Introduction
Cancer is a leading cause of mortality worldwide,
with 8.2 million cancer-associated mortalities being reported in
2012. Gastric cancer (GC) is the fifth most common type of cancer,
with 952,000 new cases per year, and is also the third leading
cause of cancer-associated mortality, of which 70% are reported in
developing countries, particularly those in East Asia and Latin
America (1). The number of cases in
these countries is expected to rise in the coming years due to the
aging of the population and the lack of public strategies being
undertaken to reduce the risk factors.
There is an urgent requirement to develop new
methods for the early detection of this neoplasm using minimally
invasive procedures with high sensitivity and specificity. Previous
research has suggested that circulating levels of microRNAs are
specific and sensitive for the detection of GC lesions (2). MicroRNAs are stable due to their
packaging in exosomes and apoptotic bodies and their ability to
form complexes with lipoproteins and binding proteins, which make
them resistant to RNase activity (3).
Furthermore, certain microRNAs have been implicated in the
development of cancer, and demonstrate specific expression patterns
in various types of tumor (4–6), including GC (7–9).
Studies have suggested that microRNAs may be
candidate biomarkers for use in the detection of cancer. A recent
meta-analysis revealed that circulating microRNAs can be used as
targets for the detection of various types of gastrointestinal
cancers, and that plasma samples have a higher accuracy in this
regard compared with serum samples (10). Li et al (11) identified hsa-miR-223 and hsa-miR-21 to
be overexpressed and hsa-miR-218 underexpressed in the plasma of
patients with GC, and suggested that these microRNAs are involved
in the processes of tumorigenesis and metastasis. Gorur et
al (12) conducted an analysis of
the expression profiles of 740 different microRNAs in patients with
GC, and reported that only hsa-miR-195-5p was underexpressed in
sera. Song et al (13)
identified 16 microRNAs that were overexpressed in the sera of
patients with GC; however, following additional investigation, the
authors suggested that only hsa-miR-221, hsa-miR-744 and
hsa-miR-376c were potential biomarkers. A study based on a Chinese
population revealed that hsa-miR-148a, hsa-miR-142-3p, hsa-miR-26a
and hsa-miR-195 were downregulated in patients with GC; in
particular, the plasma levels of hsa-miR-26a were significantly
reduced (14). Furthermore, a
meta-analysis revealed that 35 microRNAs have been reported as
being candidate biomarkers for GC detection, but that hsa-miR-21 is
the only microRNA to be consistently reported (15).
This inconsistency of results may be a result of
differences in the GC subtypes (9) or
populations being studied, differences in microRNA extraction
methods or differences in the methods of detection used (16). A critical drawback is the lack of
targets to which data from sera or plasma samples may be
normalized, similar to the used of β-actin or GAPDH levels to
normalize intracellular RNAs. Several studies have proposed to use
5S ribosomal RNA (17), hsa-miR-93,
hsa-miR-191, RNU6-2, the small nucleolar RNAs: SNORD48, SNORD61,
SNORD68, SNORD72, SNORD95 and SNORD96a (18), and hsa-miR-16-5p (19) for normalization. However, a number of
these genes have been dismissed due to their instability in
circulation (20,21). The present study presents evidence
suggesting that the standardization of microRNA data expression
must be established according to the neoplasm and the type of
sample. Steady levels of hsa-miR-18a-5p and hsa-miR-29a-3p were
identified in the panel of samples used in the present study, and
the use of those microRNAs as normalizers of the array data allowed
the identification of hsa-miR-200c-3p and hsa-miR-26b-5p
underexpression in plasma samples from patients with GC.
Materials and methods
Patients and samples
The present study included 6 adult patients (5 males
and 1 female) with distal GC, of which 3 had intestinal-type (IGC)
and 3 had diffuse-type (DGC), without any other type of neoplasia
(Table I). None of the patients had
undergone treatment. The comparison (control) group comprised
plasma samples from 2 patients with non-atrophic gastritis
(Table I). Plasma samples were stored
at −70°C until use.
 | Table I.Clinical characteristics of patients
with GC or non-atrophic gastritis. |
Table I.
Clinical characteristics of patients
with GC or non-atrophic gastritis.
| A, GC samples |
|---|
|
|---|
| Sample ID | Age, years | Gender | Cancer type |
|---|
| 1GC | 72 | Male |
Intestinalb |
| 2GC | 58 | Male |
Diffuseb |
| 3GC | 91 | Male |
Intestinalb |
| 4GC | 76 | Male |
Diffuseb |
| 5GC | 76 | Male |
Diffusea,
b |
| 6GC | 52 | Female | Intestinal |
|
| B, Gastritis
samples |
|
| Sample ID | Age, years | Gender | Diagnosis |
|
| 1C | 64 | Male | Non-atrophic
gastritisb |
| 2C | 62 | Male | Non-atrophic
gastritisb |
The inclusion criteria for the study were
individuals who attended the Oncology Hospital and the General
Hospital of the XXI Century National Medical Center (Mexican
Institute of Social Security, Mexico City, Mexico) due to gastric
symptoms and were diagnosed with either GC or non-atrophic
gastritis. The exclusion criteria were as follows: Individuals and
samples that did not have a diagnosis of GC or non-atrophic
gastritis; patients with other types of cancer; patients with
recurrent cancer; and patients who had already received cancer
therapy.
Samples were not included in the study if they
lacked the RNA quantity and/or quality for the microRNA analysis,
or if a sufficient quality of complementary DNA (cDNA) was not
identified in the GAPDH multiplex reverse transcription
(RT)-polymerase chain reaction (PCR).
The present study was approved by the Scientific and
Ethics Committees of the Mexican Institute of Social Security, and
patients were informed regarding the nature of the study and asked
to sign a consent letter. The study was conducted according to the
best clinical practices of our institution and the identity of the
patients was anonymized for the duration of the study.
