Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most common types of malignant tumors of the digestive tract,
and it has the worst prognosis of all major malignancies, with a
5-year survival rate of 6% and a median survival of 6 months
subsequent to diagnosis (1,2). Although numerous advances in prevention,
surgical resection and adjuvant chemoradiotherapy have led to a
decline in the overall mortality due to PDAC, the survival rates
for patients with metastatic disease have not significantly
improved (3,4). Therefore, the identification of an ideal
marker for early diagnosis and prognosis evaluation of PDAC has
been an important research field in clinical examination.
Nicotinamide adenine dinucleotide phosphate (NADPH):
quinone oxidoreducase 1 (NQO1) is located on chromosome 16q22, and
consists of six exons and five introns (5). NQO1 is a flavin adenine
dinucleotide-dependent direct two-electron reductase that can use
NADH or NADPH as reducing cofactors to reduce quinones to
hydroquinones (6). Several functions
of NQO1 have been reported, including xenobiotic detoxification,
superoxide scavenging, modulation of p53, maintenance of endogenous
antioxidants and proteasomal degradation (7), suggesting that NQO1 is important in
protecting normal cells against oxidative injury and carcinogenesis
(8). NQO1 protein was also observed
to be abnormally expressed in numerous human tumors (9,10).
However, there is limited research investigating the associations
between NQO1 expression and PDAC development and progression.
Thus, the current study aimed to demonstrate the
association between NQO1 expression and clinicopathological
characteristics of patients with PDAC. The present results revealed
that NQO1 was significantly upregulated in PDAC, which was
associated with tumor grade, tumor node-metastasis (TNM) stage,
lymph node (LN) metastasis and overall survival (OS) of patients
with PDAC. NQO1 may be an independent prognostic biomarker for
patients with PDAC.
Materials and methods
Ethics statement
The present study complied with the principles of
the Declaration of Helsinki, and was approved by the human ethics
and research ethics committees of Yanbian University Medical
College (Yanji, China). Patients provided written informed consent
and were informed that resected specimens were stored by the
hospital, and potentially used for scientific research, and their
privacy would be maintained. Follow-up survival data were collected
retrospectively through medical record analyses.
Clinical specimens
A total of 181 tissue specimens were studied,
including 126 PDAC tissues and 55 normal pancreas specimens. All
tissues were obtained from Shanghai Outdo Biotech Co., Ltd.
(Shanghai, China) and the Tissue Bank of Yanbian University Medical
College. All tissues were fixed in 10% buffered formalin and
paraffin embedded. The present study protocol was approved by the
institutional review board of Yanbian University Medical College.
Pathological parameters consisted of age, gender, tumor size,
differentiation, pathological grading, TNM stage, LN metastasis and
survival data. In total, 74 patients were >50 years old and 52
patients were below 50 years of age. TNM staging was assessed
according to the staging system established by the American Joint
Committee on Cancer (AJCC) (11–13). The
male-to-female ratio was 71:55. Staging was performed according to
the TNM stage, and 72 patients were stage I–II while 54 patients
were stage III–IV. In addition, the ratio of tumor grading was
36(G1):37(G2):53(G3). Among the 126 patients with PDAC, 74 patients
had LN metastasis while 52 patients did not have LN metastasis. In
the 126 patients with PDAC, the follow-up time was 30–67
months.
Immunofluorescence (IF) staining for
NQO1 protein in BxPC-3 cells
BxPC-3 was purchased from the Korean Cell Line Bank
(Seoul, Korea). The cells were grown on glass coverslips in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) media with 10% heat inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The
cells were incubated at 37°C in an atmosphere containing 5%
CO2 and at 95% humidity, to between 70 and 80%
confluence. The cells were fixed 4% paraformaldehyde for 20 min at
room temperature (RT) and permeabilized with 0.1% Triton X-100 for
10 min. The cells were then washed with PBS, and 5% bovine serum
albumin was next added for 30 min at RT. The cells were
subsequently incubated with anti-NQO1 antibody (dilution, 1:200;
cat. no. A180:sc-32793; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at 4°C overnight. The cells were then incubated with Alexa
Fluor® 568 Goat Anti-Mouse IgG (H+L) secondary antibody
(dilution, 1:200; cat. no. A11004; Invitrogen; Thermo Fisher
Scientific, Inc.) for 1 h at RT. Subsequent to washing with PBS,
cells were counterstained with DAPI (cat. no. C1006; Beyotime
Institute of Biotechnology, Shanghai, China), and the coverslips
were mounted with Antifade Mounting Medium (cat. no. P0126;
Beyotime Institute of Biotechnology). Finally, the IF signals were
visualized and recorded with a TCS SP5 II confocal microscope
(Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Immunohistochemical (IHC)
analysis
IHC analysis was performed using the LSAB kit (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA). Prior to IHC
staining, all sections were deparaffinized, rehydrated and
incubated with 3% H2O2 in methanol for 15 min
at RT. The antigen was retrieved at 95°C for 20 min by placing the
slides in 0.01 M sodium citrate buffer (pH 6.0). The slides were
then incubated with the aforementioned anti-NQO1 monoclonal
antibody (dilution, 1:200) at 4°C overnight. Subsequent to
incubation with a biotinylated secondary antibody (pre-diluted;
cat. no. PV9000; OriGene Technologies, Inc., Beijing, China) at RT
for 30 min, the slides were incubated with a
streptavidin-peroxidase complex (cat. no. PV9000; OriGene
Technologies, Inc.) at RT for 30 min. IHC staining was developed
using 3,3′-diaminobenzidine, and Mayer's hematoxylin (cat. no.,
ZLI9610; OriGene Technologies, Inc.) was used for counterstaining.
