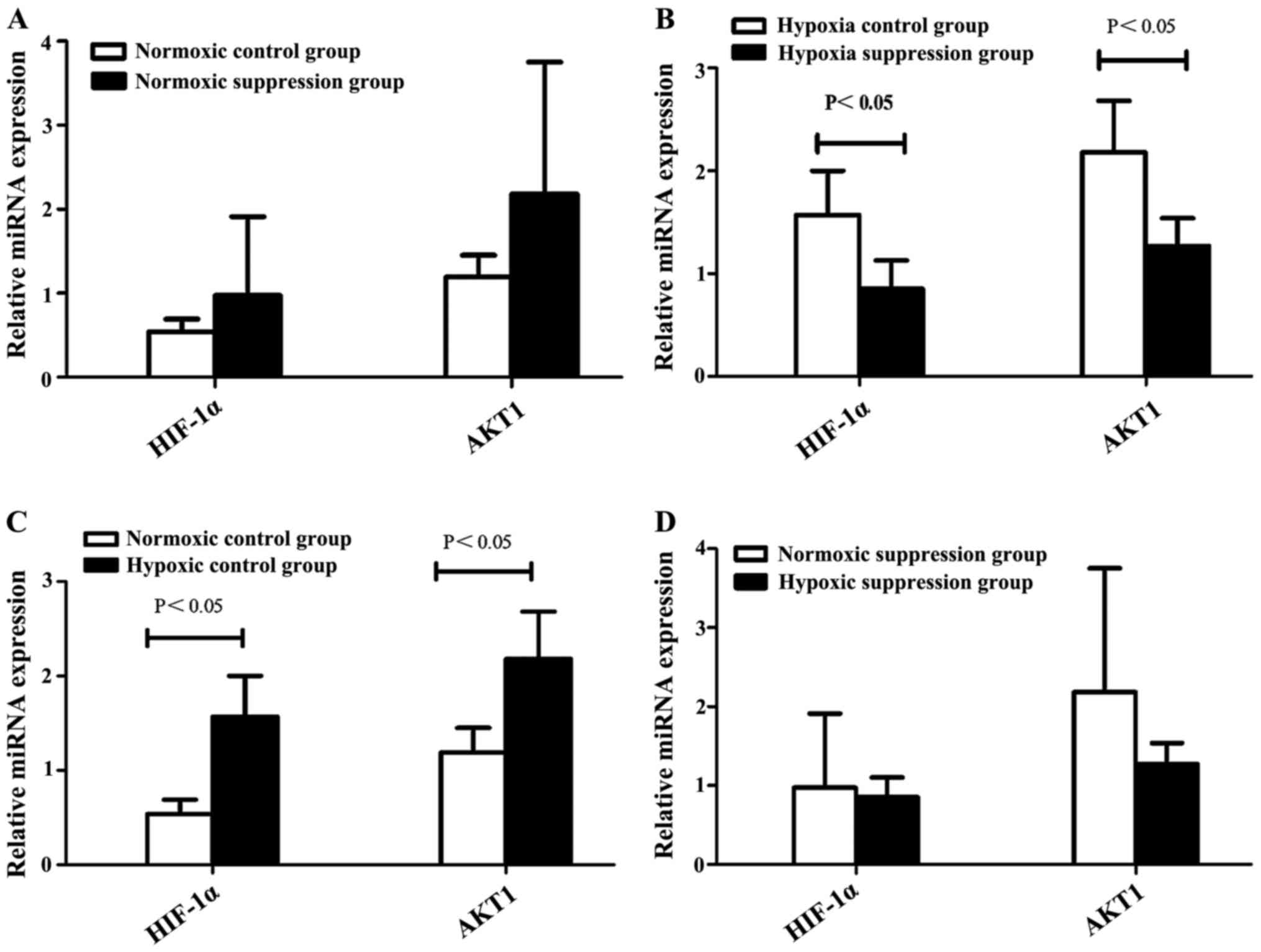
Compared to the normoxic control group, the levels
of HIF-1α and AKT1 mRNA were higher in the hypoxic control group.