RNA extraction
RNAs were extracted from 100 µl plasma samples using
the miRNeasy Serum/Plasma kit (Qiagen Inc., Valencia, CA, USA)
according to the manufacturer's protocol. The RNA concentration and
purity [using the optical density (OD) 260/280 nm ratio] were
determined in an Epoch spectrophotometer (BioTek Instruments, Inc.,
Winooski, VT, USA).
cDNA synthesis
cDNA synthesis was performed using a miScript II RT
kit (Qiagen GmbH, Hilden, Germany). Mature microRNAs were
polyadenylated by poly-A polymerase followed by cDNA synthesis
using oligo-dTs. The PCR reaction mixture (20 µl) contained 1X
miScript HiSpec buffer, 1X miScript Nucleics mix, 1X miScript
reverse transcriptase mix and 250 ng of RNA as template. The
mixture was incubated for 60 min at 37°C for polymerization,
followed by 5 min at 95°C to inactivate the miScript reverse
transcriptase.
GAPDH multiplex PCR
cDNA quality was determined using a Multiplex PCR
kit (Qiagen GmbH). In this reaction, 7 fragments (100–700 bp) of
the GAPDH gene were amplified, and the sequences of the primers
were described previously (22). The
PCR reaction mixture (12.5 µl) contained 1X Master mix, 0.2 µM of
each primer and 5 µl of cDNA. Amplification was performed in a
MasterCycler GSX1 (Eppendorf, Hamburg, Germany) as follows: 95°C
for 15 min; followed by 40 cycles of 94°C for 30 sec, 57°C for 90
sec and 72°C for 90 sec; and a final extension at 72°C for 10 min.
PCR products were separated by electrophoresis on a 2.0% agarose
gel with a 100 bp DNA ladder (Promega Corporation, Madison, WI,
USA), stained with 1X GelRed™ (Biotium, Inc., Hayward, CA, USA) and
visualized under an ultraviolet light transilluminator and
photodocumenter system (Syngene, Frederick, MD, USA).
RT-quantitative PCR (qPCR) array
The microRNA expression profile was analyzed using a
miScript SYBR® Green PCR kit (Qiagen GmbH) and a Serum
and Plasma miScript miRNA PCR Array for the detection of microRNAs
in human serum or plasma (Qiagen GmbH; catalog no. MIHS-106ZF). The
array comprises microRNAs that have been reported in blood
circulation in various types of cancer, including GC; these
microRNAs include members of the let-7 and microRNA-200 families.
The following RNAs are used as controls in the array: One oligo for
the detection of miR-39 Caenorhabditis elegans; 5 small nucleolar
RNAs (snoRNAs: SNORD61, SNORD68, SNORD72, SNORD95, SNORD96A) and 1
small nuclear RNA (snRNA: RNU6-6P) for data normalization; a
control for the RT reaction (miRTC) and a positive PCR control
(PPC). The PCR reaction mixture (25 µl/well) contained 1X
QuantiTect SYBR® Green PCR Master Mix, 1X miScript
Universal Primer-T, and 1 µl/well of cDNA. The following thermal
cycling was employed: Initial activation at 95°C for 15 min;
followed by 45 denaturation cycles at 94°C for 15 sec, annealing at
55°C for 30 sec and extension at 70°C for 30 sec. The reaction was
performed using a Roche® LightCycler® 480
(Roche Diagnostics, Indianapolis, IN, USA).
Stability analysis
A stability analysis of the microRNAs was performed
using the NormFinder algorithm (23).
This software determined the overall expression variation of
microRNA targets and controls between samples.
Analysis of RT-qPCR data
Data were analyzed using the miScript miRNA PCR
Array Data Analysis tool (http://pcrdataanalysis.sabiosciences.com/mirna; free
access). The relatively quantified levels of the expression of
microRNAs, based on their quantification cycle (Cq) were estimated
using the ΔΔCq method (24). Firstly,
microRNAs with a Cq>35 were discarded. The remaining microRNAs
complied with the values established in the miRTC (ΔCq<7) and
PPC (Cq=19±2) controls. Cq values were normalized independently
using the snoRNAs and snRNA panel and the microRNAs identified
using the stability analysis.
Pathways associated with microRNAs and
GC
In silico analyses were performed to determine the
pathways associated with the microRNAs of interest; miRPath DIANA
software (version 3.0) (25) was used
to perform a Kyoto Encyclopedia of Genes and Genome (KEGG) pathway
analysis based on the TarBase database (version 7.0).
Statistical analysis
A non-parametric Mann-Whitney U test/Wilcoxon
rank-sum test was performed to assess differences between the
normalization strategies employed in this study. Analyses were
performed using the statistical software package SPSS (version
22.0; IBM SPSS, Armonk, NY, USA) and the R Project (version 3.2.3;
https://CRAN.R-project.org/doc/FAQ/R-FAQ.html) to
corroborate the results. Following the in silico analysis of the
targets and pathways associated with microRNAs, a false discovery
rate (FDR) correction with a P-value <0.05 was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients and samples
In total, 3 IGC, 3 DGC and 2 non-atrophic gastritis
samples were analyzed. The pathological characteristics of the
samples were determined by two independent pathologists. The
clinical characteristics of the patients are shown in Table I.
RNA extraction
RNA extraction produced on average 90±3 ng/µl RNA in
a final volume of 50 µl. RNA quality was determined by the OD
260/280 nm, which produced a value of 1.9±0.1.
GAPDH multiplex PCR
The quality of the cDNA was tested by a GAPDH
multiplex PCR, from which the shortest fragment amplified in all
samples was of 200 bp, indicating homogeneous cDNA quality between
samples.
RT-qPCR array
Following the array, the miRTC (ΔCq=3±1) and PPC
(Cq=18±1) values obtained complied with the standard of quality. By
contrast, the snoRNAs and snRNA control panel included in the array
demonstrated inconsistent Cq values, with some of the samples
yielding positive readings only following 35 cycles (Table II); those samples were scored as
‘undetermined’ for the snoRNAs and snRNA normalizing controls.
 | Table II.Cq values for 6 quantitative
polymerase chain reaction controls, including 5 small nucleolar
RNAs (SNORD61, SNORD68, SNORD72, SNORD95 and SNORD96A) and a small
nuclear RNA (RNU6-6P) for data normalization. |
Table II.