Mouse immunoglobulin G (pre-diluted; cat. no. PV9000; OriGene
Technologies, Inc.) was used as an isotype control, incubation at
RT for 30 min. A negative control was utilized by processing the
tissue sections without the primary antibody.
Evaluation of IHC staining
All tissue specimens were blindly examined by two
pathologists. In case of discrepancies, a final score was
established by reassessment on a double-headed microscope.
Immunostaining for NQO1 was semi-quantitatively scored as: -,
<5% positive cells; +, 5–25% positive cells; ++, 26–50% positive
cells; and +++, >50% positive cells. Cytoplasmic or nuclear NQO1
staining was considered to be positive staining. Tissue sections
scored as ++, and +++ were considered to be strong positive
staining. For survival data analysis, ++ or +++ scored samples were
considered high-level NQO1 expression, while - or + scored samples
were considered to exhibit low levels of NQO1 expression.
Statistical analysis
All statistical analyses were performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA) software.
χ2 test and Fisher's exact test, were used to assess the
association between clinicopathological characteristics and the
expression of studied protein. The survival rates following tumor
removal were calculated using the Kaplan-Meier estimator and
differences in survival curves were analyzed using log-rank tests.
Multivariate survival analysis was performed on all the significant
characteristics measured by univariate survival analysis through
the Cox proportional hazards regression model. P<0.05 was
considered to indicate a statistically significant difference.
Results
High expression of NQO1 protein in
PDACs
The positive rate of NQO1 protein expression in PDAC
tissue was significantly increased compared with that in normal
pancreatic tissues (83.3%, 105/126, P<0.01). Similarly, the
strongly positive rate of NQO1 expression in PDAC tissue was
increased compared with that in normal pancreatic tissues (65.9%,
83/126, P<0.01; Table I). IF
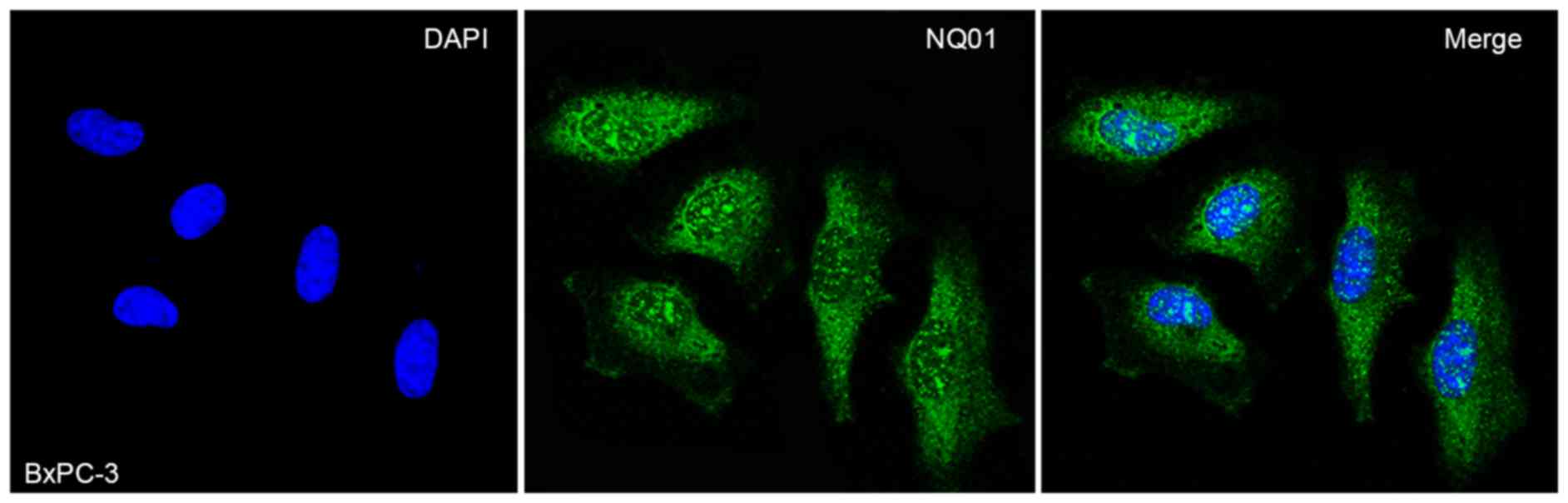
staining revealed that NQO1 protein was mainly located in the
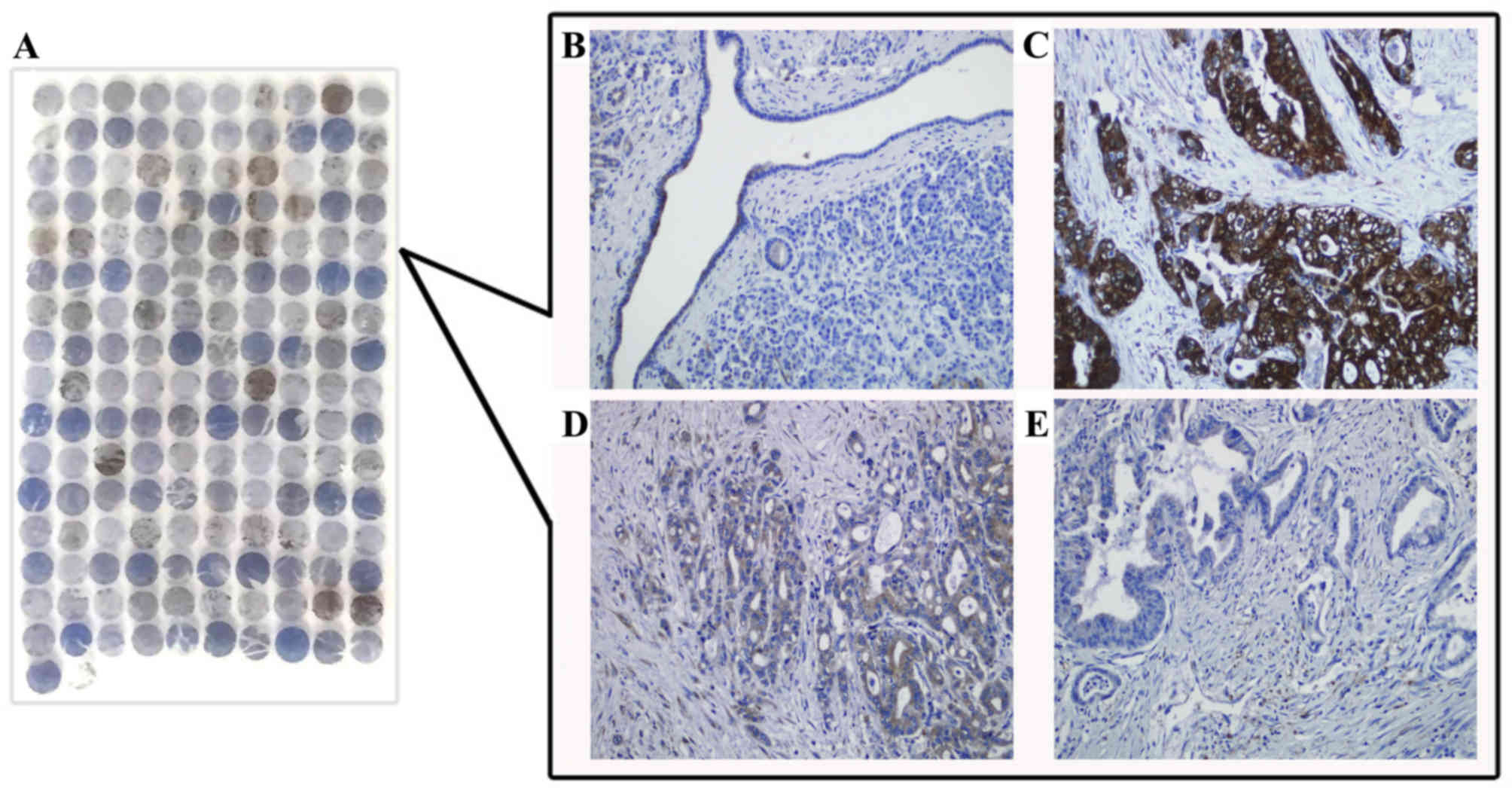
cytoplasm and nucleus of BxPC-3 cells (Fig. 1). Consistently, IHC staining
demonstrated that NQO1 protein localized to the cytoplasm of PDAC
tissues (Fig. 2).
 | Table I.Nicotinamide adenine dinucleotide
phosphate:quinone oxidoreductase 1 protein expression in pancreatic
ductal adenocarcinoma. |
Table I.
Nicotinamide adenine dinucleotide
phosphate:quinone oxidoreductase 1 protein expression in pancreatic
ductal adenocarcinoma.