However, compared to the hypoxic suppression group, the levels of
HIF-1α and AKT1 mRNA were higher than in the hypoxic control group
(Table I and Fig. 3).
 | Table I.Effect of hypoxia and PI3K inhibitor
LY294002 on HIF-1α and AKT1 mRNA expression (mean ± SD, n=9). |
Table I.
Effect of hypoxia and PI3K inhibitor
LY294002 on HIF-1α and AKT1 mRNA expression (mean ± SD, n=9).
|
| Normoxic | Hypoxic |
|---|
|
|
|
|
|---|
| Group | HIF-1α | AKT1 | HIF-1α | AKT1 |
|---|
| Control | 0.54±0.15 | 1.19±0.26 |
1.57±0.43a |
2.18±0.50a |
| Suppression | 0.97±0.94 | 2.18±1.57 | 0.85±0.28 | 1.27±0.27 |
| t-test | −1.050 | −1.469 |
0.3716 | 5.183 |
| P-value | 0.353 | 0.216 | 0.021 | 0.007 |
Effect of hypoxia and LY294002 on
levels of angiogenic factors
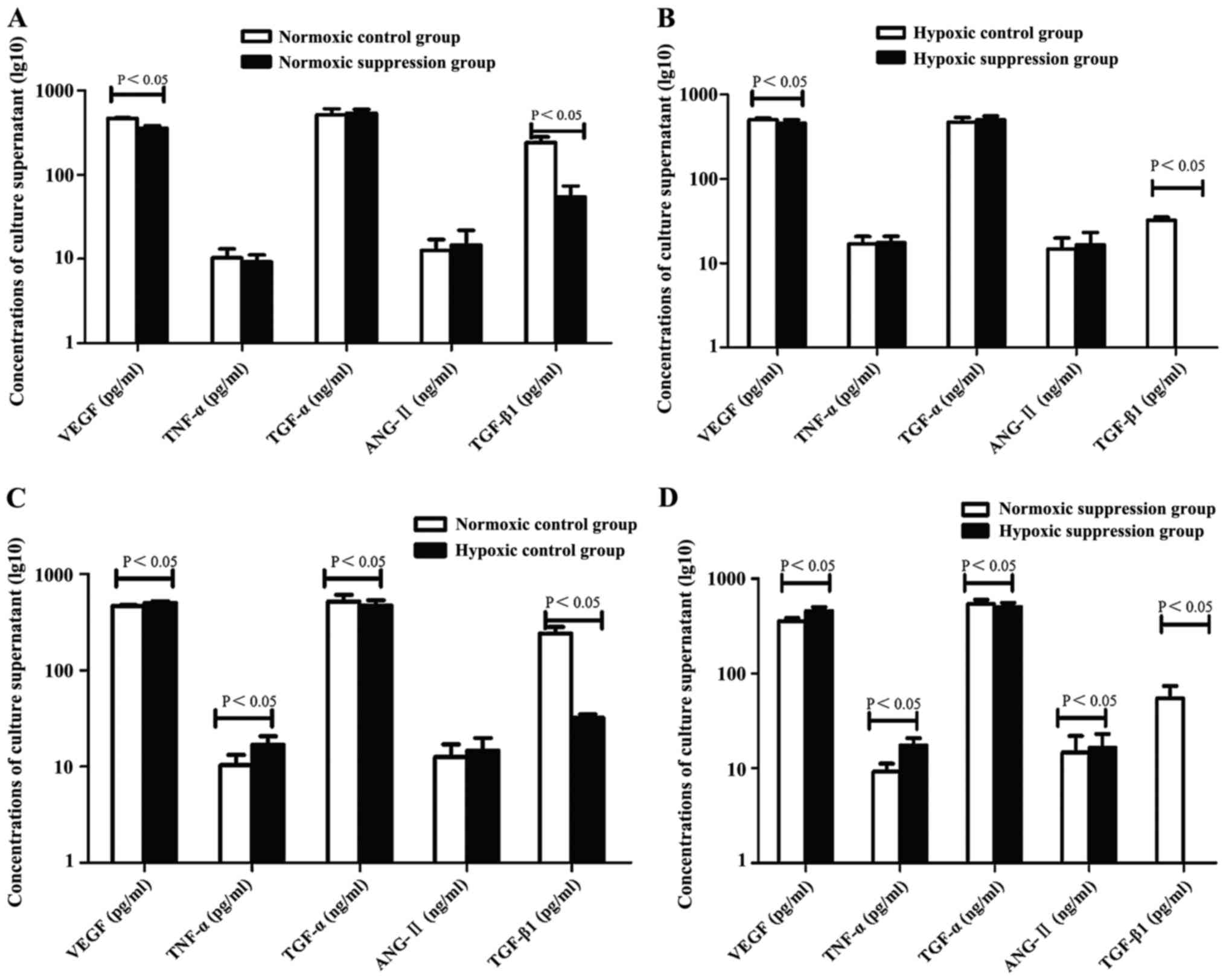
Compared to the normoxic control group and normoxic
suppression group, the concentrations of VEGF and TNF-α in culture
supernatant were higher in the hypoxic control group and hypoxic
suppression group. However, TGF-α and TGF-β1 showed an opposite
trend of expression. The concentration of ANG-II in the hypoxic
suppression group was higher than in the normoxic suppression
group. In addition, compared to the normoxic control group and
hypoxic control group, the concentrations of VEGF and TGF-β1 in
supernatant were lower in the normoxic suppression group and in the
hypoxic suppression group, respectively (Table II and Fig.
4).
 | Table II.Effect of hypoxia and PI3K inhibitor
LY294002 on the concentrations of angiogenic factors (mean ± SD,
n=9). |
Table II.
Effect of hypoxia and PI3K inhibitor
LY294002 on the concentrations of angiogenic factors (mean ± SD,
n=9).
| Group | Items | Control | Suppression | t-test | P-value |
|---|
| Normoxic | VEGF (pg/ml) | 468.26±9.93 | 360.59±24.50 | 13.670 | 0.000 |
|
| TNF-α (pg/ml) | 10.34±2.89 | 9.22±1.94 | 1.148 | 0.281 |