Cq values for 6 quantitative
polymerase chain reaction controls, including 5 small nucleolar
RNAs (SNORD61, SNORD68, SNORD72, SNORD95 and SNORD96A) and a small
nuclear RNA (RNU6-6P) for data normalization.
| Sample ID | SNORD61 | SNORD68 | SNORD72 | SNORD95 | SNORD96A | RNU6-6P |
| 1C | UD | UD | UD | UD | 31.66 | 32.84 |
| 2C | 33.55 | 28.87 | UD | 31.81 | 32.78 | 34.50 |
| 1GC | 33.70 | 32.98 | UD | UD | 33.99 | UD |
| 2GC | 31.98 | 32.60 | UD | 30.48 | 31.86 | 32.72 |
| 3GC | UD | UD | UD | 33.42 | 34.77 | UD |
| 4GC | UD | 34.43 | UD | UD | UD | UD |
| 5GC | UD | 31.72 | UD | 34.42 | UD | UD |
| 6GC | 31.83 | 32.40 | UD | 32.23 | UD | UD |
Stability analysis
Since a single snoRNA and/or snRNA with positive
values across all of the samples was not obtained, other targets
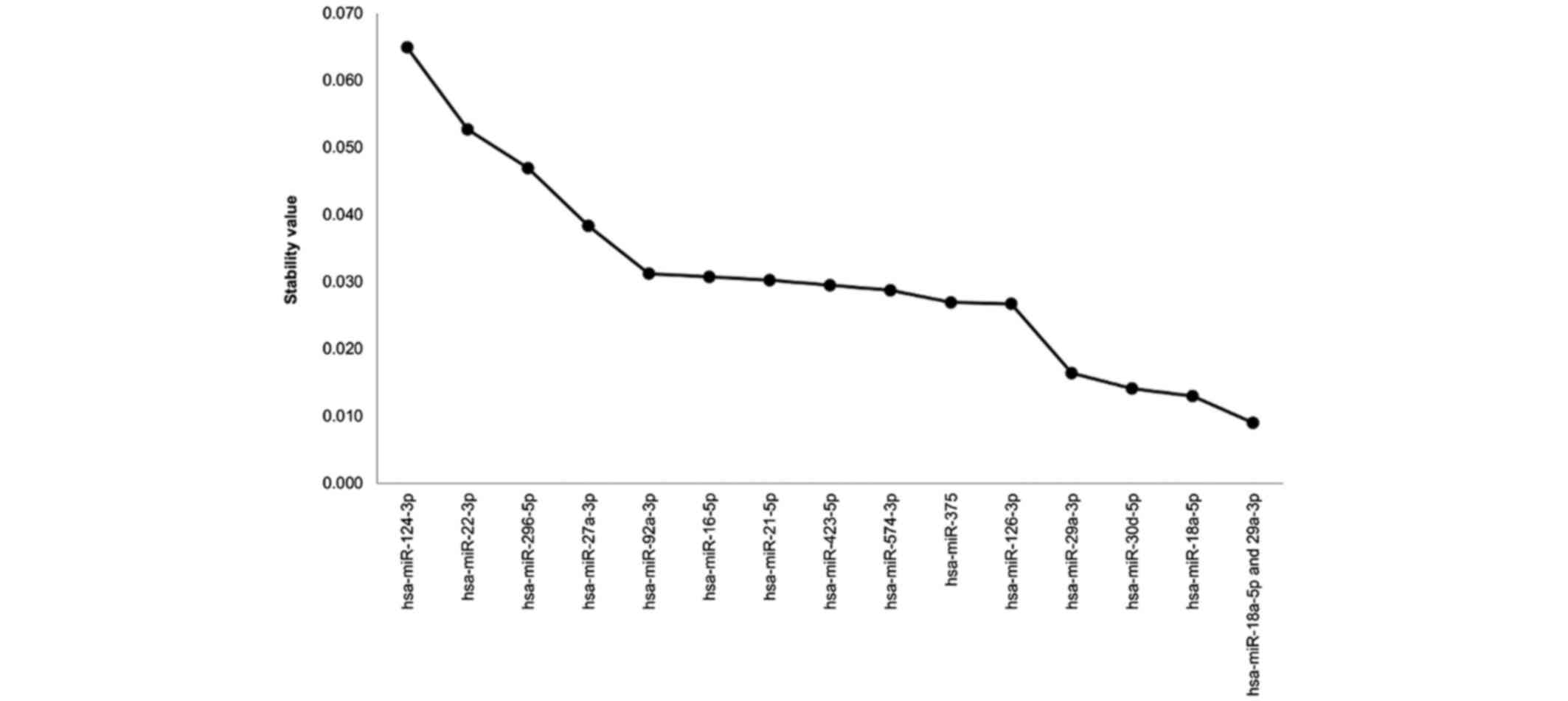
were investigated for normalization. A total of 14 microRNAs with
positive Cq values in all samples were identified, and their
stability values were determined using the NormFinder algorithm.
Smaller stability values are indicative of fewer variations in
expression. A total of 3 microRNAs with a score <0.02 were
identified: hsa-miR-18a-5p, hsa-miR-30d-5p, and hsa-miR-29a-3p
(Fig. 1). The NormFinder algorithm
demonstrated that a combination of hsa-miR-18a-5p and
hsa-miR-29a-3p had a stability value of 0.009, and this combination
was selected as the best array data normalizer.