|
|
| Positive
patients |
|
|
|---|
|
|
|
| Positive patient
rates, % | Strongly positive
rates,% |
|---|
| Diagnosis | Patients, n | − | + | ++ | +++ |
|
|
|---|
| PDAC tissue | 126 | 21 | 22 | 45 | 38 | 83.3a | 65.9a |
| Normal pancreas | 55 | 36 | 13 | 6 | 0 | 34.5 | 10.9 |
Clinicopathological significance of
NQO1 expression in patients with PDAC
The present study investigated the association
between NQO1 expression and the clinicopathological features of
patients with PDAC. According to the clinical parameters listed in
Table II, it was shown that NQO1
overexpression was not associated with patient's age (P=0.153),
gender (P=0.931), tumor size (P=0.192) or differentiation
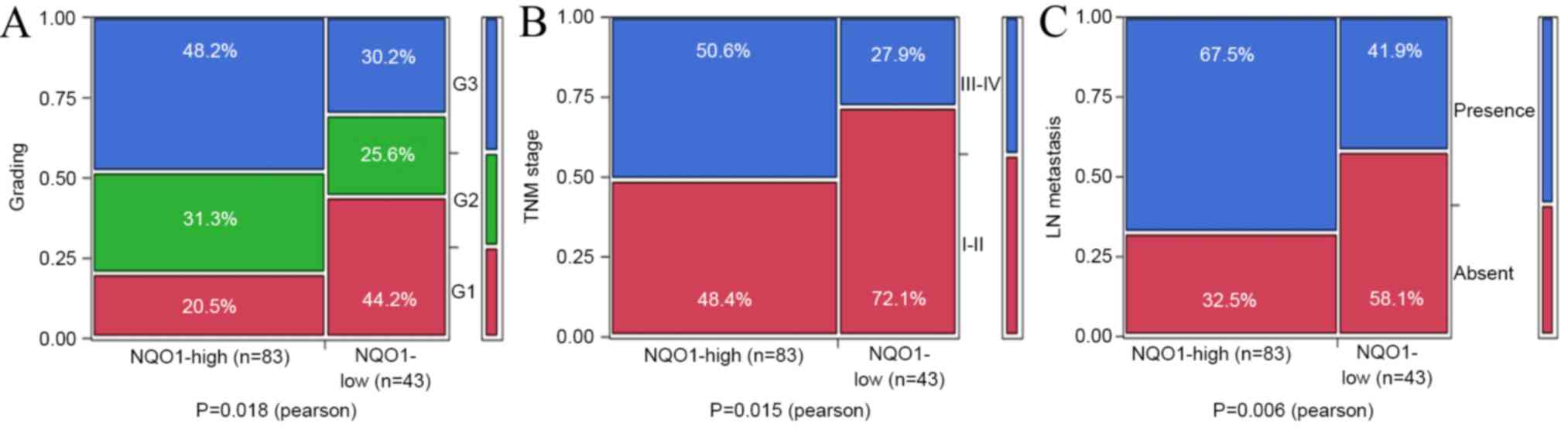
(P=0.050). However, it was observed that the positive rates of NQO1
expression in grade 2 (G2; 70.3%, 26/37) and grade 3 (G3; 75.5%,
40/53) were significantly increased compared with those in grade 1
(G1; 47.2%, 17/36, P=0.018). Additionally, NQO1 expression was
higher in TNM stage III–IV (77.8%, 42/54) compared with that in
stage I–II (56.9%, 41/72, P=0.015). In addition, NQO1 expression
was higher in PDAC patients with LN metastasis (75.7%, 56/74) than
in patients without LN metastasis (51.9%, 27/52; P<0.01;
Table II and Fig. 3).
 | Table II.Association between high expression of
NQO1 protein and clinicopathological parameters of patients with
pancreatic ductal adenocarcinoma. |
Table II.
Association between high expression of
NQO1 protein and clinicopathological parameters of patients with