|
| TGF-α (ng/ml) | 520.93±90.74 | 541.49±60.64 | −1.208 | 0.258 |
|
| ANG-II (ng/ml) | 12.66±4.39 | 14.67±7.29 | −1.666 | 0.130 |
|
| TGF-β1 (pg/ml) | 242.07±40.31 | 54.49±19.28 | 14.425 | 0.000 |
| Hypoxic | VEGF (pg/ml) |
502.90±23.90a |
457.83±44.82a | 3.565 | 0.007 |
|
| TNF-α (pg/ml) |
16.88±3.84a |
17.40±3.49a | −0.321 | 0.756 |
|
| TGF-α (ng/ml) |
471.21±62.82a |
504.04±52.58a | −2.091 | 0.066 |
|
| ANG-II (ng/ml) | 14.66±5.25 |
16.47±6.67a | −1.715 | 0.121 |
|
| TGF-β1 (pg/ml) |
32.32±2.58a |
|
|
|
Discussion
Lung cancer ranks as the primary cause of cancer
death worldwide, and is the most commonly diagnosed cancer
worldwide. In 2012, there were 1.8 million lung cancer diagnoses
representing 13% of the total (7).
The most common cause of death in 2012 was lung cancer. The number
of deaths from lung cancer were 1.6 million and this represented
19.4% of total deaths in 2012 (7).
Previous studies have demonstrated that the biological behavior of
solid tumor growth includes invasion and metastasis as well as
tumor- related angiogenesis and remodeling. The PI3Ks are a family
of lipid kinases whose primary biochemical function is to generate
second messengers by phosphorylating the 3-hydroxyl group of
phosphatidylinositols (8). Akt
(protein kinase B) is a serine/threonine kinase activated
downstream of PI3K-α, that is involved in promoting cell
differentiation, inhibition of cell death and other important
biological functions (8). Studies
have shown that the overexpression rate of PI3K/Akt pathway was
84.75% in non-small cell lung cancer and was related with high
proliferative activity of tumors (9).
The results of the present study demonstrate that A549 cell
migration was not significantly affected by hypoxia, while
migration after treatment with LY294002 significantly decreased.