Data normalization to evaluate
microRNA expression levels
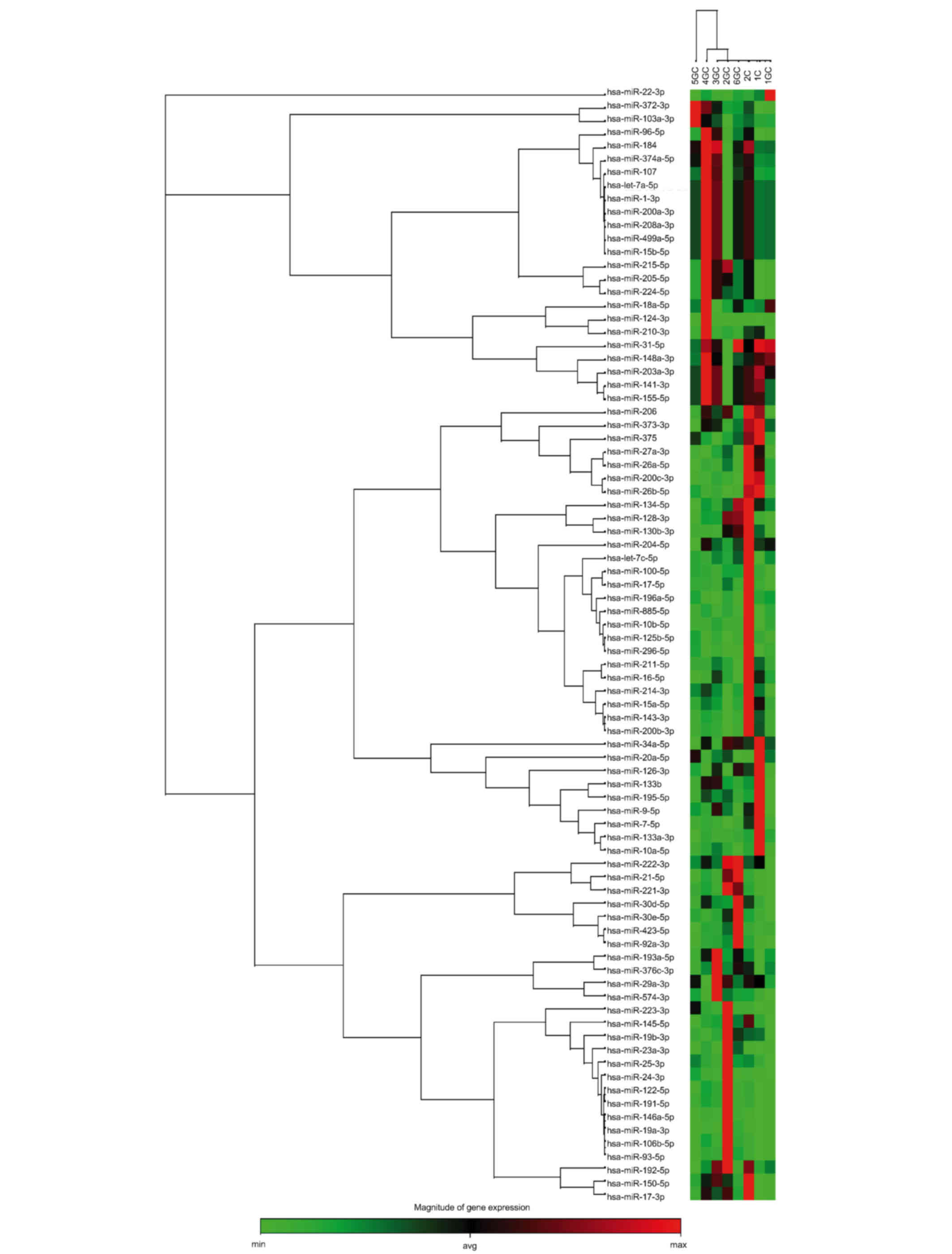
The relative expression of 84 microRNAs was
determined in GC and non-atrophic gastritis samples. Briefly, the
relative expression levels were used to construct a clustergram
(http://pcrdataanalysis.sabiosciences.com/mirna) to
observe the microRNA expression profiles of the samples. Firstly,
the snoRNAs and snRNA normalizer control panel provided by the
array was used. Following this normalization strategy, no specific
expression profile was observed that would allow the distinction of
non-atrophic gastritis from GC, or IGC from DGC (Fig. 2). Subsequently, each microRNA was
evaluated manually and normalized against the combination of
hsa-miR-18a-5p and hsa-miR-29a-3p. More consistent results were
observed following normalization against these stable microRNAs,
which allowed the differentiation between GC and non-atrophic
gastritis by the expression profiles of hsa-miR-200c-3p (ΔΔCq and
Cq P=0.00293) and hsa-miR-26b-5p (ΔΔCq and Cq P=0.00293), which
were significantly decreased in GC samples. A comparison of the
clustergrams following normalization with hsa-miR-18a-5p and
hsa-miR-29a-3p is presented in Fig.
3. There is a difference between CG and non-atrophic gastritis
in data that has been normalized to hsa-miR-18a-5p and
hsa-miR-29a-3p (Fig. 3A) compared
with those that have been obtained with the snoRNA/snRNA panel
(Fig. 3B).