pancreatic ductal adenocarcinoma.
| Variables | Patients, n | NQO1 strongly
positive patients, n (%) | P-value |
|---|
| Age, years |
|
|
|
| ≥50 | 74 | 45 (60.8) | 0.153 |
|
<50 | 52 | 38 (73.1) |
|
| Gender |
|
|
|
| Male | 71 | 47 (66.2) | 0.931 |
|
Female | 55 | 36 (65.4) |
|
| Tumor size, cm |
|
|
|
|
<3 | 75 | 46 (61.3) | 0.192 |
| ≥3 | 51 | 37 (72.5) |
|
| Differentiation |
|
|
|
| Well | 37 | 21 (56.8) | 0.050 |
|
Moderately | 42 | 26 (61.9) |
|
|
Poorly | 47 | 36 (76.6) |
|
| Grading |
|
|
|
| G1 | 36 | 17 (47.2) | 0.018 |
| G2 | 37 | 26 (70.3) |
|
| G3 | 53 | 40 (75.5) |
|
| TNM stage |
|
|
|
| I–II | 72 | 41 (56.9) | 0.015 |
| III–IV | 54 | 42 (77.8) |
|
| LN metastasis |
|
|
|
| Absence | 52 | 27 (51.9) | 0.006 |
| Presence | 74 | 56 (75.7) |
|
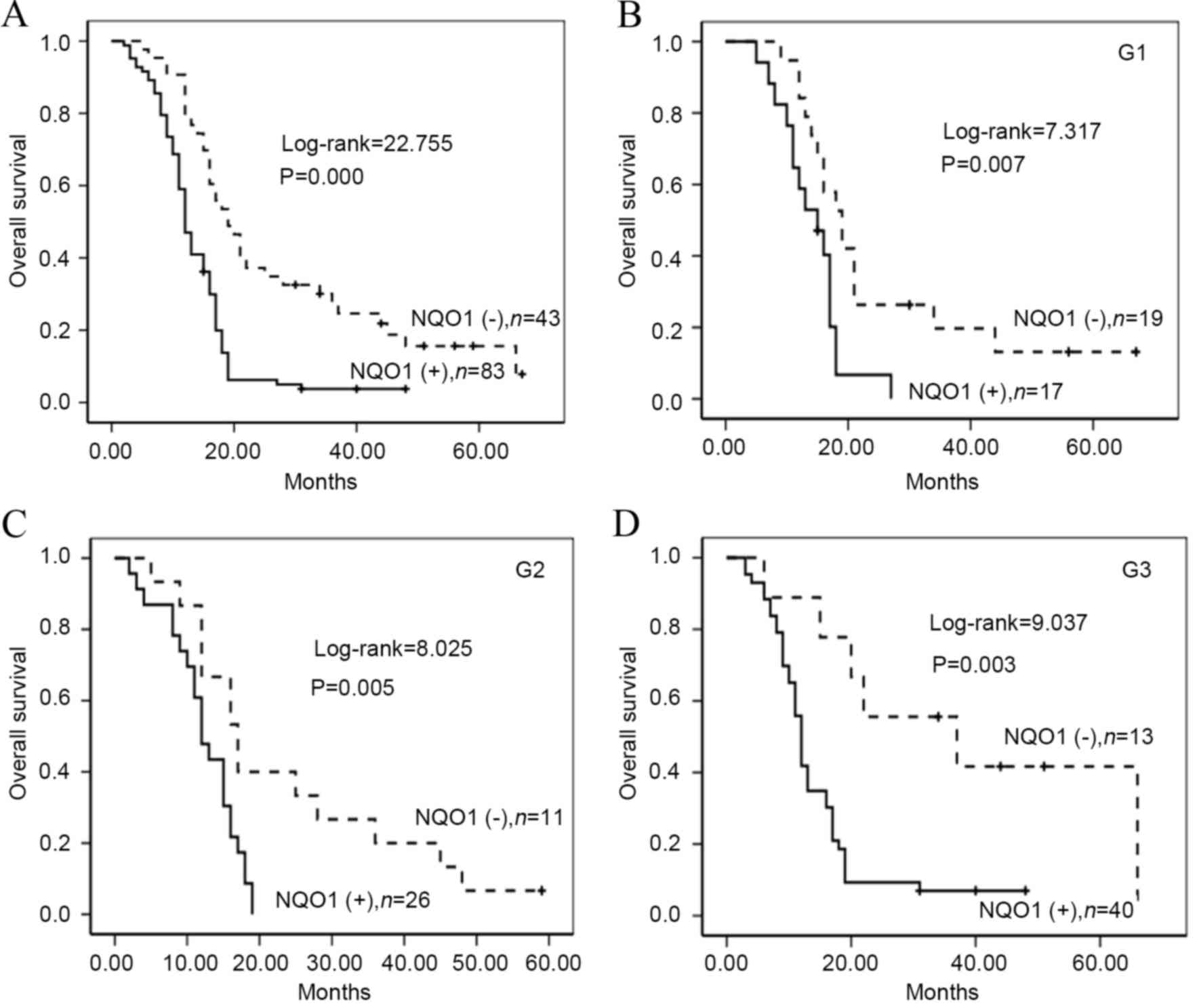
To additionally confirm the importance of NQO1 in
PDAC, the present study analyzed the OS of 162 patients with PDAC
using the Kaplan-Meier method. Patients with high NQO1 expression
exhibited a lower rate of OS compared with those with low NQO1
expression (log-rank=22.755, P<0.001; Fig. 4A). Similarly, survival of patients
with G1 (log-rank=7.317, P<0.01), G2 (log-rank=8.025, P<0.01)
and G3 (log-rank=9.037, P=0.003) was significantly lower in PDAC
tissues exhibiting high NQO1 expression compared with those that in
tissues exhibiting low NQO1 expression (Fig. 4B-D). Patients with PDAC and NQO1
positive expression had lower OS rates compared with those without