Although hypoxia had no effect on the migration of A549 cells,
RT-qPCR showed that hypoxia increased levels of HIF-1α and AKT1
mRNA and treatment with LY294002 reduced the levels of HIF-1α and
AKT1 under hypoxic conditions. However, there were no such changes
under normoxic conditions. These findings suggest that hypoxia can
activate PI3K/Akt signaling in A549 cells and the migratory ability
of these cells is related to the PI3K/Akt pathway (3).
More significant is the observation that hypoxia
stimulated A549 cells to secrete VEGF and TNF-α and reduce the
expression of TGF-α and TGF-β1. ANG-II displayed a trend of
increasing in the hypoxic control group compared to the normoxic
control group, but there was no statistically significant
difference. Hypoxia stimulated A549 cells treated by LY294002 to
secrete VEGF and TNF-α and to reduce expression of TGF-α and
TGF-β1, while increasing the secretion of ANG-II. This indicates
that hypoxia can stimulate A549 cells to secrete VEGF and TNF-α and
to inhibit TGF-α and TGF-β1. The ability of A549 cells to secrete
VEGF and TGF-β1 is partially regulated by PI3K/Akt and ANG-II
expression may be dependent on the PI3K/Akt pathway under hypoxic
conditions. The present study shows that the PI3K/Akt signaling
pathway is related to invasion and metastasis of lung cancer cells
(3,10,11), and
VEGF plays an important role in angiogenesis and invasion.
A549 cells treated with the PI3K/AKT inhibitor,
LY294002 in vitro, under normoxic or hypoxic conditions,
were significantly inhibited in their ability to secrete VEGF and
TGF-β1, and it was more pronounced under normoxic conditions. The
levels of TGF-β1 in A549 cell supernatant after treatment with
LY294002 were below the lower detection limit of the ELISA under
hypoxic conditions. This indicates that the PI3K/Akt signaling
pathway affected more than the levels of VEGF and TGF-β1. Studies
have shown that VEGF also activates PI3K/Akt/Forkhead signaling to
inhibit apoptosis, promote DNA synthesis and transition from G1 to
S phase in endothelial cells (12).
In addition to angiogenesis, research suggests that the phenomenon
of vascular mimicry was a part of cancer pathogenesis in lung
tissue (13,14). This was related to patient prognosis.
Together with high expression of matrix metalloproteinases,
degradation of the extracellular matrix in highly malignant tumor
cells in a hypoxic microenvironment formed a vessel-like structure.
PI3K inhibitors also inhibited the ability to form pipeline tumor
cells connected to each other by inhibiting matrix
metalloproteinase (MMP)-2 and MMP-9 and extracellular matrix
degradation, which inhibited vasculogenic mimicry (15,16). These
observations suggested that tumor angiogenesis was related to a
number of factors. There is insufficient evidence that targeting
VEGF or the VEGF receptor has a therapeutic effect related to the
PI3K/Akt pathway (17–19).
However, other studies reported that large doses of
LY294002 did not completely block VEGF transcription, suggesting
that other factors are involved in the regulation of VEGF
expression. Multiple signaling pathways communicate with each
other, thus forming a signaling network. This phenomenon is
limiting in regards to the efficacy of a single target drug to have
an effect. Previous findings have also shown that when
microvascular lung endothelial cells and squamous or adenocarcinoma
lung cancer cells are co-cultured in vitro, this increased
the blood supply to each other, suggesting that non-angiogenic
factors cannot be ignored in tumor therapy (20). Thus, the effect of single target tumor
therapy has limitations. In practice, we need to consider both
tumor molecular biology and pathology in order to select targeted
drugs to achieve individualized treatment and improve efficacy.
Acknowledgements
The present study was funded by Foundation Research
Project of Qinghai Provincial Health and Family Planning Commission
and Qinghai Province Key Specialty (respiratory).
References
|
1
|
Nourse MB, Halpin DE, Scatena M, et al:
VEGF induces differentiation of functional endothelium from human
embryonic stem cells: implications for tissue engineering.