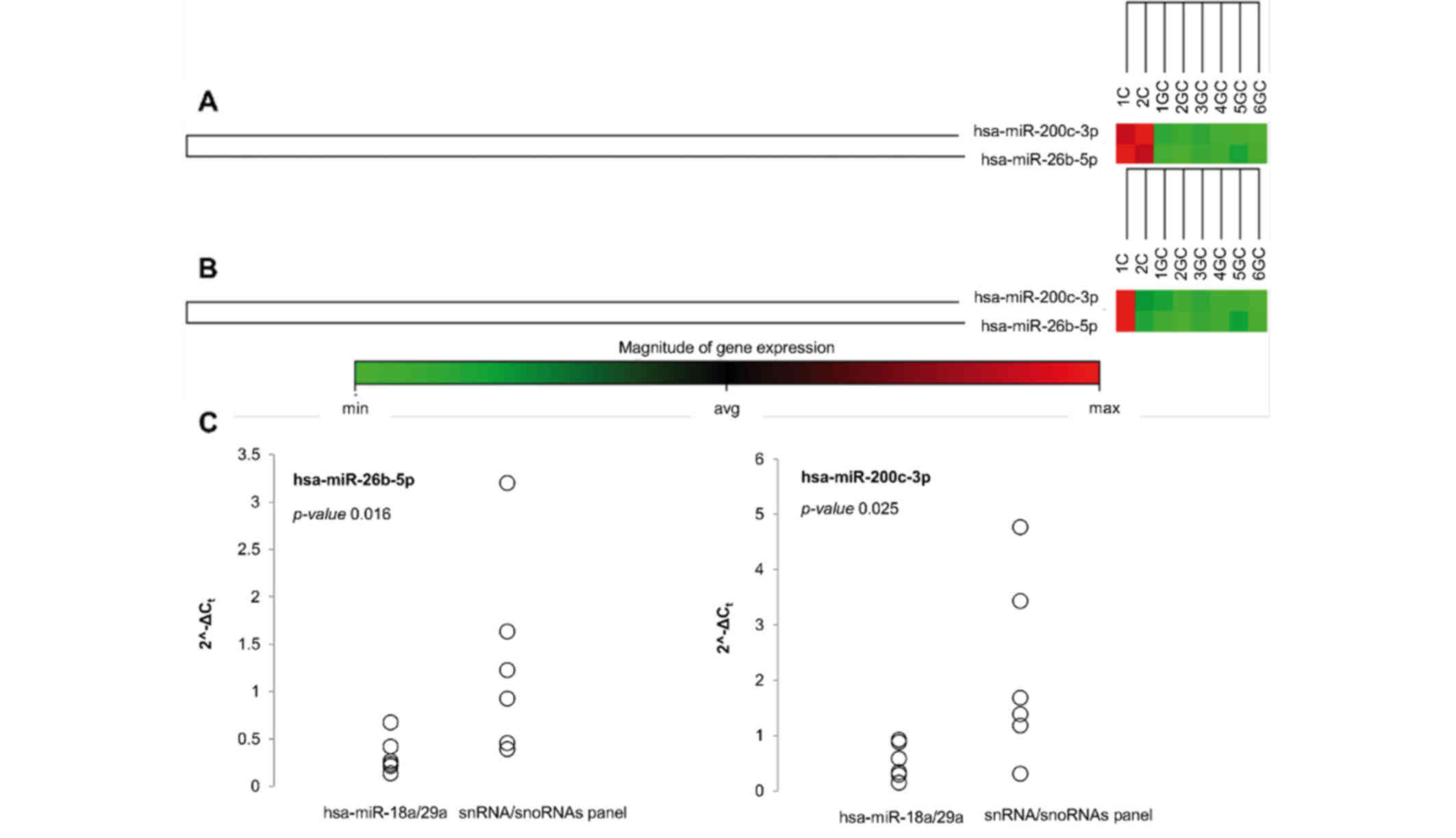 | Figure 3.Differential expression levels of
hsa-miR-200c-3p and hsa-miR-26b-5p obtained by two different
methods of normalization in plasma samples from patients with GC or
non-atrophic gastritis. (A and B) Clustergrams showing
hsa-miR-200c-3p and hsa-miR-26b-5p expression levels in patients
with GC (1-6GC) or non-atrophic gastritis (1C and 2C) normalized
using (A) the stable microRNAs hsa-miR-18a-5p and hsa-miR-29a-3p,
or (B) the snoRNA/snRNA panel. (C) Distribution of 2-∆∆Cq values of
downregulated microRNAs in the plasma samples of patients with GC,
obtained by the two different normalization methods. Circles
represent the values of 2-∆∆Cq for hsa-miR-200c-3p and
hsa-miR-26b-5p normalized using hsa-miR-18a-5p/hsa-miR-29a-3p or
the snoRNA/snRNA panel. For the two microRNAs assessed, a smaller
amount of variation was observed when using
hsa-miR-18a-5p/hsa-miR-29a-3p for normalization compared with the
use the snoRNA/snRNA panel for normalization. The P-values obtained
for each of the microRNAs indicates a significant difference
between the two methods of normalization (P<0.05). GC, gastric
cancer; snoRNA, small nucleolar RNA; snRNA, small nuclear RNA; min,
minimum; avg, average; max, maximum; Cq, quantification cycle. |
A scatter analysis was also performed in order to
observe the distribution of hsa-miR-26b-5p and hsa-miR-200c-3p
expression levels determined by normalization with hsa-miR-18a-5p
and hsa-miR-29a-3p, or with the snoRNAs and snRNA panel; this
revealed that the distribution of the expression values was
narrower when hsa-miR-18a-5p and hsa-miR-29a-3p were used for
normalization (Fig. 3C).
Pathways associated with
hsa-miR-200c-3p and hsa-miR-26b-5p expression
DIANA miRPath reported a total of 841 targets for
hsa-miR-200c-3p and 2,904 targets for hsa-miR-26b-5p using the
TarBase database. The KEGG analysis identified 22 pathways in which
hsa-miR-200c-3p and hsa-miR-26b-5p are most likely to have an
important regulatory role, of which 6 were found to have
significant P-values (Table III).
These 6 identified pathways were mainly involved in processes such
as cell adhesion, cell cycle and cancer.
 | Table III.KEGG pathways associated with
hsa-miR-200c-3p and/or hsa-miR-26b-5p. |
Table III.
KEGG pathways associated with
hsa-miR-200c-3p and/or hsa-miR-26b-5p.
| KEGG pathway | P-value | Number of
genes | Associated
microRNAs |
|---|
| microRNAs in
cancer | 2.18163×10–39 | 71 | hsa-miR-200c-3p and
hsa-miR-26b-5p |
| Hippo signaling
pathway | 6.73503×10–7 | 46 | hsa-miR-200c-3p and
hsa-miR-26b-5p |
| p53 signaling
pathway | 3.60141×10–6 | 33 | hsa-miR-200c-3p and
hsa-miR-26b-5p |
| Glycosaminoglycan
biosynthesis-chondroitin sulfate/dermatan sulfate | 1.96115×10–5 | 9 | hsa-miR-26b-5p |
| TGF-β signaling
pathway | 3.86866×10–5 | 29 | hsa-miR-200c-3p and
hsa-miR-26b-5p |
| Cancer
pathways | 5.33533×10–5 | 116 | hsa-miR-200c-3p and
hsa-miR-26b-5p |
Discussion
The detection of microRNAs is increasingly being
considered as a reliable tool to assess cancer diagnosis and
prognosis, and the detection of circulating microRNAs has the
additional benefit of overcoming the necessity for invasive
procedures (26). However,
uncertainty remains regarding the best candidate targets for data
normalization, since different types of tissues and cancers secrete
variable levels of microRNAs into the circulation. As microRNAs are
critical regulators of cellular processes, their own expression is
strongly regulated. In GC, varying profiles of expression should be
expected from tumors of different histological types or arising
from different regions of the stomach, or depending on whether the
patient is positive or negative for H. pylori. Furthermore, the
stability of secreted microRNAs is variable.