NQO1 expression in the absence (log-rank=19.291, P<0.001) and
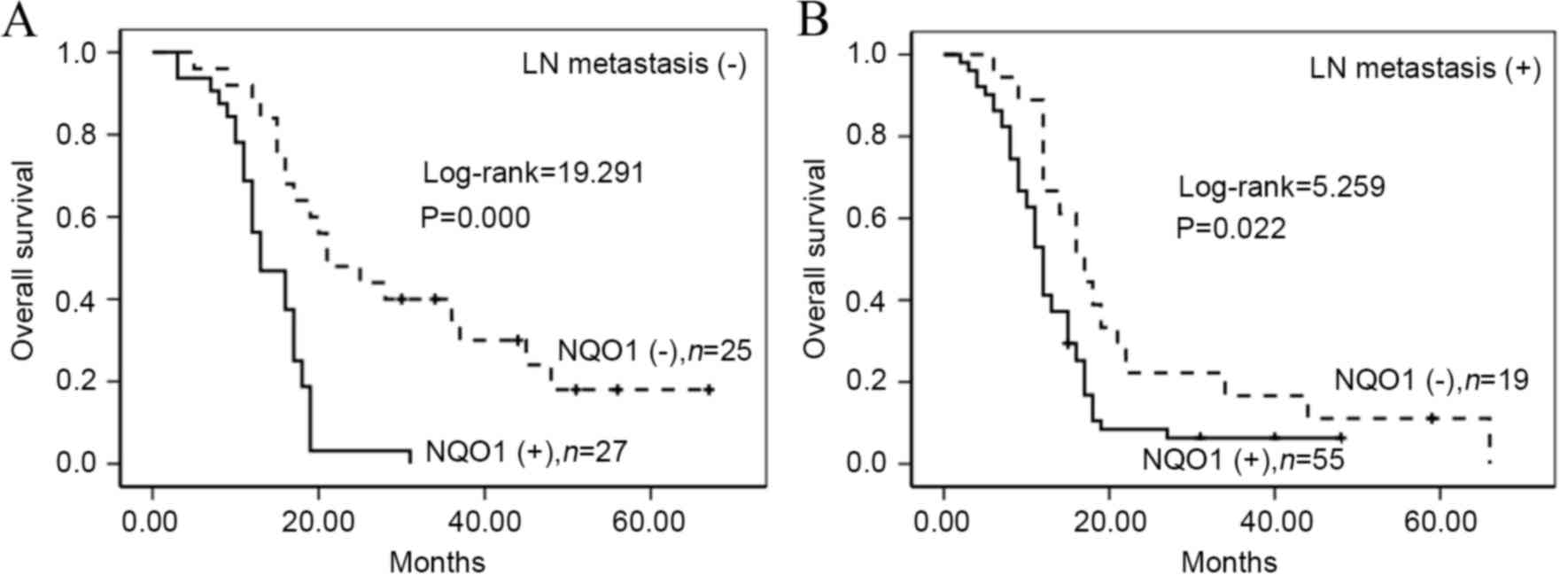
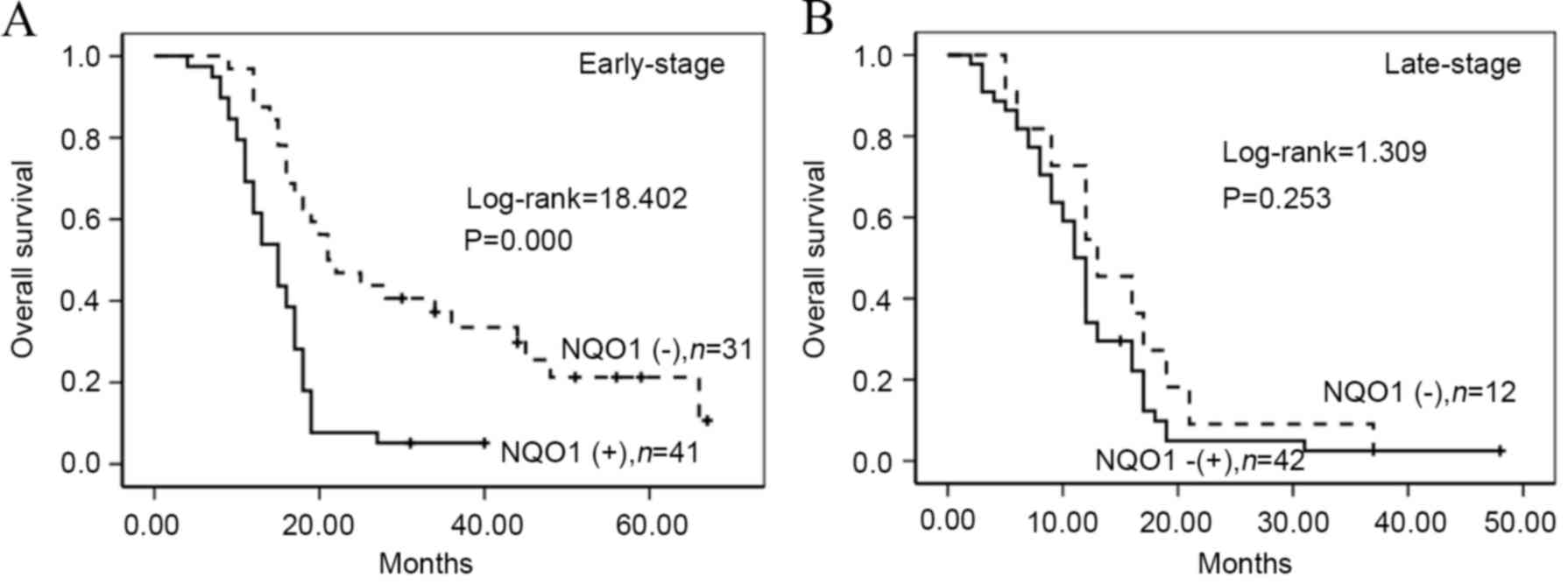
presence of LN metastasis (log-rank=5.259, P=0.022) (Fig. 5). Furthermore, patients with PDAC
exhibiting high NQO1 expression displayed decreased OS compared
with those with low NQO1 expression in early stage patients
(log-rank=18.402, P<0.001; Fig.
6).
NQO1 expression is an independent
prognostic biomarker in PDAC
Univariate analysis demonstrated that PDAC patients
with NQO1 positive expression exhibited significant lower OS rate
[hazard ratio (HR)=1.715, 95% confidence interval (CI)=1.802–4.091,
P<0.001) compared with those with NQO1 negative expression.
Additionally, tumor size (HR=1.444, 95% CI=1.008–2.068, P=0.045),
TNM stage (HR=2.148, 95% CI=1.487–3.102, P<0.001) and LN
metastasis (HR=1.466, 95% CI=1.028–2.091, P=0.035) were all
significantly associated with OS rates of PDAC patients.
Furthermore, multivariate analysis was performed using the Cox
proportional hazards model for all the variables examined in the
univariate analysis. It was observed that NQO1 expression emerged
as a significant independent prognostic factor for OS rates in
patients with PDAC (HR=2.340, 95% CI=1.409–3.884, P=0.001) along
with TNM stage (HR=1.962, 95% CI=1.334–2.817, P=0.002; Table III).
 | Table III.Univariate and multivariate analyses
of clinicopathological factors for the overall survival rate of 126
patients with pancreatic ductal adenocarcinoma. |
Table III.