Arterioscler Thromb Vasc Biol. 30:80–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Avraham-Davidi I, Yona S, Grunewald M,
Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M,
Strauss-Ayali D, Mack M, et al: On-site education of VEGF-recruited
monocytes improves their performance as angiogenic and arteriogenic
accessory cells. J Exp Med,. 210:2611–2625. 2013. View Article : Google Scholar
|
|
3
|
Park JJ, Hwang SJ, Park JH and Lee HJ:
Chlorogenic acid inhibits hypoxia-induced angiogenesis via
down-regulation of the HIF-1α/AKT pathway. Cell Oncol (Dordr).
38:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K,
Kim TJ, Ham J, Jang HJ, Kang KS and Ko H: Stereospecific effects of
ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal
transition and suppresses lung cancer migration, invasion and
anoikis resistance. Toxicology. 322:23–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Z: Study on PI3K as an anti-cancer
angiogenesis and vascular mimicry common targets (unpublished PhD
thesis). Huazhong University of Science and Technology. 2010.
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Health Organization: Latest world
cancer statistics: Global cancer burden rises to 14.1 million new
cases in 2012: Marked increase in breast cancers must be addressed.
WHO; Geneva: 2013
|
|
8
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao DW, Wang L, Zhang XG and Liu MQ:
Expression and significance of PTEN/PI3K signal
transduction-related proteins in non-small cell lung cancer. Ai
Zheng. 25:1238–1242. 2006.(In Chinese). PubMed/NCBI
|
|
10
|
Zhang Q, Tang X, Zhang ZF, Velikina R, Shi
S and Le AD: Nicotine induces hypoxia-inducible factor-1alpha
expression in human lung cancer cells via nicotinic acetylcholine
receptor-mediated signaling pathways. Clin Cancer Res.
13:4686–4694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Savai R, Schermuly RT, Voswinckel R,
Renigunta A, Reichmann B, Eul B, Grimminger F, Seeger W, Rose F and
Hänze J: HIF-1α attenuates tumor growth in spite of augmented
vascularization in an A549 adenocarcinoma mouse model. Int J Oncol.
27:393–400. 2005.PubMed/NCBI
|
|
12
|
Abid MR, Guo S, Minami T, Spokes KC, Ueki
K, Skurk C, Walsh K and Aird WC: Vascular endothelial growth factor
activates PI3K/Akt/forkhead signaling in endothelial cells.
Arterioscler Thromb Vasc Biol. 24:294–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu S, Cheng Z, Yu L, Song W and Tao Y:
Expression of CD82/KAI1 and HIF-1α in non-small cell lung cancer
and their relationship to vasculogenic mimicry. Zhongguo Fei Ai Za
Zhi. 14:918–925. 2011.(In Chinese). PubMed/NCBI
|
|
14
|
Han Y, Jing W, Wang G and Ao Q: Lung
cancer angiogenesis mimicry and expression and significance of
HIF-lα. J Clin Exp Pathol. 24:269–272. 2008.(In Chinese). doi:
10.13315/j.cnki.cjcep.2008.03.011.
|
|
15
|
Chetty C, Lakka SS, Bhoopathi P and Rao
JS: MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated
PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer.
127:1081–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khoufache K, Bazin S, Girard K,
Guillemette J, Roy MC, Verreault JP, Al-Abed Y, Foster W and Akoum
A: Macrophage migration inhibitory factor antagonist blocks the
development of endometriosis in vivo. PLoS One. 7:e372642012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munagala R, Aqil F and Gupta RC: Promising
molecular targeted therapies in breast cancer. Indian J Pharmacol.
43:236–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Tourneau C, Faivre S, Serova M and
Raymond E: mTORC1 inhibitors: Is temsirolimus in renal cancer
telling us how they really work? Br J Cancer. 99:1197–1203. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji Y, Chen S, Li K, Li L, Xu C and Xiang
B: Signaling pathways in the development of infantile hemangioma. J
Hematol Oncol. 7:132014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaessmeyer S, Bhoola K, Baltic S, Thompson
P and Plendl J: Lung cancer neovascularisation: Cellular and
molecular interaction between endothelial and lung cancer cells.
Immunobiology. 219:308–314. 2014. View Article : Google Scholar : PubMed/NCBI
|