Wang et al (27) identified different profiles of
microRNAs present in H. pylori-positive gastritis and intestinal
metaplasia samples, supporting the use of microRNAs as biomarkers
for the progression of gastric lesions (27). However, despite several different
lines of evidence implicating numerous microRNAs as potential
biomarkers for GC, hsa-miR-21 expression is the only microRNA that
has been consistently identified to exhibit altered expression in
GC (15). The present study also
identified hsa-miR-21 to be overexpressed, but only in 2 of the GC
samples (Fig. 2). By contrast, the
remaining 4 GC samples demonstrated similar values to those of the
non-atrophic gastritis samples. Therefore, this microRNA did not
fulfill the objective of this study to identify microRNAs that are
differentially expressed between patients with non-atrophic
gastritis and those with GC.
Considering the differential expression and the
stability of circulating microRNAs, it has been recommended to
establish the normalizing microRNAs within the set of samples being
tested (16). In the present study,
using the NormFinder algorithm, improved stability values were
observed for hsa-miR-18a-5p and hsa-miR-29a-3p compared with the
snoRNA/snRNA panel provided by the array. Data normalized with
these stable microRNAs were more narrowly distributed, and allowed
the identification of downregulated expression of hsa-miR-200c-3p
and hsa-miR-26b-5p in all GC samples studied, compared with the
non-atrophic gastritis samples. These microRNAs have been reported
to be dysregulated in GC and other types of cancer in previous
studies (28–41). Therefore, the present study also
supports normalization against the most stable microRNAs within the
set of samples analyzed.
hsa-miR-200c belongs to the microRNA-200 family,
which includes hsa-miR-200a, hsa-miR-200b, hsa-miR-200c,
hsa-miR-141 and hsa-miR-429, whose increased expression has been
associated with negative regulation of the
epithelial-to-mesenchymal transition (EMT) via the downregulation
of master regulators of EMT, zinc finger E-box binding homeobox
(ZEB) 1 and 2 (28). In agreement
with this, it has been observed that the overexpression of
hsa-miR-200c can suppress metastasis following the orthotopic
xenotransplantation of liver cancer cells (28). In a clinical colorectal cancer study
by Hur et al (29), miR-200c
underexpression was observed at the invasive front of the primary
tumors, whereas its overexpression as detected in liver metastases.
A study revealed a complex pattern of expression of this family of
microRNAs in which lower levels of expression may promote EMT,
facilitating cell invasion and metastasis, whilst higher levels of
them may promote the reversion of EMT and the colonization of
distant organs (30). A similar axis
of regulation between microRNA-200, ZEB transcription factors and
EMT has been previously reported in GC cell lines (31). The underexpression of hsa-miR-200c has
also been reported in GC samples derived from aggressive tumors and
in invasive GC-derived cell lines with higher cell growth capacity
(32). The reported targets of
hsa-miR-200b and hsa-miR-200c are DNA methyltransferase (DNMT) 3A,
DNMT3B and SP1 (a transactivator of the DNMT1 gene); thus, the loss
of these microRNAs has been associated with increased global DNA
methylation, and their re-expression associated with concomitant
re-expression of genes encoding E-cadherin and p16 through promoter
hypomethylation (32). The present
data agree with these studies, as they also support the finding of
a loss of hsa-miR-200c during the progression from non-atrophic
gastritis to GC. However, the overexpression of hsa-miR-200c has
also been reported in the sera of patients with GC when compared
with sera from healthy controls, and the same study also reported
that high levels of hsa-miR-200c were markers of poor prognosis
(33). The reasons for this
discrepancy of data, and the question of how a suppressor of EMT
could be associated with poor prognosis, is not currently
understood.
microRNAs are therefore divided into two types,
oncogenic microRNA (onco-microRNA) and tumor-suppressive microRNA.
microRNA-26 has also been documented as being a critical regulator
of tumor initiation and progression (onco-microRNA) and as a tumor
suppressor microRNA. microRNA-26 has been observed to be
downregulated in several types of cancer, including bladder, breast
and lung cancer, as well as in hepatocellular carcinoma (34). In prostate cancer hsa-miR-26b was
underexpressed and acted as a tumor suppressor by regulating the
oncogene La ribonucleoprotein domain family member 1 together with
hsa-miR-26a (35). By contrast, two
studies have observed an overexpression of hsa-miR-26b-5p in GC
tissue samples when compared with non-tumor adjacent tissue
(42,43). However, neither of these studies
associated hsa-miR-26b expression with clinical features. It has
been observed that hsa-miR-26a serves a critical role in the
carcinogenesis of different tissues by regulating various
processes, including angiogenesis, invasion, cellular
proliferation, metastasis and energy metabolism (37). Additionally, the therapeutic use of
hsa-miR-26b has been suggested, since its overexpression is
associated with decreased cellular proliferation, migration and
invasion in hepatocellular carcinoma (38), osteosarcoma (39), epithelial ovarian carcinoma (40), prostate (35) and lung cancer (41). Qiu et al (14) also reported the downregulation of
hsa-miR-26a in the plasma of patients with GC, identifying this
microRNA as an interesting candidate for use as a GC biomarker.