Univariate and multivariate analyses
of clinicopathological factors for the overall survival rate of 126
patients with pancreatic ductal adenocarcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.241
(0.864–1.864) | 0.242 | 1.306
(0.876–1.876) | 0.189 |
| Gender | 1.346
(0.935–1.935) | 0.110 | 0.799
(0.526–1.526) | 0.295 |
| Tumor size | 1.444
(1.008–2.008) | 0.045 | 1.294
(0.890–1.890) | 0.177 |
|
Differentiation | 1.086
(0.878–1.878) | 0.446 | 1.067
(0.842–1.842) | 0.592 |
| Grading | 1.094
(0.887–1.887) | 0.399 | 0.856
(0.678–1.678) | 0.192 |
| TNM stage | 2.148
(1.487–3.487) | 0.000 | 1.962
(1.334–2.334) | 0.002 |
| LN metastasis | 1.466
(1.028–2.028) | 0.035 | 1.441
(0.986–2.986) | 0.059 |
| NQO1
expression | 2.715
(1.802–4.802) | 0.000 | 2.340
(1.409–3.409) | 0.001 |
Discussion
NQO1 was identified by Ernster and Navazio in 1958
(14) as a cytosolic flavoenzyme that
reduces quinones to less toxic hydroquinones in a single
two-electron transfer step (15).
Subsequent to decades of research, it is currently known that NQO1
can induce a series of defensive factors to prevent the damage
caused by exogenous substances, oxidants, and ultraviolet and
ionizing radiation (16). NQO1 is
additionally involved in the maintenance of the active forms of
coenzyme Q and vitamin E, the regulation of the immune response and
autoimmunity and the stabilization of the tumor suppressors p53,
p73a and p33 (17).
As a cell protector, there is evidence that NQO1
expression was significantly increased in the cytoplasm and nucleus
of certain human solid tumors (18).
Malkinson et al (19) observed
that NQO1 was highly expressed in human lung cancer tissues. Cheng
et al (20) demonstrated that
NQO1 expression was significantly increased in primary melanomas
compared with that in dysplastic nevi, and that this may occur in
the initiation stage of melanoma development. Our previous studies
have shown that the expression of NQO1 protein was significantly
high in non-small cell lung cancer (21), serous ovarian carcinoma (22) and gastric adenocarcinoma (23), indicating that the expression of NQO1
was associated with the occurrence of tumors, and that it may be a
significant prognostic or predictive marker. However, the role of
NQO1 as a biomarker in PDAC is unclear.
In the present study, IHC staining of NQO1 protein
in PDAC tissue was performed, and it was observed that staining of
NQO1 is localized in the cytoplasm. IF staining also revealed that
NQO1 protein localized in the cytoplasm and nucleus of BxPC-3
cells. In these tissues, the strongly positive rate of NQO1 protein
was 65.9% (83/126) in PDAC tissue, which was significantly greater
than that in normal pancreas tissue, indicating that NQO1 may serve
an important role in the progression of PDAC. The current study
analyzed the association between high expression of NQO1 protein
and the clinicopathological parameters of PDAC; the results
indicated that the rate of strongly positive NQO1 protein
expression in G2 and G3 PDAC was increased compared with that in G1
PDAC. Additionally, high NQO1 expression was significantly
associated with LN metastasis and TNM stage, which is consistent
with the study by Mikami et al (24), suggesting that NQO1 upregulation
promotes the invasion and metastasis of PDAC.
Buranrat et al (25) reported a significant association
between high level of NQO1 expression and short OS time of patients
with cholangiocarcinoma, which raised the possibility of using NQO1
as a tumor marker. In the present study, univariate survival
analysis revealed that tumor size (P=0.045), TNM stage
(P<0.001), LN metastasis (P=0.035) and NQO1 expression
(P<0.001) status were all significantly associated with OS of
patients with serous PDAC. Additionally, multivariate survival
analysis revealed that NQO1 expression was a prognostic factor of
PDAC, along with TNM stage. Overall, the present results indicate
that NQO1 may be a biomarker for early diagnosis and prognosis, and
a potential molecular target in patients with PDAC.
Recent studies have reported that anti-tumor agents
such as β-lapachone and deoxynyboquinone (DNQ) effectively kill
cancer cells through NQO1 specific activation (26,27). Huang
et al reported that the potency and NQO1-dependent
therapeutic window of DNQ and its apparent reduced metabolism by
one-electron oxidoreductases make DNQ or its derivatives a
promising avenue for study (28).
In conclusion, NQO1 is important in the occurrence
of PDAC, and may serve as an important marker and potential
therapeutic target for PDAC. Therefore, additional research may
verify whether NQO1 inhibitors could be used in the treatment of
patients with PDAC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81460399
and 61371067).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michl P and Gress TM: Current concepts and
novel targets in advanced pancreatic cancer. Gut. 62:317–326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Charpentier M and Martin S: Interplay of
stem cell characteristics, EMT and microtentacles in circulating
breast tumor cells. Cancers (Basel). 5:1545–1565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuppone F, Bria E, Vaccaro V, Puglisi F,
Fabi A, Sperduti I, Carlini P, Milella M, Nisticò C, Russillo M, et
al: Magnitude of risks and benefits of the addition of bevacizumab
to chemotherapy for advanced breast cancer patients:
Meta-regression analysis of randomized trials. J Exp Clin Cancer
Res. 30:542011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosvold EA, McGlynn KA, Lustbader ED and
Buetow KH: Re: Detection of a point mutation in NQO1
(DT-diaphorase) in a patient with colon cancer. J Natl Cancer Inst.