In the present study, the DIANA miRPath and KEGG
analyses revealed that hsa-miR-200c-3p and hsa-miR-26b-5p microRNAs
were associated with cancer pathways, including p53 signaling,
hippo signaling, transforming growth factor-β signaling, and
glycosaminoglycan biosynthesis-chondroitin sulfate/dermatan
sulfate. Cooney et al (44)
described that chondroitin sulfate glycosaminoglycans serve an
important role in metastasis of breast cancer cells, and proposed
that these polysaccharides could be targets for novel
anti-metastatic therapies (44).
A critical limitation of the present study is the
low number of samples tested. Despite this, the two microRNAs
identified have been previously suggested to be involved in cancer,
and the in silico analysis supported the involvement of
hsa-miR-200c-3p and hsa-miR-26b-5p in cancer pathways, confirming
these microRNAs as interesting candidates to be evaluated as
potential biomarkers for GC progression in larger scale studies.
The present study therefore serves as a pilot study, providing an
initial exploration of circulating microRNAs that may mirror
oncogenic processes occurring in the gastric mucosa. Although an
extension of this study is planned, we consider the observations
regarding the instability of the array normalization targets to be
a valuable contribution for researchers aiming to quantify microRNA
levels. There are already reports demonstrating that normalization
based on the values of the snoRNA/snRNA of the array is not precise
(16–18,20–23). The
present study has provided a strategy to overcome those problems,
and this method of analysis based on a stability test allowed
reliable data to be obtained, since the two microRNAs identified to
be downregulated in GC patients have previously been reported in
other GC studies of circulating and tissue microRNAs. In addition
to increasing the number of samples analyzed, it is important to
perform in silico and in vitro analyses of the target genes
of hsa-miR-200c and hsa-miR-26b, in order to improve the
understanding of their participation in gastric carcinogenesis.
In conclusion, the present data supports the use of
a test to identify the most stable microRNAs within a set of
samples. Using a stability test, hsa-miR-18a-5p and hsa-miR-29a-3p
were identified as the most stable microRNAs across all the
samples. hsa-miR-18a-5p and hsa-miR-29a-3p microRNAs were
subsequently used to normalize samples derived from patients with
gastric lesions, which led to the finding that the expression
levels of hsa-miR-200c-3p and hsa-miR-26b-5p were downregulated in
sera from GC patients compared with sera from patients with
symptomatic non-atrophic gastritis. This finding suggests that
hsa-miR-200c-3p and hsa-miR-26b-5p may be lost throughout the
progression of gastric lesions.
Acknowledgements
The present study was supported by the Coordination
of Health Research-Mexican Institute of Social Security (grant nos.
FIS/IMSS/PROT/PRIO/13/027 and FIS/IMSS/PROT/G16/1573); the
Secretariat for Research and Postgraduate Studies-National
Polytechnic Institute-Mexico (grant no. 20161878) and the National
Council for Science and Technology (grant no. 576518). This study
constitutes partial fulfillment of the Graduate Program of Master
Degree in Sciences, Biomedicine and Molecular Biotechnology,
National School of Biological Sciences, National Polytechnic
Institute (Mexico City, Mexico). The authors wish to thank Ms.
Margaret Reynolds for her assistance with English editing.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Creemers EE, Tijsen AJ and Pinto YM:
Circulating MicroRNAs: Novel biomarkers and extracellular
communicators in cardiovascular disease? Circ Res. 110:483–495.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munker R and Calin GA: MicroRNA profiling
in cancer. Clin Sci (Lond). 121:141–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shrestha S, Hsu SD, Huang WY, Huang HY,
Chen W, Weng SL and Huang HD: A systematic review of microRNA
expression profiling studies in human gastric cancer. Cancer Med.
3:878–888. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang R, Wen H, Xu Y, Chen Q, Luo Y, Lin Y,
Luo Y and Xu A: Circulating microRNAs as a novel class of
diagnostic biomarkers in gastrointestinal tumors detection: A
meta-analysis based on 42 articles. PLoS One. 9:e1134012014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Zhao Y, Guo G, Li W, Zhu ED, Luo X,
Mao XH, Zou QM, Yu PW, Zuo QF, et al: Plasma microRNAs, miR-223,
miR-21 and miR-218, as novel potential biomarkers for gastric
cancer detection. PLoS One. 7:e416292012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gorur A, Fidanci Balci S, Dogruer Unal N,
Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS and Tamer
L: Determination of plasma microRNA for early detection of gastric
cancer. Mol Biol Rep. 40:2091–2096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li
JY, Yuasa Y, Kang D, Kim YS and You WC: Identification of serum
microRNAs as novel non-invasive biomarkers for early detection of
gastric cancer. PLoS One. 7:e336082012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu X, Zhang J, Shi W, Liu S, Kang M, Chu
H, Wu D, Tong N, Gong W, Tao G, et al: Circulating MicroRNA-26a in
plasma and its potential diagnostic value in gastric cancer. PLoS
One. 11:e01513452016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X, Lv M, Wang H and Guan W:
Identification of circulating MicroRNAs as novel potential
biomarkers for gastric cancer detection: A systematic review and
meta-analysis. Dig Dis Sci. 59:911–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mestdagh P, Van Vlierberghe P, De Weer A,
Muth D, Westermann F, Speleman F and Vandesompele J: A novel and
universal method for microRNA RT-qPCR data normalization. Genome
Biol. 10:R642009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peltier HJ and Latham GJ: Normalization of
microRNA expression levels in quantitative RT-PCR assays:
Identification of suitable reference RNA targets in normal and
cancerous human solid tissues. RNA. 14:844–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sperveslage J, Hoffmeister M, Henopp T,
Klöppel G and Sipos B: Establishment of robust controls for the
normalization of miRNA expression in neuroendocrine tumors of the
ileum and pancreas. Endocrine. 46:226–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juzėnas S, Saltenienė V, Kupcinskas J,
Link A, Kiudelis G, Jonaitis L, Jarmalaite S, Kupcinskas L,
Malfertheiner P and Skieceviciene J: Analysis of deregulated
microRNAs and their target genes in gastric cancer. PLoS One.
10:e01323272015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benz F, Roderburg C, Cardenas Vargas D,
Vucur M, Gautheron J, Koch A, Zimmermann H, Janssen J,
Nieuwenhuijsen L, Luedde M, et al: U6 is unsuitable for
normalization of serum miRNA levels in patients with sepsis or
liver fibrosis. Exp Mol Med. 45:e422013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng G, Wang H, Zhang X, Yang Y, Wang L,
Du L, Li W, Li J, Qu A, Liu Y and Wang C: Identification and
validation of reference genes for qPCR detection of serum microRNAs
in colorectal adenocarcinoma patients. PLoS One. 8:e830252013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Beers EH, Joosse SA, Ligtenberg MJ,
Fles R, Hogervorst FB, Verhoef S and Nederlof PM: A multiplex PCR
predictor for aCGH success of FFPE samples. Br J Cancer.
94:333–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vlachos IS, Zagganas K, Paraskevopoulou
MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T and
Hatzigeorgiou AG: DIANA-miRPath v3.0: Deciphering microRNA function
with experimental support. Nucleic Acids Res. 43:W460–W466. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pritchard CC, Cheng HH and Tewari M:
MicroRNA profiling: Approaches and considerations. Nat Rev Genet.
13:358–369. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XW, Wu Y, Wang D and Qin ZF: MicroRNA
network analysis identifies key microRNAs and genes associated with
precancerous lesions of gastric cancer. Genet Mol Res.
13:8695–8703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong CM, Wei L, Au SL, Fan DN, Zhou Y,
Tsang FH, Law CT, Lee JM, He X, Shi J, et al: MiR-200b/200c/429
subfamily negatively regulates Rho/ROCK signaling pathway to
suppress hepatocellular carcinoma metastasis. Oncotarget.
6:13658–13670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hill L, Browne G and Tulchinsky E:
ZEB/miR-200 feedback loop: At the crossroads of signal transduction
in cancer. Int J Cancer. 132:745–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Xu L, Li C, Zhao L, Ma Y, Zheng H,
Li Z, Zhang Y, Wang R, Liu Y and Qu X: Ubiquitin ligase Cbl-b
represses IGF-I-induced epithelial mesenchymal transition via ZEB2
and microRNA-200c regulation in gastric cancer cells. Mol Cancer.
13:1362014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang H, Deng M, Tang Y and Xie X, Guo J,
Kong Y, Ye F, Su Q and Xie X: MiR-200b and miR-200c as prognostic
factors and mediators of gastric cancer cell progression. Clin
Cancer Res. 19:5602–5612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang HP, Sun FB and Li SJ: Serum miR-200c
expression level as a prognostic biomarker for gastric cancer.
Genet Mol Res. 14:15913–15920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao J and Liu QG: The role of miR-26 in
tumors and normal tissues (Review). Oncol Lett. 2:1019–1023.
2011.PubMed/NCBI
|
|
35
|
Kato M, Goto Y, Matsushita R, Kurozumi A,
Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M,
Ichikawa T and Seki N: MicroRNA-26a/b directly regulate La-related
protein 1 and inhibit cancer cell invasion in prostate cancer. Int
J Oncol. 47:710–718. 2015.PubMed/NCBI
|
|
36
|
Ramachandran K, Saikumar J, Bijol V,
Koyner JL, Qian J, Betensky RA, Waikar SS and Vaidya VS: Human
miRNome profiling identifies MicroRNAs differentially present in
the urine after kidney injury. Clin Chem. 59:1742–1752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen J, Zhang K, Xu Y, Gao Y, Li C, Wang R
and Chen L: The role of microRNA-26a in human cancer progression
and clinical application. Tumor Biol. 37:7095–7108. 2016.
View Article : Google Scholar
|
|
38
|
Li H, Sun Q, Han B, Yu X, Hu B and Hu S:
MiR-26b inhibits hepatocellular carcinoma cell proliferation,
migration, and invasion by targeting EphA2. Int J Clin Exp Pathol.
8:4782–4790. 2015.PubMed/NCBI
|
|
39
|
Zheng WD, Zhou FL and Lin N: MicroRNA-26b
inhibits osteosarcoma cell migration and invasion by
down-regulating PFKFB3 expression. Genet Mol Res. 14:16872–16879.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin J, Zhang L, Huang H, Huang Y, Huang L,
Wang J, Huang S, He L, Zhou Y, Jia W, et al: MiR-26b/KPNA2 axis
inhibits epithelial ovarian carcinoma proliferation and metastasis
through downregulating OCT4. Oncotarget. 6:23793–23806. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia M, Duan ML, Tong JH and Xu JG: MiR-26b
suppresses tumor cell proliferation, migration and invasion by
directly targeting COX-2 in lung cancer. Eur Rev Med Pharmacol Sci.
19:4728–4737. 2015.PubMed/NCBI
|
|
42
|
Xie J, Chen M, Zhou J, Mo MS, Zhu LH, Liu
YP, Gui QJ, Zhang L and Li GQ: miR-7 inhibits the invasion and
metastasis of gastric cancer cells by suppressing epidermal growth
factor receptor expression. Oncol Rep. 31:1715–1722.
2014.PubMed/NCBI
|
|
43
|
Inoue T, Iinuma H, Ogawa E, Inaba T and
Fukushima R: Clinicopathological and prognostic significance of
microRNA-107 and its relationship to DICER1 mRNA expression in
gastric cancer. Oncol Rep. 27:1759–1764. 2012.PubMed/NCBI
|
|
44
|
Cooney CA, Jousheghany F, Yao-Borengasser
A, Phanavanh B, Gomes T, Kieber-Emmons AM, Siegel ER, Suva LJ,
Ferrone S, Kieber-Emmons T and Monzavi-Karbassi B: Chondroitin
sulfates play a major role in breast cancer metastasis: A role for
CSPG4 and CHST11 gene expression in forming surface P-selectin
ligands in aggressive breast cancer cells. Breast Cancer Res.
13:R582011. View Article : Google Scholar : PubMed/NCBI
|