87:1802–1803. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schlager JJ and Powis G: Cytosolic
NAD(P)H: (Quinone-acceptor)oxidoreductase in human normal and tumor
tissue: Effects of cigarette smoking and alcohol. Int J Cancer.
45:403–409. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buranrat B, Prawan A, Kukongviriyapan U,
Kongpetch S and Kukongviriyapan V: Dicoumarol enhances
gemcitabine-induced cytotoxicity in high NQO1-expressing
cholangiocarcinoma cells. World J Gastroenterol. 16:2362–2370.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su XL, Yan MR and Yang L: Qimuge-Suyila:
NQO1 C609T polymorphism correlated to colon cancer risk in farmers
from western region of Inner Mongolia. Chin J Cancer Res.
24:317–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siegel D, Franklin WA and Ross D:
Immunohistochemical detection of NAD(P)H: Quinone oxidoreductase in
human lung and lung tumors. Clin Cancer Res. 4:2065–2070.
1998.PubMed/NCBI
|
|
10
|
Siegel D and Ross D: Immunodetection of
NAD(P)H: Quinone oxidoreductase 1 (NQO1) in human tissues. Free
Radic Biol Med. 29:246–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. 7th. Wiley Blackwell;
Bognor Regis, UK: 2009
|
|
12
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. Springer; New
York, NY: 2010
|
|
13
|
Bosman F, Carneiro F, Hruban RH and Theise
ND: Who classification of Tumours of the Digesitive System. IARC
Press; Lyon: 2010
|
|
14
|
Ernster L and Navazio F: Soluble
diaphorase in animal tissues. Acta Chem Scand. 12:595–602. 1958.
View Article : Google Scholar
|
|
15
|
Ross D, Kepa JK, Winski SL, Beall HD,
Anwar A and Siegel D: NAD(P)H: Quinone oxidoreductase 1 (NQO1):
Chemoprotection, bioactivation, gene regulation and genetic
polymorphisms. Chem Biol Interact. 129:77–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garate M, Wani AA and Li G: The NAD(P)H:
Quinone oxidoreductase 1 induces cell cycle progression and
proliferation of melanoma cells. Free Radic Biol Med. 48:1601–1609.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iskander K, Li J, Han S, Zheng B and
Jaiswal AK: NQO1 and NQO2 regulation of humoral immunity and
autoimmunity. J Biol Chem. 281:30917–30924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel D, Kepa JK and Ross D: NAD(P)H:
Quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in
human cells. PLoS One. 7:e448612012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malkinson AM, Siegel D, Forrest GL, Gazdar
AF, Oie HK, Chan DC, Bunn PA, Mabry M, Dykes DJ, Harrison SD, et
al: Elevated DT-diaphorase activity and messenger RNA content in
human non-small cell lung carcinoma: Relationship to the response
of lung tumor xenografts to mitomycin Cl. Cancer Res. 52:4752–4757.
1992.PubMed/NCBI
|
|
20
|
Cheng Y, Li J, Martinka M and Li G: The
expression of NAD(P)H: Quinone oxidoreductase 1 is increased along
with NF-κB p105/p50 in human cutaneous melanomas. Oncol Rep.
23:973–979. 2010.PubMed/NCBI
|
|
21
|
Li Z, Zhang Y, Jin T, Men J, Lin Z, Qi P,
Piao Y and Yan G: NQO1 protein expression predicts poor prognosis
of non-small cell lung cancers. BMC Cancer. 15:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui X, Li L, Yan G, Meng K, Lin Z, Nan Y,
Jin G and Li C: High expression of NQO1 is associated with poor
prognosis in serous ovarian carcinoma. BMC cancer. 15:2442015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin L, Qin Y, Jin T, Liu S, Zhang S, Shen
X and Lin Z: Significance of NQO1 overexpression for prognostic
evaluation of gastric adenocarcinoma. Exp Mol Pathol. 96:200–205.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mikami K, Naito M, Tomida A, Yamada M,
Sirakusa T and Tsuruo T: DT-diaphorase as a critical determinant of
sensitivity to mitomycin C in human colon and gastric carcinoma
cell lines. Cancer Res. 56:2823–2826. 1996.PubMed/NCBI
|
|
25
|
Buranrat B, Chau-In S, Prawan A, Puapairoj
A, Zeekpudsa P and Kukongviriyapan V: NQO1 expression correlates
with cholangiocarcinoma prognosis. Asian Pac J Cancer Prev.
13:(Suppl). S131–S136. 2012.
|
|
26
|
Kuang HN, Weng TY, Liu YL, Lu KS and Chau
YP: Sulidac compounds facilitate the cytotoxicity of β-lapachone by
up-regulation of NAD(P)H quinone oxidoreductase in human lung
cancer cells. PLoS One. 9:e881222014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS
and Kwon TK: β-lapachone induces programmed necrosis through the
RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma
SK-Kep1 cells. Cell Death Dis. 5:e12302014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang X, Dong Y, Bey EA, Kilgore JA, Bair
JS, Li LS, Patel M, Parkinson EI, Wang Y, Williams NS, et al: An
NQO1 substrate with potent antitumor activity that selectively
kills by PARP1-induced programmed necrosis. Cancer Res.
72:3038–3047. 2012. View Article : Google Scholar : PubMed/NCBI
|